Published online Nov 14, 2009. doi: 10.3748/wjg.15.5307
Revised: August 28, 2009
Accepted: September 5, 2009
Published online: November 14, 2009
AIM: To investigate the molecular mechanisms involved in coagulation factor expression and/or function during direct hyperplasia (DH)-mediated liver regeneration.
METHODS: Direct hyperplasia-mediated liver regeneration was induced in female C57BL/6 mice by administering 1,4-bis[2-(3,5-dichloropyridyloxy)] benzene (TCPOBOP), a representative hepatomitogen. Mice were weighed and sacrificed at various time points [Day 0 (D0: prior to injection), 3 h, D1, D2, D3, and D10] after TCPOBOP administration to obtain liver and blood samples. Using the RNA samples extracted from the liver, a comprehensive analysis was performed on the hepatic gene expression profiling of coagulation-related factors by real-time RT-PCR (fibrinogen, prothrombin, factors V, VII, VIII, IX, X, XI, XII, XIIIβ, plasminogen, antithrombin, protein C, protein S, ADAMTS13, and VWF). The corresponding plasma levels of coagulation factors (fibrinogen, prothrombin, factors V, VII, VIII, IX, X, XI, XII, XIII, and VWF) were also analyzed and compared with their mRNA levels.
RESULTS: Gavage administration of TCPOBOP (3 mg/kg body weight) resulted in a marked and gradual increase in the weight of the mouse livers relative to the total body weight to 220% by D10 relative to the D0 (control) ratios. At the peak of liver regeneration (D1 and D2), the gene expression levels for most of the coagulation-related factors (fibrinogen, prothrombin, factors V, VII, VIII, IX, XI, XII, XIIIβ, plasminogen, antithrombin, protein C, ADAMTS13, VWF) were found to be down-regulated in a time-dependent manner, and gradually recovered by D10 to the basal levels. Only mRNA levels of factor X and protein S failed to show any decrease during the regenerative phase. As for the plasma levels, 5 clotting factors (prothrombin, factors VIII, IX, XI, and XII) demonstrated a significant decrease (P < 0.05) during the regeneration phase compared with D0. Among these 5 factors, factor IX and factor XI showed the most dramatic decline in their activities by about 50% at D2 compared to the basal levels, and these reductions in plasma activity for both factors were consistent with our RT-PCR findings. In contrast, the plasma activities of the other coagulation factors (fibrinogen, factors V, VII, XIII, and VWF) were not significantly reduced, despite the reduction in the liver mRNA levels. Unlike the other factors, FX showed a temporal increase in its plasma activity, with significant increases (P < 0.05) detected at D1.
CONCLUSION: Investigating the coagulation cascade protein profiles during liver regeneration by DH may help to better understand the basic biology of the liver under normal and pathological conditions.
- Citation: Tatsumi K, Ohashi K, Taminishi S, Takagi S, Utoh R, Yoshioka A, Shima M, Okano T. Effects on coagulation factor production following primary hepatomitogen-induced direct hyperplasia. World J Gastroenterol 2009; 15(42): 5307-5315
- URL: https://www.wjgnet.com/1007-9327/full/v15/i42/5307.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.5307
| Symbol | Gene name | Assay ID | Amplicon length (bp) |
| Housekeeping genes | |||
| PPIA | Peptidylprolyl isomerase A | Mm02342430_g1 | 148 |
| RPL4 | Ribosomal protein L4 | Mm00834993_g1 | 129 |
| Target genes | |||
| Fbg | Fibrinogen, beta chain | Mm00805336_ml | 154 |
| F2 | Coagulation factor II (prothrombin) | Mm00438843_m1 | 68 |
| F5 | Coagulation factor V | Mm00484202_m1 | 61 |
| F7 | Coagulation factor VII | Mm00487329_m1 | 78 |
| F8 | Coagulation factor VIII | Mm00433174_m1 | 110 |
| F9 | Coagulation factor IX | Mm01308427_m1 | 74 |
| F10 | Coagulation factor X | Mm00484177_m1 | 81 |
| F11 | Coagulation factor XI | Mm00511167_m1 | 115 |
| F12 | Coagulation factor XII | Mm00491349_m1 | 64 |
| F13b | Coagulation factor XIIIβ subunit | Mm00491938_m1 | 62 |
| AT | Antithrombin | Mm00446573_m1 | 94 |
| PC | Protein C | Mm00435966_m1 | 52 |
| PS | Protein S (α) | Mm01343426_m1 | 62 |
| Plg | Plasminogen | Mm00447087_m1 | 83 |
| VWF | Von Willebrand factor | Mm00550376_m1 | 63 |
| ADAMTS13 | A disintegrin-like and metallopeptidase with thrombospondin type 1 motif, 13 | Mm01218030_g1 | 77 |
| Body weight (g) | Liver weight (g) | LW/BW × 100 | |
| D0 | 22.44 ± 0.95 | 1.06 ± 0.06 | 4.73 ± 0.14 |
| 3 h | 21.05 ± 0.88 | 1.10 ± 0.07 | 5.22 ± 0.26 |
| D1 | 28.28 ± 2.08 | 1.63 ± 0.15 | 5.70 ± 0.16 |
| D2 | 26.71 ± 1.49 | 2.05 ± 0.10 | 7.70 ± 0.12 |
| D3 | 27.55 ± 2.29 | 2.38 ± 0.19 | 8.62 ± 0.09 |
| D10 | 29.81 ± 0.91 | 3.15 ± 0.11 | 10.42 ± 0.16 |
The normal adult liver is largely quiescent and only a small percentage of the cells are undergoing cell division at any one time. However, the liver is one of the few organs in the mammalian system that can undergo rapid proliferation in response to various stimuli through a process known as liver regeneration. Liver regeneration is a well-orchestrated process in which hepatocytes and non-parenchymal cells rapidly proliferate via induction by two distinct pathways: (1) compensatory regeneration; and (2) direct hyperplasia (DH)[1,2]. In the former pathway, liver regeneration is triggered by a loss of functional liver mass, which can be observed in situations where the liver becomes necrotic due to chemical administration or portions of the liver lobes are physically removed by partial hepatectomy[3-5]. In the latter DH pathway, the liver regeneration can be initiated by a direct administration of hepatocyte mitogens, such as 1,4-bis[2-(3,5-dichloropyridyloxy)] benzene (TCPOBOP), thyroid hormone T3, phenobarbital or bile acids[3,4,6-8].
The process of liver regeneration is known to involve a complex interaction of cytokine-mediated responses as well as pro-proliferative gene expression during the initiation, propagation, and termination phase[1,2]. Liver regeneration appears to adversely affect the production of other liver-specific proteins not directly involved in the regenerative process, including albumin and ornithine transcarbamylase. In terms of hemostasis, the production and secretion of the coagulation factors, anti-coagulant factors, and fibrinolytic factors are highly dependent on the hepatocytes in the liver[9-12]. There is evidence that some of the liver-specific proteins have temporal suppression in their production during compensatory liver regeneration[5,13,14]. Although the kinetics for the coagulation and/or fibrinolytic factors during compensatory liver regeneration have been investigated in the past[15,16], comprehensive analyses assessing the gene expression profiles of the coagulation factors and their related proteins in combination with their plasma activities during DH-mediated liver regeneration have not yet been documented.
Recently, several reports have elucidated that fibrinolytic factors such as plasminogen and urokinase-type plasminogen activator are largely involved in promoting cancer cell invasion and liver regeneration[17,18]. It could thus be speculated that coagulation factors, which have an opposite function to fibrinolytic factors, might participate in modulating the liver regeneration process. In this context, elucidation of the coagulation factor profiles during the liver regeneration process may provide an important clue in clarifying the mechanism of liver regeneration and in understanding the biological process of the blood coagulation system. To date, coagulation factor profiles during the TCPOBOP-induced liver regeneration process have yet to be documented, to the best of our knowledge.
In the present study, we induced liver regeneration in C57BL/6 mice by administering TCPOBOP; the most commonly used experimental procedure to induce DH-mediated liver regeneration. During this liver regeneration process, we investigated the gene expression profiles of coagulation factors and fibrinolytic factors as well as plasma activities of these factors at different time points following TCPOBOP administration.
A total of 48 female C57BL/6 mice (10-12 wk old; The Jackson Laboratory, Bar Harbor, ME) were housed in an environmentally controlled room with alternating 12-h dark/light cycles (8:00 am lights on/8:00 pm lights off). The mice had ad libitum access to food and water. Female mice were exclusively used in this study, since there was a previous report documenting a gender-dependent effect by the liver in the response to TCPOBOP[19]. Experimental protocols were developed in accordance with the guidelines outlined by the local animal committee at Nara Medical University.
DH to the liver was induced by a single intragastric injection of a primary hepatomitogen, TCPOBOP (kindly provided by Dr. BA Diwan) as previously described[3,8,20]. Briefly, TCPOBOP was dissolved in dimethyl sulfoxide (DMSO)/corn oil solution and administered by gavage at a dosage of 3 mg/kg body weight.
Mice were weighed and sacrificed at various time points [Day 0 (D0: prior to injection), 3 h, D1, D2, D3, and D10] after the injection of TCPOBOP (n = 8 mice/time point). Before sacrifice, blood samples were obtained from the retro-orbital plexus and anti-coagulated with 0.1 vol of 3.8% sodium citrate. The blood solution was centrifuged at 4°C, and plasma was separated for storage at -80°C until analysis. After sacrifice, the livers were harvested and weighed, and the liver lobes were snap-frozen in liquid nitrogen and kept at -80°C to preserve the integrity of the RNA until extraction.
Total RNA was extracted from each tissue sample using the RNeasy Mini Kit (QIAGEN, Hilden, Germany) according to manufacturer’s instructions. DNase I was used to digest and remove genomic DNA contamination. Aliquots of the total RNA samples were diluted in TE buffer and the concentration of each sample was measured at a wavelength of 260 nm (A260) using the UV-1600 spectrophotometer (Shimadzu, Kyoto, Japan). Gene expression analyses were performed only when the A260/A280 ratios ranged between 1.9 and 2.1, and the integrity of each RNA sample was confirmed by agarose gel electrophoresis.
Total RNA (1 μg) was reverse-transcribed using oligod(T)16 primers as described by the manufacturer (Omniscript RT Kit, QIAGEN). First-strand cDNA samples were subjected to quantitative PCR amplification using the StepOne Real-time PCR System (Applied Biosystems Japan Ltd., Tokyo, Japan). Each of the cDNA samples was examined to assess gene expression levels for housekeeping and coagulation factor genes. To normalize the gene expression of the coagulation cascade genes between the various time points following the activation of liver proliferation, the geometric mean of the two genes, peptidylprolyl isomerase A (PPIA) and ribosomal protein L4 (RPL4), were utilized as previously identified by our lab[20,21]. The coagulation factors analyzed in our study included fibrinogen (Fbg), prothrombin (PT), factor V (FV), factor VII (FVII), factor FVIII (FVIII), factor IX (FIX), factor X (FX), factor XI (FXI), factor XII (FXII), factor XIIIβ subunit (FXIIIβ), and von Willebrand factor (VWF). In addition, gene expressions of plasminogen (Plg), antithrombin (AT), protein C (PC), protein S (PS), and a disintegrin-like and metalloproteinase with thrombospondin type 1 motif 13 (ADAMTS13) were also analyzed. TaqMan probes and primers for the target genes were purchased from Applied Biosystems (TaqMan Gene Expression Assay, Table 1). All the PCR analyses were performed using the following cycling conditions: 10 min at 95°C (initial melt), followed by 40 cycles of 15 s at 95°C and 1 min at 60°C. The specificity of the primers was verified by 2% agarose gel electrophoresis of the amplicons derived from naïve mouse liver cDNAs. For quantification of gene expression, the cDNAs derived from total RNA extracted from pooled normal mouse livers were serially-diluted, and used to generate the reference standard curves.
Plasma activity of coagulation factors was measured by one-stage clotting assay using human plasma that was deficient for each specific coagulation factor (Sysmex, Kobe, Japan). The activities of FVIII, FIX, FXI, and FXII were measured based on the activated partial thromboplastin time (aPTT) and those of PT, FV, FVII, and FX were measured based on the prothrombin time (PT) using the KC10A Coagulometer (Amelung, Lemgo, Germany). Plasma mouse VWF antigen levels were assayed by ELISA using a primary antibody against human VWF (Dako, Glostrup, Denmark), and a secondary goat anti-human VWF-HRP antibody (Dako). Fibrinogen levels were measured by the Clauss method using bovine thrombin, and FXIII levels were measured by the Berichrom FXIII chromogenic assay (Dade Behring, Marburg, Germany). For all the assessments, pooled plasma collected from 50 normal C57Bl/6 mice was used as a standard.
Significant differences between two groups were analyzed by two-tailed Wilcoxon t-test using Excel (Microsoft) with ystat2006 software (Igakutosyosyuppan, Tokyo, Japan). P < 0.05 was considered significant.
As shown in Table 2, the mouse livers treated with TCPOBOP were found to display an increase in organ size over defined time points as determined by the quantitative changes in the liver weight to body weight (LW/BW) ratio expressed as a percentage. Naive LW/BW ratio (D0) was measured at 4.73% ± 0.14%, and there was a significant (P < 0.05) increase in the ratio to 5.22% ± 0.26% as early as 3 h post-TCPOBOP administration. Over the 10 d period following the TCPOBOP injection, the ratio gradually increased (5.70% ± 0.16%, 7.70% ± 0.19%, 8.62% ± 0.09%, and 10.42% ± 0.16%, at D1, D2, D3, and D10, respectively). At D10, the LW/BW ratio increased to 220% of the naïve LW/BW ratio at D0. We also observed that the body weight increased during the 10 d period, but its magnitude of increase was far less than that of liver weight. Furthermore, administering only the solvent (DMSO/corn oil) to the mice did not affect the LW/BW ratio significantly, confirming that the regenerative responses were caused by TCPOBOP (data not shown).
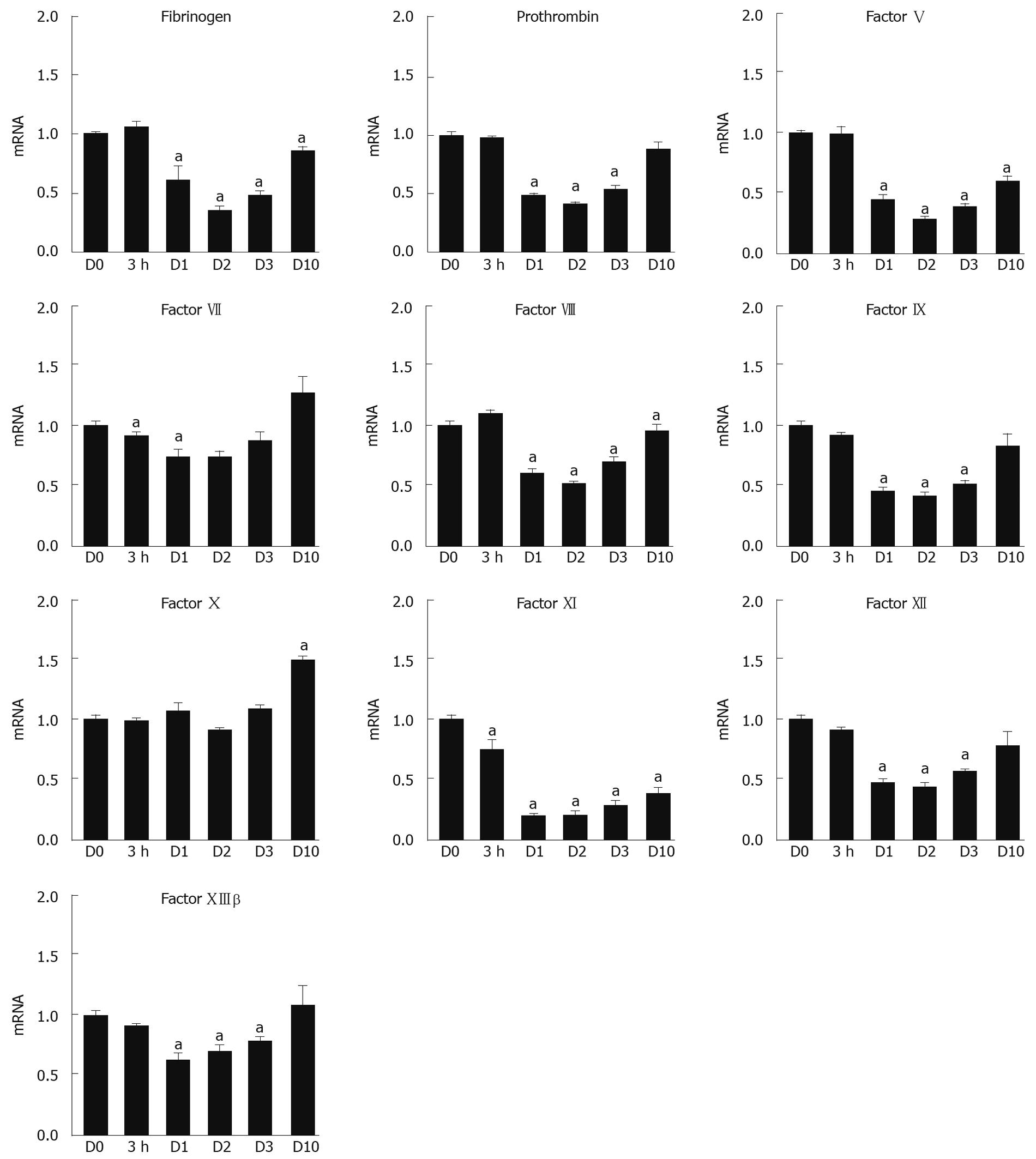
Initial experiments were performed to assess the mRNA expression profile of 10 different coagulation factors (Fbg, PT, FV, FVII, FVIII, FIX, FX, FXI, FXII, and FXIIIβ) following TCPOBOP injection at distinct time points (n = 8 livers/time point) using real-time RT-PCR. All of the data were normalized to the geometric mean of two housekeeping genes, PPIA and RPL4, and then calculated as a comparative ratio to the quiescent (control) liver at D0. Compared with values at D0, mRNA expression levels of all the analyzed coagulation factors, except for FX, were significantly (P < 0.05) but temporally down-regulated during the most active phase of DH-mediated liver regeneration as shown in Figure 1. FX mRNA levels did not decrease during the DH-liver regenerative phase, and ultimately, the levels were significantly increased at D10 compared with D0. For most of the coagulation factor mRNAs that were down-regulated, the mRNA levels gradually recovered by D10 to the levels observed at D0, but there were several factors, particularly FXI, that remained significantly lower (P < 0.05) until D10.
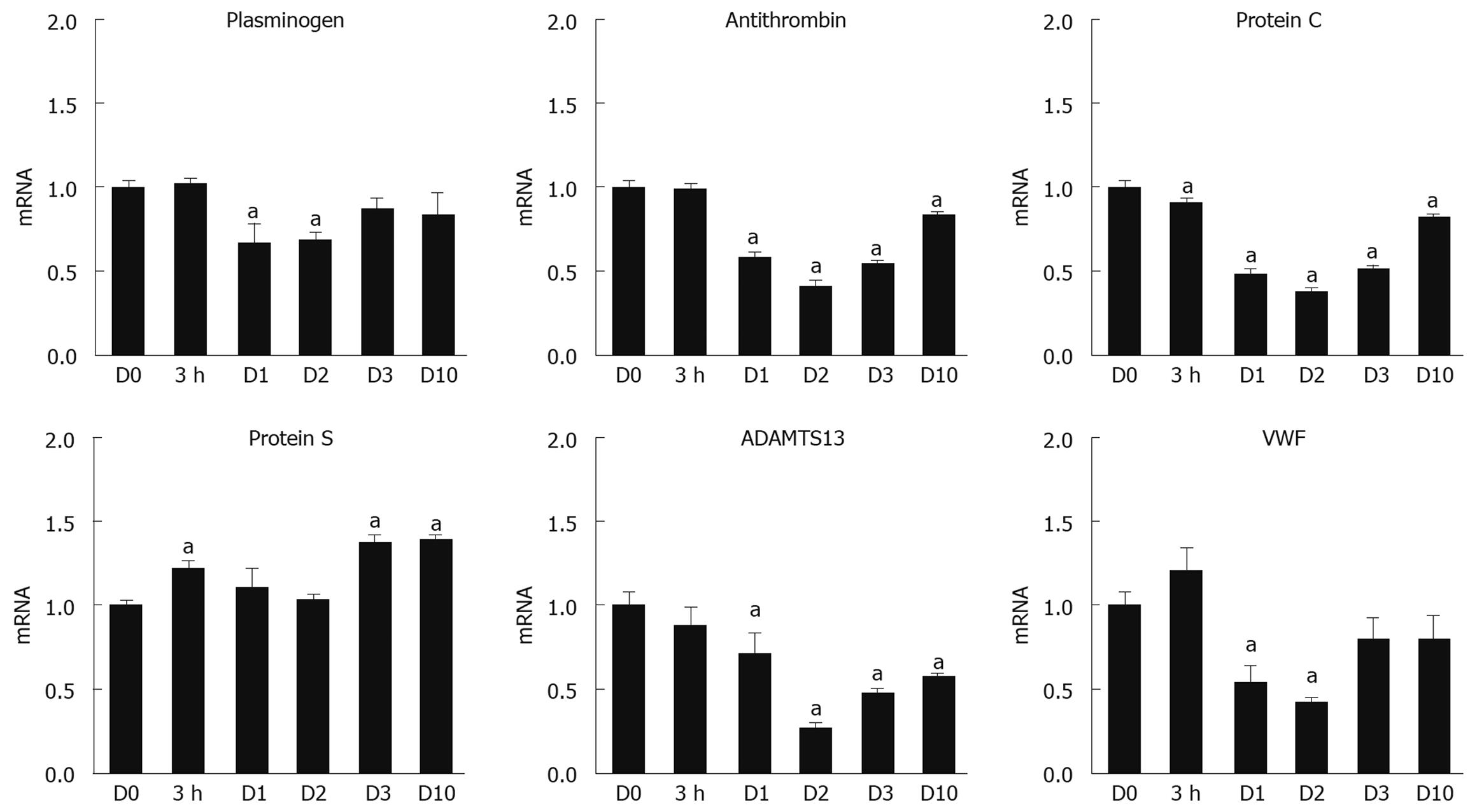
In addition to coagulation factors, we assessed the mRNA expression levels for other factors involved in the regulation of the coagulation cascade, including Plg, AT, PC, PS, VWF, and ADAMTS13. As shown in Figure 2, the mRNA levels of Plg, AT, PC, and ADAMTS13 were down-regulated at D1 and D2 after the TCPOBOP injection, and slowly recovered by D10 to the levels detected in the quiescent state (D0). PS mRNA tended to be increased and, at times, significantly (P < 0.05) elevated, throughout the DH-mediated liver regenerative phase.
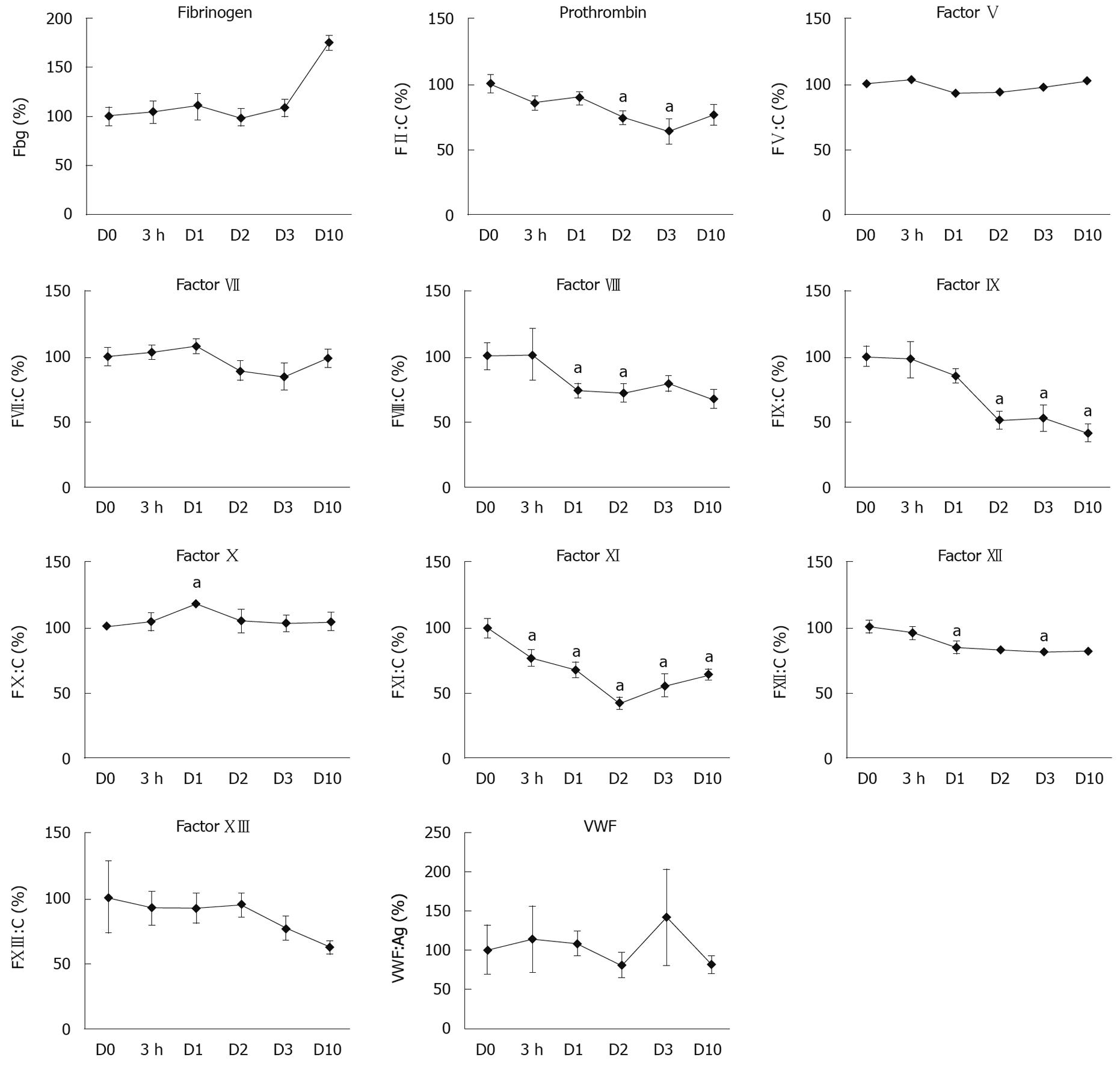
We then determined the changes in the plasma activity of the coagulation factors during the DH-mediated liver regenerative phase. As shown in Figure 3, we detected a significant decrease (P < 0.05) in the plasma activities for 5 of the clotting factors (PT, FVIII, FIX, FXI, and FXII) during the regeneration phase compared with D0. Among these 5 factors, FIX and FXI showed the most dramatic decline in plasma activity by about 50% at D2 compared to the D0 levels. These reductions in plasma activity for FIX and FXI were consistent with our RT-PCR findings (Figure 1) following TCPOBOP injection. In contrast, the plasma activities of the other coagulation factors, Fbg, FV, FVII, and FXIII, as well as VWF, were not significantly reduced despite the reduction in the liver mRNA levels. Unlike the other coagulation factors, FX showed a temporal increase in its plasma activity, with significant increases (P < 0.05) detected at D1.
The present study demonstrated a comprehensive gene expression profile in the liver with corresponding plasma activities of coagulation factors and fibrinolytic factors during the liver regeneration phase induced by TCPOBOP, which is a hepatomitogenic compound. At the peak regenerative phase (i.e. D2 after TCPOBOP injection), the mRNA expression levels for most of the analyzed genes were temporally down-regulated followed by a compensatory recovery back towards the normal levels upon the completion of the regenerative activity (i.e. D10 after TCPOBOP injection). In contrast to the mRNA expression profile, the plasma activities for the coagulation factors showed varied responses. More specifically, PT, FVIII, FIX, FXI, and FXII demonstrated a temporal decrease in activity, FX showed a temporal increase in activity, and Fbg, FV, FVII, FXIII, and VWF did not markedly change throughout the regenerative period.
Liver regeneration is known to be an intrinsic function that can be induced through two distinct pathways: (1) compensatory regeneration and (2) direct hyperplasia (DH). In compensatory regeneration, tumor necrosis factor, interleukin-6, nuclear factor-κB, STAT3, and AP-1 are associated with the initiation of hepatocyte proliferation, but the process of DH-mediated liver regeneration appears to be independent of these cytokines[7,22]. At the level of gene expression, there are distinct genetic pathways that are activated depending on the mode of liver regeneration. For example, there is a rapid induction of cyclin D1 as early as 8 h after the induction of the mode of DH, whereas cyclin D1 does not appear to be induced at any time point during compensatory regeneration[23].
In this paper, we focused on the DH mode of liver regeneration, which is a unique process that mediates hepatocellular proliferation due to inoculation with primary hepatocyte mitogens[6,8]. Following the administration of the mitogens, hepatocytes and other liver cell types demonstrate a rapid activation of DNA synthesis in which the peak of the cell division occurs between D1 to D2 leading to a subsequent increase in liver size and weight. Among the currently available hepatocyte mitogens, TCPOBOP, a synthetic ligand to the constitutive androstane receptor (CAR), has been shown to produce robust proliferation and growth in mouse livers[6,8]. The rate of regenerative activity at the peak phase of DH-mediated proliferation following TCPOBOP administration is more robust compared to the regenerative effect attributed to a partial hepatectomy-induced compensatory regeneration as observed by the BrdU labeling of proliferating hepatocytes[8]. Consequently, an active proliferation of hepatocytes at the same levels of previous reports was induced appropriately by the administration of TCPOBOP in the present study (Table 2)[8,20].
In the face of a proliferative response in the liver, liver function can be adversely affected. It has been reported that gene expression levels of liver-specific proteins are temporally suppressed, which suggests that the regenerating liver sacrifices the production of liver-specific proteins, mainly plasma proteins, in order to promote the protein production required for cell division and growth[24,25]. These findings are supported by our previous report that gene expression of essential structural proteins for the cell, including β-actin, were significantly increased during liver regeneration[5,20]. In contrast to compensatory liver regeneration, there remains a paucity of information regarding the molecular changes in gene expression and production of liver-specific proteins, including coagulation factors, during liver regeneration through the DH mode. Since the majority of the coagulation factors and anti-coagulation factors are produced by hepatocytes, analysis of the liver to profile the gene expression of these factors would provide a comprehensive determination of the effect of proliferation on gene expression. In our analyses, we demonstrated that livers in the active phase of liver regeneration (D1 and D2) significantly down-regulated the gene expression of almost all of the coagulation and fibrinolytic factors analyzed (Figures 1 and 3). The reduction in the coagulation cascade gene expression is consistent with previous studies in our lab whereby TCPOBOP-induced hepatocyte proliferation led to a marked down-regulation of other liver-specific genes, including albumin and ornithine transcarbamylase[8,20]. Interestingly, the decrease in gene expression for the coagulation cascade proteins did not necessarily correlate with the plasma protein activity for many of the analyzed genes, such as Fbg, FV, FVII, FX and FXIII. For these proteins, the plasma protein activity appeared to be maintained or elevated during the liver proliferation and growth phase even though the mRNA levels of these proteins were significantly reduced. The lack of reduced plasma activity could be related to a number of factors, including the threshold of mRNA expression needed for efficient translation of the coagulation factor, an extended half-life of the protein, enhanced translation and subsequent secretion of the proteins into the circulation, or the mass of liver needed to produce the coagulation proteins. Unlike the other coagulation factors, FIX activity remained significantly reduced throughout the liver regenerative phase even though the FIX mRNA profile returned towards normal levels in the face of increased liver mass. In order for FIX to become available in a biologically active and secretable form, it has been described that several post-translational modification steps are required within the hepatocytes. It may be speculated that the disparity between the low plasma FIX activity and revived liver mRNA levels is the result of insufficient recovery of such post-translational modification systems in the regenerated hepatocytes. Since FIX is a critical protein for mediating blood coagulation[11,12], further investigation needs to be addressed for the elucidation of the mechanism producing the biologically active FIX during the regeneration phase.
The effect of proliferative stimuli on coagulation factors not produced in the hepatocytes is not well-established. VWF is known to be an important glycoprotein for platelet adhesion to wound sites as well as a carrier protein for FVIII, and to be largely produced in endothelial cells throughout the body, including the liver[26]. ADAMTS13, which is produced exclusively by hepatic stellate cells, is a VWF-cleaving enzyme essential for preventing excessive thrombotic events in the body[27]. These proteins were found to have their mRNA expression significantly down-regulated during the active phase of DH-mediated regeneration (Figures 1 and 3). Proliferation of non-parenchymal cells in the liver has been shown to occur following DH-mediated stimuli, but the peak level of proliferation was found to occur 1-2 d following the peak normally observed in hepatocytes[8]. One possible explanation for the down-regulation of VWF and ADAMTS13 at the early regeneration phase could be associated with the early initiation, but lagging pace, of cell-cycle progression in the non-parenchymal cells.
In conclusion, this is the first detailed study to investigate the liver gene expression profiles with corresponding plasma protein activity levels for a panel of coagulation factors and related coagulation cascade proteins during liver regeneration mediated by direct hyperplasia. Temporal down-regulation of gene expression was found to be associated with this mode of liver regeneration, while plasma activity levels showed mixed changes in activity depending on the factor being examined. These results suggest that there may be a common feature between two mechanisms involved in liver regeneration whereby the liver in the proliferative phase sacrifices the production of liver-specific proteins that are not essential for hepatocyte division and growth until the liver has completed or nearly completed the regenerative event. However, it is clear that the genetic profiles of these proteins cannot be simply answered by determining the expression and protein activity, but that other mechanisms are involved requiring further investigation as to how liver-specific proteins not involved in cell division and growth are regulated during DH-mediated liver regeneration.
Liver regeneration is a well-orchestrated process in which hepatocytes and non-parenchymal cells undergo rapid proliferation via induction by two distinct pathways: (1) compensatory regeneration; and (2) direct hyperplasia (DH). In the former pathway, liver regeneration is triggered by a loss of functional liver mass, which can be observed in situations where the liver becomes necrotic due to chemical administration or portions of the liver lobes are physically removed by partial hepatectomy. In the latter DH pathway, the liver regeneration can be initiated by direct administration of hepatocyte mitogens, such as 1,4-bis[2-(3,5-dichloropyridyloxy)] benzene (TCPOBOP), thyroid hormone T3, phenobarbital or bile acids.
There is evidence that some of the liver-specific proteins have temporal suppression in their production during compensatory liver regeneration. Although the kinetics for the coagulation and/or fibrinolytic factors during compensatory liver regeneration have been investigated, comprehensive analyses assessing the gene expression profiles of the coagulation factors and their related proteins in combination with their plasma activities during the DH-mediated liver regeneration have not yet been documented.
In the present study, the authors induced liver regeneration in C57BL/6 mice by administering TCPOBOP; the most commonly used experimental procedure to induce DH-mediated liver regeneration. During this liver regeneration process, they investigated the gene expression profiles of coagulation factors and fibrinolytic factors as well as plasma activities of these factors at different time points following TCPOBOP administration.
The systematic and thorough investigation of the molecular changes in the coagulation cascade during liver regeneration by DH will help to better understand the basic biology of the liver under normal and pathological conditions.
TCPOBOP is one of the currently available hepatocyte mitogens, and functions as a synthetic ligand to the constitutive androstane receptor, which has been shown to produce robust proliferation and growth in mouse livers.
In this study, the authors investigated the changes in expression and activity of several factors of the coagulation cascade during liver regeneration. The study is well presented and the figures are convincing.
| 1. | Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45-S53. |
| 2. | Michalopoulos GK. Liver regeneration. J Cell Physiol. 2007;213:286-300. |
| 3. | Ohashi K, Waugh JM, Dake MD, Yokoyama T, Kuge H, Nakajima Y, Yamanouchi M, Naka H, Yoshioka A, Kay MA. Liver tissue engineering at extrahepatic sites in mice as a potential new therapy for genetic liver diseases. Hepatology. 2005;41:132-140. |
| 4. | Ohashi K, Yokoyama T, Yamato M, Kuge H, Kanehiro H, Tsutsumi M, Amanuma T, Iwata H, Yang J, Okano T. Engineering functional two- and three-dimensional liver systems in vivo using hepatic tissue sheets. Nat Med. 2007;13:880-885. |
| 5. | Tatsumi K, Ohashi K, Taminishi S, Okano T, Yoshioka A, Shima M. Reference gene selection for real-time RT-PCR in regenerating mouse livers. Biochem Biophys Res Commun. 2008;374:106-110. |
| 6. | Columbano A, Ledda-Columbano GM. Mitogenesis by ligands of nuclear receptors: an attractive model for the study of the molecular mechanisms implicated in liver growth. Cell Death Differ. 2003;10 Suppl 1:S19-S21. |
| 7. | Columbano A, Ledda-Columbano GM, Pibiri M, Piga R, Shinozuka H, De Luca V, Cerignoli F, Tripodi M. Increased expression of c-fos, c-jun and LRF-1 is not required for in vivo priming of hepatocytes by the mitogen TCPOBOP. Oncogene. 1997;14:857-863. |
| 8. | Ohashi K, Park F, Kay MA. Role of hepatocyte direct hyperplasia in lentivirus-mediated liver transduction in vivo. Hum Gene Ther. 2002;13:653-663. |
| 9. | Biron-Andréani C, Bezat-Bouchahda C, Raulet E, Pichard-Garcia L, Fabre JM, Saric J, Baulieux J, Schved JF, Maurel P. Secretion of functional plasma haemostasis proteins in long-term primary cultures of human hepatocytes. Br J Haematol. 2004;125:638-646. |
| 10. | Boost KA, Auth MK, Woitaschek D, Kim HS, Hilgard P, Nadalin S, Blaheta RA. Long-term production of major coagulation factors and inhibitors by primary human hepatocytes in vitro: perspectives for clinical application. Liver Int. 2007;27:832-844. |
| 11. | Tatsumi K, Ohashi K, Kataoka M, Tateno C, Shibata M, Naka H, Shima M, Hisanaga M, Kanehiro H, Okano T. Successful in vivo propagation of factor IX-producing hepatocytes in mice: potential for cell-based therapy in haemophilia B. Thromb Haemost. 2008;99:883-891. |
| 12. | Tatsumi K, Ohashi K, Shima M, Nakajima Y, Okano T, Yoshioka A. Therapeutic effects of hepatocyte transplantation on hemophilia B. Transplantation. 2008;86:167-170. |
| 13. | Fukuhara Y, Hirasawa A, Li XK, Kawasaki M, Fujino M, Funeshima N, Katsuma S, Shiojima S, Yamada M, Okuyama T. Gene expression profile in the regenerating rat liver after partial hepatectomy. J Hepatol. 2003;38:784-792. |
| 14. | Kurumiya Y, Nozawa K, Sakaguchi K, Nagino M, Nimura Y, Yoshida S. Differential suppression of liver-specific genes in regenerating rat liver induced by extended hepatectomy. J Hepatol. 2000;32:636-644. |
| 15. | Antović J, Djordjević V, Kocić G, Koraćević D, Bjelaković G, Bakić M. Blood coagulation factors changes during liver regeneration in rats. Arch Int Physiol Biochim Biophys. 1993;101:357-359. |
| 16. | Zhao LF, Zhang WM, Xu CS. Expression patterns and action analysis of genes associated with blood coagulation responses during rat liver regeneration. World J Gastroenterol. 2006;12:6842-6849. |
| 17. | McMahon B, Kwaan HC. The plasminogen activator system and cancer. Pathophysiol Haemost Thromb. 2008;36:184-194. |
| 18. | Shimizu M, Hara A, Okuno M, Matsuno H, Okada K, Ueshima S, Matsuo O, Niwa M, Akita K, Yamada Y. Mechanism of retarded liver regeneration in plasminogen activator-deficient mice: impaired activation of hepatocyte growth factor after Fas-mediated massive hepatic apoptosis. Hepatology. 2001;33:569-576. |
| 19. | Ledda-Columbano GM, Pibiri M, Concas D, Molotzu F, Simbula G, Cossu C, Columbano A. Sex difference in the proliferative response of mouse hepatocytes to treatment with the CAR ligand, TCPOBOP. Carcinogenesis. 2003;24:1059-1065. |
| 20. | Takagi S, Ohashi K, Utoh R, Tatsumi K, Shima M, Okano T. Suitable reference genes for the analysis of direct hyperplasia in mice. Biochem Biophys Res Commun. 2008;377:1259-1264. |
| 21. | Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:RESEARCH0034. |
| 22. | Ledda-Columbano GM, Curto M, Piga R, Zedda AI, Menegazzi M, Sartori C, Shinozuka H, Bluethmann H, Poli V, Ciliberto G. In vivo hepatocyte proliferation is inducible through a TNF and IL-6-independent pathway. Oncogene. 1998;17:1039-1044. |
| 23. | Ledda-Columbano GM, Pibiri M, Loi R, Perra A, Shinozuka H, Columbano A. Early increase in cyclin-D1 expression and accelerated entry of mouse hepatocytes into S phase after administration of the mitogen 1, 4-Bis[2-(3,5-Dichloropyridyloxy)] benzene. Am J Pathol. 2000;156:91-97. |
| 24. | Cajone F, Bernelli-Zazzera A. Protein synthesis in regenerating liver. Int J Biochem. 1980;12:537-544. |
| 25. | Lewan L. Protein, nucleic acids and cell structure in the regenerating mouse liver. Z Zellforsch Mikrosk Anat. 1972;129:56-64. |
| 26. | Sadler JE, Budde U, Eikenboom JC, Favaloro EJ, Hill FG, Holmberg L, Ingerslev J, Lee CA, Lillicrap D, Mannucci PM. Update on the pathophysiology and classification of von Willebrand disease: a report of the Subcommittee on von Willebrand Factor. J Thromb Haemost. 2006;4:2103-2114. |
Peer reviewer: Dr. Katja Breitkopf, Department of Medicine II, University Hospital Mannheim, University of Heidelberg, Theodor-Kutzer-Ufer 1-3, 68167 Mannheim, Germany
S- Editor Li LF L- Editor Logan S E- Editor Yin DH