Published online Nov 14, 2009. doi: 10.3748/wjg.15.5287
Revised: July 28, 2009
Accepted: August 5, 2009
Published online: November 14, 2009
AIM: To assess the mucosa-associated bacterial microflora and mucus layer in adolescents with inflammatory bowel disease (IBD).
METHODS: Sixty-one adolescents (mean age 15 years, SD ± 4.13) were included in the study. Intestinal biopsies from inflamed and non-inflamed mucosa of IBD patients and from controls with functional abdominal pain were cultured under aerobic and anaerobic conditions. The number of microbes belonging to the same group was calculated per weight of collected tissue. The mucus thickness in frozen samples was measured under a fluorescent microscope.
RESULTS: The ratios of different bacterial groups in inflamed and non-inflamed mucosa of IBD patients and controls were specific for particular diseases. Streptococcus spp. were predominant in the inflamed mucosa of Crohn’s disease (CD) patients (80% of all bacteria), and Lactobacillus spp. were predominant in ulcerative colitis patients (90%). The differences were statistically significant (P = 0.01-0.001). Lower number of bifidobacteria was observed in the whole IBD group. A relation was also found between clinical and endoscopic severity and decreased numbers of Lactobacillus and, to a lesser extent, of Streptococcus in biopsies from CD patients. The mucus layer in the inflamed sites was significantly thinner as compared to controls (P = 0.0033) and to non-inflamed areas in IBD patients (P = 0.031).
CONCLUSION: The significantly thinner mucosa of IBD patients showed a predominance of some aerobes specific for particular diseases, their numbers decreased in relation to higher clinical and endoscopic activity of the disease.
- Citation: Fyderek K, Strus M, Kowalska-Duplaga K, Gosiewski T, Wędrychowicz A, Jedynak-Wąsowicz U, Sładek M, Pieczarkowski S, Adamski P, Kochan P, Heczko PB. Mucosal bacterial microflora and mucus layer thickness in adolescents with inflammatory bowel disease. World J Gastroenterol 2009; 15(42): 5287-5294
- URL: https://www.wjgnet.com/1007-9327/full/v15/i42/5287.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.5287
| Bacterial groups (taxons) | Children with UC (n =12) | Children with CD (n = 22) | Children in control group (n = 24) | ||
| Inflamed site | Non-inflamed | Inflamed site | Non-inflamed | ||
| Enterobacteriacae | 4.92 × 105 | 5.55 × 106 | 2.17 × 107 | 9.71 × 106 | 1.94 × 107 |
| Enterococcus | 1.80 × 106 | 1.27 × 106 | 1.36 × 106 | 1.43 × 106 | 6.82 × 105 |
| Streptococcus | 6.83 × 105 | 1.07 × 106 | 1.29 × 108b | 1.33 × 106 | 5.39 × 106 |
| Lactobacillus | 9.28 × 107a | 7.30 × 106 | 3.37 × 106 | 7.76 × 106 | 1.01 × 107 |
| Bifidobacterium | 5.56 × 106a | 7.14 × 107 | 8.62 × 106a | 3.15 × 107 | 1.99 × 108 |
| Bacteroides | 1.63 × 106 | 5.13 × 102 | 1.24 × 105 | 2.31 × 105 | 1.63 × 106 |
| Total number of cultured bacteria | 5.83 × 108a | 4.79 × 108 | 3.93 × 108 | 2.30 × 108 | 1.77 × 108 |
The term inflammatory bowel disease (IBD), used to describe the chronic intestinal processes, includes ulcerative colitis (UC) and Crohn’s disease (CD). Despite very extensive studies, the pathogenesis of IBD still remains unclear. Genetic, environmental and immunological factors seem to play a substantial role[1]. However, an increasing number of both clinical and laboratory studies support the idea of the leading role of bacterial microflora in the onset and persistence of inflammation[2]. It is well known that the anatomic sites with the highest concentration of luminal bacteria are most often involved in the inflammatory process[3]. Giaffer et al[4] showed in 1991 that intestinal flora of patients with active CD is different from that of quiescent patients, UC subjects and controls with functional abdominal pain. Patients with Crohn’s disease presented with elevated numbers of aerobic bacteria, mainly Escherichia coli, and some anaerobic bacteria. There was also evidence of decreased numbers of Bifidobacteria. The results of research based on animal models support the importance of luminal bacteria in experimental colitis. Transgenic mice kept under germ free conditions did not develop inflammation until bacteria were introduced[5]. In recent years, the results of several reviews on gut microflora and IBD have suggested that inflammation may be due to a shift in the balance of healthy flora, which leads to dysregulation of the microflora and triggers or maintains the inflammation[6,7]. Moreover, it is also known, that microbes which were in close contact with the epithelium were different from those found in stools, and probably more important in the process of gut inflammation[8].
There is very little information about the specificity of the gut microbiota in adolescents with IBD. Up to now, only one study in the literature by Conte et al[9] where both culture and PCR amplification methods were used, found higher numbers of mucosa-associated bacteria in biopsies from IBD children in comparison to controls. On the other hand, the occurrence of strict anaerobes including Bacteroides was low in specimens from patients with CD, UC and indeterminate colitis.
It has also been demonstrated that mucus which is an integral part of the mucosal barrier may play an important role in hindering penetration of the mucosa by luminal bacteria and their products thus preventing the inflammatory process. A study by Pullan et al[10] showed that a reduction of mucus layer thickness in adult IBD patients caused increased exposure of the microbes to the gut immune system, which in turn sustained the inflammation.
Both mucus and mucosa-associated bacterial microflora form a specific environment in the gut and its disruption may play a crucial role in the development of IBD, promoting specific bacterial colonization and immunological response.
Assessment of the multiple interactions among bacterial microflora and mucus in the very early stage of IBD, seen in newly diagnosed disease, is essential for a better understanding of disease pathogenesis and may help in suggesting therapeutic options such as the use of specific probiotics or antimicrobial treatment.
The aim of our study was to assess mucosa-associated bacterial microflora and mucus layer thickness in relation to the inflammatory process in adolescents with IBD in comparison to controls.
The trial was approved by the Jagiellonian University Bioethical Committee and informed consent was obtained from all patients’ legal guardians and/or patients over 16 years of age enrolled in the study.
Sixty-one adolescents (25 boys and 36 girls, age: 15 ± 4.13 years) were enrolled in the study from January 2004 to October 2006. There were 12 patients with UC (5 boys and 7 girls, age: 14.74 ± 2.9 years), 22 with CD (9 boys and 13 girls, age: 16.22 ± 3.8 years) and 3 with indeterminate colitis (IC) (1 boy and 2 girls, age: 15.01 ± 7.9 years) in the study group and 24 control subjects (10 boys, 14 girls, age: 14.13 ± 4.4 years) who underwent colonoscopy because of chronic abdominal pain, which was finally diagnosed as functional abdominal pain, without any organic abnormalities. The mean duration of symptoms in the UC group was 96.5 ± 75.1 d, 153 ± 146.7 d in the CD group and 40.5 ± 27.6 d in the IC group. IC patients were not considered for further analysis as a separate group due to the small number of subjects. Patients with UC were younger at disease onset (11.64 ± 4.44 years) than those with CD (13.67 ± 3.28 years). Nutritional status of the patients assessed according to the Cole index was comparable in both groups (UC group - 84.57, CD - 82.89). Abdominal pain as the main complaint was more frequent in the group with CD (85.7%) than UC (81.1%), whereas bloody diarrhea and sideropenic anemia were more frequent in UC (81.2% and 36.4%, respectively) than CD patients (40.9% and 19.1%, respectively). Only 9% of UC patients presented with fever at disease onset, as compared to 27.2% of CD patients.
The diagnosis of CD or UC was based on endoscopic, histopathological, immunological and radiological criteria. Disease activity was assessed according to the Pediatric Crohn’s Disease Activity Index (PCDAI) for CD patients and the modified Trulove-Witts score for UC patients[11]. Depending on the score value the patients were classified into three subgroups of disease activity: mild, moderate and severe. The majority of patients in both groups were classified as having mild (UC - 4 and CD - 8) to moderate (UC - 8 and CD - 12) stages of clinical activity. Only 2 patients with CD (and none with UC) were classified as having severe clinical activity. For the evaluation of endoscopic changes we used Roth’s score[12]. Seven UC patients showed severe endoscopic activity compared to 6 patients with CD. A minority of patients in the UC group showed mild (2 children) to moderate (3 patients) endoscopic activity, as compared to the CD group (7 and 6 patients, respectively). Histology was assessed blindly by an independent histopathologist. All patients with IBD were in the active phase of the disease.
The use of antibiotics 30 d prior to enrolment, infectious diarrhea, malabsorption, immunodeficiencies and intestinal enteropathies were the exclusion criteria.
All subjects underwent the same type of preparation prior to colonoscopy, with oral sodium phosphate at a dose of 0.6-0.8 mL/kg (up to 45 mL) and bowel cleansing, consisting of 4 saline enemas. During colonoscopy, patients received intravenous sedation or general anesthesia, as required. Biopsy samples from IBD patients were obtained from both, the inflamed and the non-inflamed colonic mucosa. Four biopsy specimens were taken from both sites: two for culture, and one each for standard histopathological assessment and mucus thickness measurement. In the control group, the biopsy samples were taken from a normal sigmoid colon for the same assessments. The biopsy samples were transferred directly into Schaedler Anaerobic Broth (SAB) medium (Difco, BD, Franklin Lakes, USA) with 10% of glycerol. The samples were immediately snap frozen on dry ice and kept at -80°C or on dry ice until analysis. All procedures were performed as fast as possible, using sterile instruments and ensuring the integrity of the intestinal tissue. The codes of the biopsy samples were blinded before performing microbiological analysis.
The frozen samples were thawed, weighed, homogenized in 1 mL of SAB and quantitatively analyzed for the main bacterial constituents by cultures on differential media in aerobic and anaerobic conditions[9]. All these manipulations were done aseptically in an anaerobic chamber (MACS - MG 500 Work Station, DW Scientific, Shipley, UK) in N (85%) + H2 (10%) + CO2 (5%) atmosphere. Homogenized samples were serially diluted with SAB and 100 μL aliquots plated on the following media: McConkey Agar (Oxoid, Basingstoke, UK) for Enterobacteriaceae, Columbia Blood Agar (Difco) with 5% sheep blood for streptococci, Enterococcosel Agar (BBL, BD, Franklin Lakes, USA) for enterococci, MRS agar (Oxoid) for lactobacilli and other lactic acid bacteria (LAB), BL agar for bifidobacteria[13], and Wilkins-Chalgren Agar Base with supplements for Bacteroides.
The dilutions were then spread over the plate surface using a glass rod and the plates were then incubated aerobically at 37°C for 24 h, except for the cultures for anaerobic bacteria, which were kept in the anaerobic chamber for up to 4 d depending on the type of medium. The morphology of the grown colonies was analyzed under magnifying glass and several colony picks of each morphological type were subcultured on appropriate aerobic and anaerobic media and Gram-stained. After further incubation and culture purity checks, phenotypic identification was performed using commercial identification systems (API 20E, API20A, APIStaph, APIStrept, bioMerieux, Marcy l'Etoile, France; BBL Crystal ID System, BD, Franklin Lakes, USA).
The frozen biopsy samples, collected as described above, were cut into 5 μm sections, thawed and fixed to microscopic slides and stained with fluorescein labeled Maackia amurensis lectin MAA lectin (EY Labs, San Mateo, USA) which binds to 2-3 linked sialic acid[14]. Briefly, the lectin solution (20 μg/mL in 0.05% sodium azide solution in a buffer supplied by the producer) was applied in a 50 μL volume to each slide and incubated under cover in a dark moist chamber at room temperature for 0.5 h. Then the unbound lectin was washed out with the buffer and the slides were counterstained with DAPI (Sigma, St. Louis, USA), washed again and dried. The mucus thickness was measured under a fluorescent microscope (Olympus BX51) with 100 × magnification.
Comparisons were made using the Student’s t test for variables with a normal distribution and χ2 test. For comparison of bacterial populations and ratios of particular bacterial groups the likelihood ratio was used. The Wilcoxon test and Wilcoxon signed-rank test for matching pairs were used to compare mucus thickness. These statistical methods were chosen because data distribution was significantly different from normal distribution. All analyses were conducted using SAS 9.1 package and SAS Enterprise Quide 3.0 (SAS Institute, USA). Data are expressed as mean ± SD.
All biopsy specimens from IBD patients and controls showed the presence of all groups of bacteria in the cultures although of different populations. The total number of mucosa-associated bacteria was higher in IBD patients compared to the controls with functional abdominal pain, however, the differences were statistically significant only in the UC group. Because of high diversities among the isolated bacteria and their numbers, the ratios of bacterial groups present were calculated as pooled numbers of bacteria weighted by the number of patients in the groups (Table 1).
The ratios of different bacterial groups cultured from biopsies obtained both from inflamed and non-inflamed sites in IBD patients were different in samples taken from children with specific diseases. The differences were statistically significant (χ2 test, P < 0.0001). Streptococci were the predominant group of bacteria in inflamed mucosa of CD patients. About 80% of all microbiota cultured from the tissue belonged to these genera. On the other hand, patients with UC showed predominance of Lactobacillus (90%) in inflamed sites (Table 1). Both CD and UC patients showed a significant reduction in the ratios for anaerobes such as Bifidobacterium in samples taken from inflamed compared to non-inflamed mucosa. Considering the ratios of bacteria found in non-inflamed mucosa of IBD patients as compared with those found in controls, it appeared that bifidobacteria were the predominant group in all these sites (Table 1).
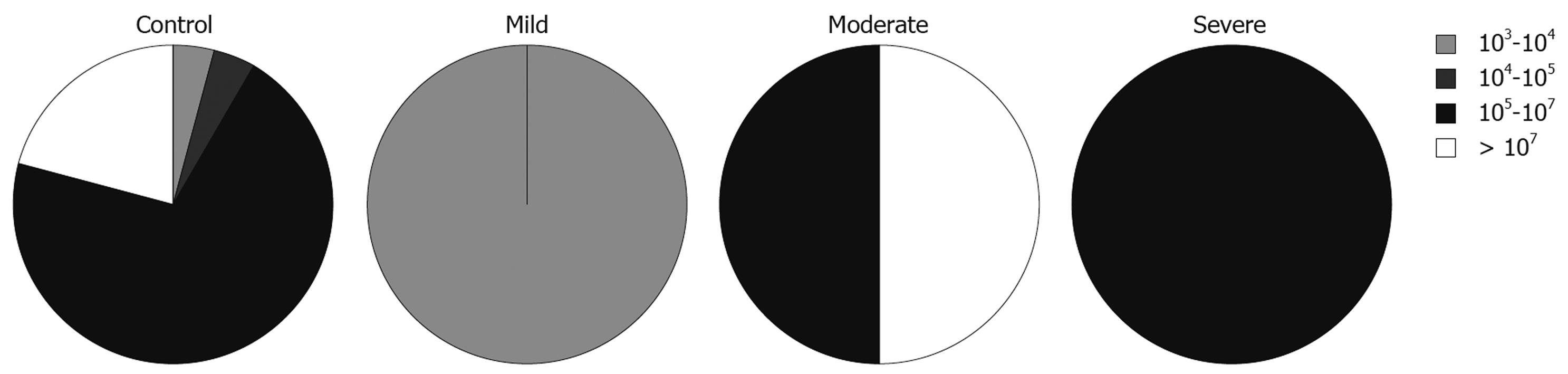
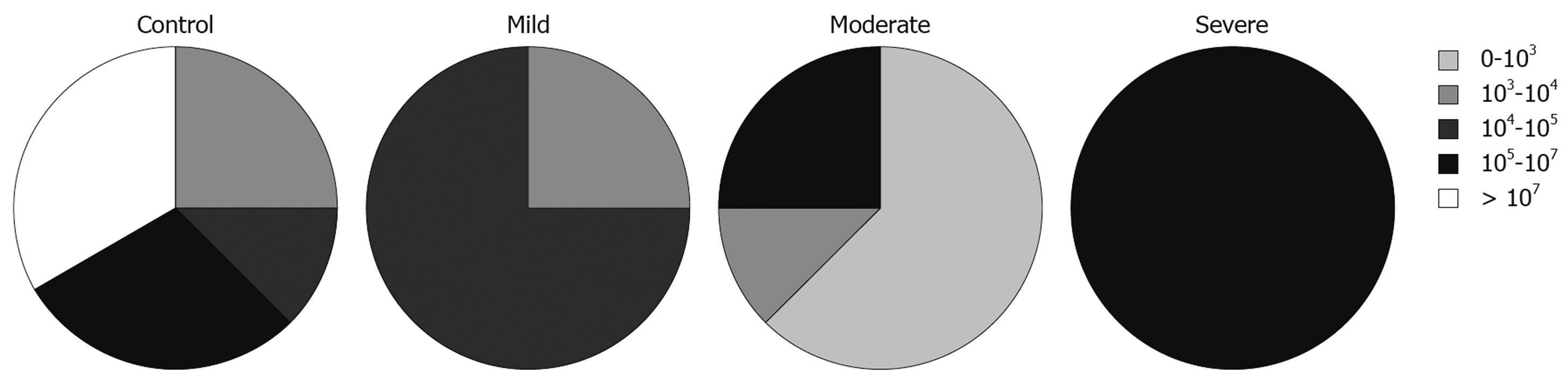
We also looked at the relationship between the total numbers of bacteria and disease activity, measured using PCDAI for CD patients and the modified Trulove-Witts score for UC in addition to the Roth’s endoscopic scoring index. To express these relationships we adopted the five interval scale of bacterial populations from Swidsinski et al[15]: 0-103 cfu/g, 103-104 cfu/g, 104-105 cfu/g, 105-107 cfu/g, and > 107 cfu/g.
The total numbers of cultured bacteria found in biopsies from inflamed mucosa of UC patients was increased with severity of the disease estimated by endoscopic activity (Figure 1). This relationship was statistically significant (likelihood ratio = 9.535, P = 0.0490). In relation to specific groups of bacteria, this analysis showed that the number of lactobacilli, which was the predominant group in the samples from inflamed mucosa of patients with UC, was lower in samples taken from patients with severe endoscopic activity. This was, however, not statistically significant (likelihood ratio = 6.959, P = 0.1381).
There was no relationship between the total numbers of bacteria in samples from inflamed sites and severity of the disease in CD patients. In relation to various groups of bacteria, the analysis showed that numbers of lactobacilli were lower in samples with higher clinical activity (likelihood ratio = 13.209, P = 0.0328, Figure 2). The same tendency, although less significant, was observed for numbers of streptococci in samples from CD patients (likelihood ratio = 13.889, P = 0.0847).
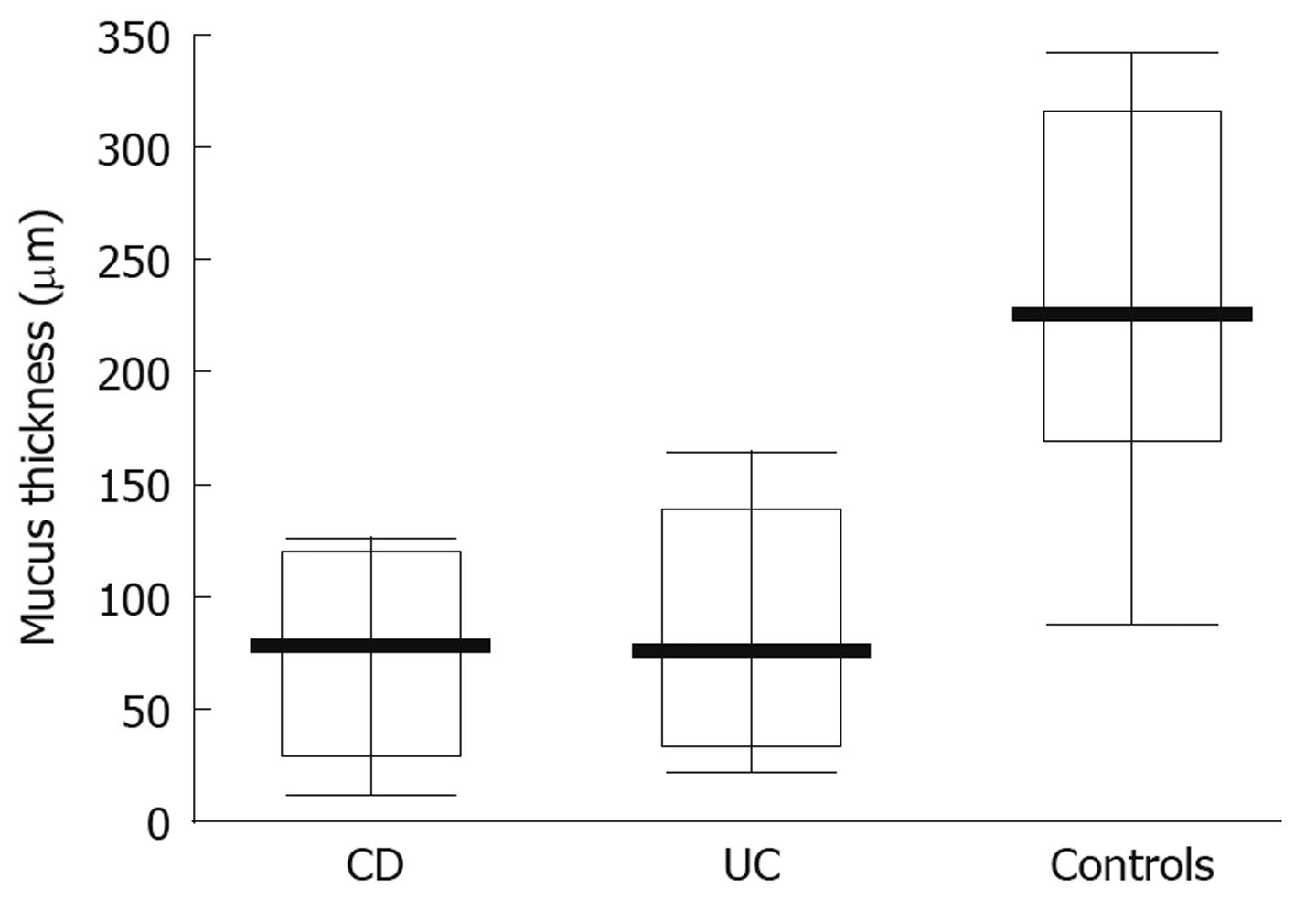
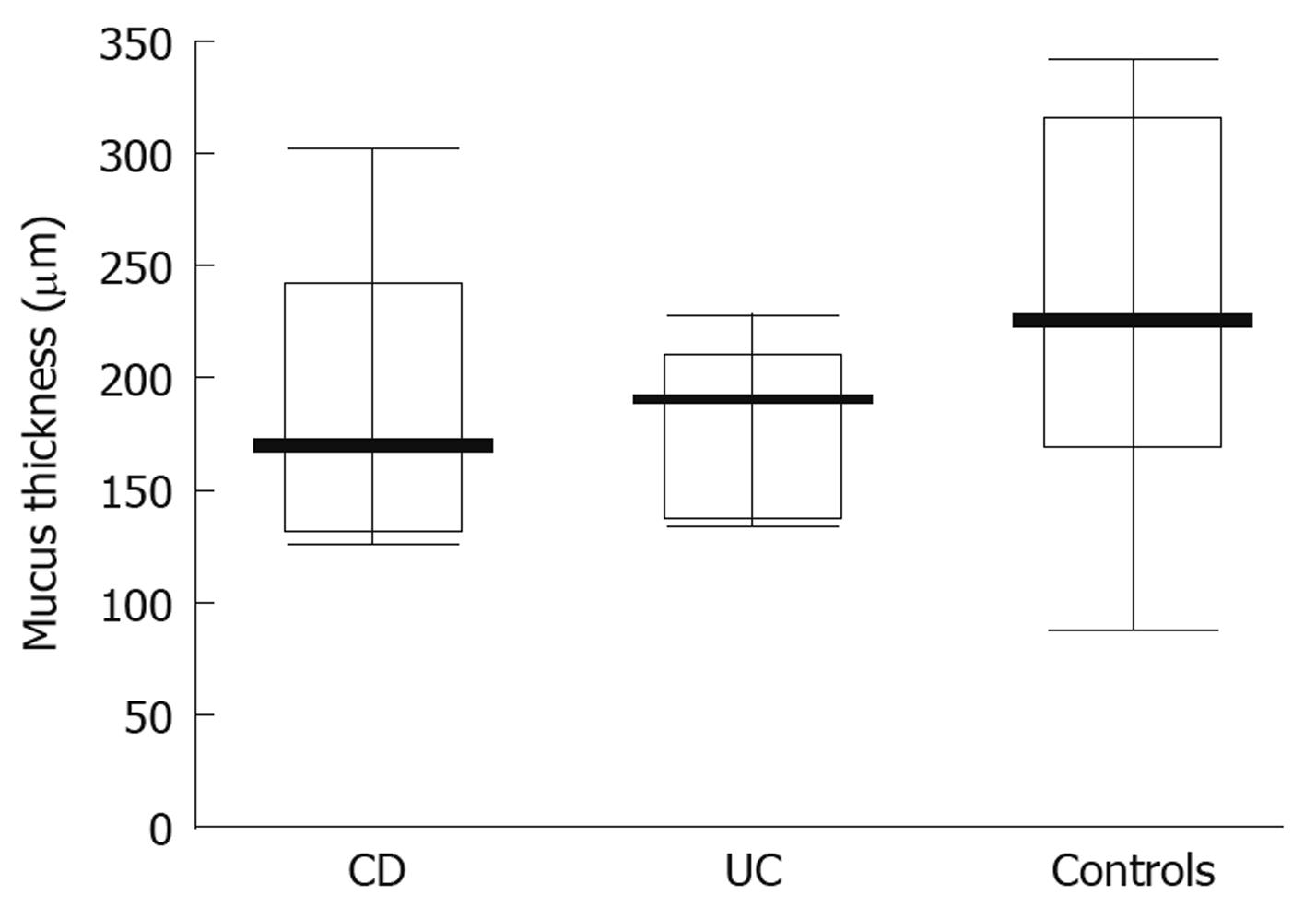
When studying the thickness of the mucus layer in biopsies taken from inflamed sites of the gut in adolescents with IBD, it appeared thinner when compared to the controls (CD-74 ± 40 μm; UC-83.35 ± 49.93 μm; controls- 218 ± 81.07 μm) and these differences were statistically significant (P = 0.0047 and P = 0.0093, respectively) (Figure 3). Also, the matching pair test analysis showed a statistically significant thinner mucus layer in inflamed as compared to non-inflamed sites in the same IBD patients (Z = 10.50, P = 0.031). Focusing on specific IBD groups and comparing inflamed to non-inflamed sites, the difference was significant in CD (P = 0.0109), but not in UC patients (P = 0.0662). On the other hand, there was no difference between the CD and control group (P = 0.7003) and the UC and control group (P = 0.3137) (Figure 4) when comparing the thickness of the mucus layer in biopsies from non-inflamed areas in IBD patients to those of the control group.
When trying to assess the histopathological activity and goblet cell depletion according to the scoring index proposed by Kudo et al[16], we found no statistically significant differences between thinner and thicker mucus layer sites both in the UC and CD groups. However, histopathological activity and goblet cell depletion was more severe in the UC group (77% and 61.5%, respectively) than in the CD group (59.1% and 32%, respectively).
Considering the numbers of bacteria contained in the biopsy samples, their ratios and the mucus thickness no relationship was found between age, gender and disease status of the patients.
It is well known that bacterial microflora in the human gastrointestinal tract play an important role in maintaining human health and may also contribute to the pathogenesis of IBD[17]. The data from animal models of IBD suggest that intestinal inflammation is dependent on the presence of intestinal microflora, although no specific pathogen has been identified and confirmed to be responsible for this process. Some studies performed on adult patients showed a strong correlation between disease activity and increased numbers of bacteria attached to the mucosa[18,19]. Although in our study we did not observe a significant increase in microflora contained in the biopsy samples in IBD adolescents in relation to controls, we did find a higher number of bacteria in biopsies taken from UC patients, particularly in those with the most advanced stage of changes as estimated at endoscopy. Increasing disease activity was inversely related to the numbers of lactobacilli in samples from CD (but not from UC patients) and to a minor degree to Streptococcus.
In various studies carried out on adult IBD patients, it is possible to find conflicting results relating to the numbers of specific groups or genera of bacteria with respect to inflammation. Some authors reported increased numbers of aerobic Gram-negative rods (E. coli), while others found increased numbers of non-spore forming anaerobes (Proteobacteria and Bacteroides) and decreased numbers of spore-forming anaerobes like Clostridium[20-24]. There are also papers in which the authors found no differences between the bacterial flora in inflamed and non-inflamed tissue in the same patient[25,26]. On the contrary, Sepehri et al[27] using a PCR method showed significant differences in bacterial flora between the inflamed and non-inflamed mucosa, and an increase in microbial diversity in controls and the non-inflamed tissue from adult IBD patients.
We found differences in microflora composition between endoscopically inflamed and non-inflamed mucosal sites in newly diagnosed IBD adolescents which were specific for each form of the disease. In the controls and in the endoscopically non-inflamed mucosa of IBD children there were high numbers of bifidobacteria compared with decreased populations of these bacteria in inflamed sites of IBD patients. Thus, our observations are in concordance with the earlier findings of Seksik et al[28] who also showed decreased numbers of Bifidobacterium and Enterobacteriaceae in adult patients with active and inactive CD. Our CD and UC patients also had decreased numbers of Bifidobacterium in inflamed mucosal sites compared with controls. It should also be noted that the numbers of lactobacilli, known for their protective effect in IBD, were lower in samples from CD adolescents with severe inflammation in our study[29]. Seksik et al[28] showed that the numbers of Lactobacillus strains were low in active and inactive CD adult patients.
Conte et al[9] recently reported the first, to our knowledge, study on gut-associated bacterial microflora in children with IBD. They found higher numbers of aerobic and facultative anaerobic bacteria in biopsy specimens from IBD children in comparison to controls, but lower numbers of Bacteroides in CD patients.
It should be noted that more than 50% of intestinal bacteria are not cultivable and this may represent a limitation of the study. Furthermore, molecular analytical methods have utility in discriminating between bowel microbiota of altered compositions and could be used in future studies in children. These techniques enable characterization as well as quantification of the microbiota; composition of the microbiota may be identified with clone libraries, fingerprinting techniques may be used to analyze microbial community structure and dot blot hybridization or fluorescent in situ hybridization (FISH) may be used to analyze a multitude of given taxa[30-32]. In our previous pilot study (results not included here) we used FISH to analyze colonic microflora in children with IBD, however, this approach was limited by low sensitivity of the method.
Considering the conflicting results on microflora in IBD presented in the literature one should remember that the patient populations as well as stages of the illness in various studies were different. Other factors that could strongly affect the results and cause obvious discrepancies were: different sites from which the biopsies were taken, the cleaning procedures and microbiological methods used to identify the microflora. Moreover, it is possible that gut bacterial flora in children is generally less stable than in adults. All these factors could be responsible for the observed variations.
We have carried out our investigation using samples of tissue and feces which were stored at -80°C during transport from the ward to the laboratory. According to Achá et al[33] and Dan et al[34], freezing does not influence viability of fecal samples.
Colonic mucus is an adherent, water insoluble gel that has several functions, including protection of the epithelium from mechanical trauma, toxins, allergens and from microbial invasion[35]. Thus, the mucus layer plays a protective role in the intestinal mucosa[36,37]. The gut mucus lubricates the passage of food and protects the epithelial cells from direct contact with bacterial flora. The mucus consists of glycoproteins called mucins, which are encoded by many MUC genes - to date more than 20 types of MUC genes have been described[38]. Thickness of the mucus layer is related to the dynamic equilibrium between mucus secretion and its subsequent removal into the lumen. In UC, the absence of an adherent layer in some areas may be a result of inadequate secretion or excessive removal. The findings of various studies on mucus synthesis in colitis have suggested that the epithelial layer remains intact in the presence of mild to moderate inflammation. In the presence of severe inflammation or inactivity, however, it tends to be lower than normal[10].
In our study we have shown that the thickness of the mucus layer lining the intestinal lumen of adolescents with IBD is about three times thinner in both CD and UC patients compared with the control group. A similar relationship was observed in biopsies taken from inflamed and non-inflamed mucosa in the same IBD patients and these differences were statistically significant. In addition, we have demonstrated that mucus layer thickness measured in tissue samples taken from normal sites in patients with IBD was only slightly thinner than that in the control group and the difference was not statistically significant.
At present, there is no data in the literature on the mucus layer in children and adolescents with CD. There is also no clear explanation of observed differences, however, the results of the latest studies are in accordance with our findings. The course of CD and localization of inflammatory lesions in childhood may be quite different from than in adults. The majority of CD adolescents (63.6%) in our study had colon involvement on endoscopic assessment, similar to UC which may have influenced the observed mucus layer depletion in this group of patients. Moreover, Gersemann et al[39] using a real-time PCR method in adult IBD patients, showed that goblet cells were diminished in the intestinal biopsies of both UC and CD patients, however, in the CD group enhanced differentiation was found.
In addition, using immunostaining Ardesjö et al[40] reported the presence of immunoreactive agents in the serum of IBD patients, which reacted with goblet cells in the intestine.
A reduction in mucus layer thickness leads to bacteria from the intestinal lumen having closer contact with the intestinal epithelium, also causing a different selection of microorganisms, which may induce an inflammatory process in the bowel[10]. In adult patients suffering from IBD, the numbers of bacterial cells adhering to the intestinal mucus layer are much higher as compared to controls without IBD, and this dependence is proportional to the severity of the clinical course[19,21]. Whether this is a result or cause of bowel inflammation still remains unclear, however, the protective property of the mucosa is significantly weakened by this process.
In summary, our results showed that non-inflamed mucosa in both IBD patients and controls was covered with a thick mucus layer with the attached microflora showing a predominance of Bifidobacterium. In contrast, in inflamed sites there was a reduction in mucus layer thickness with the prevalence of specific bacterial groups which were different for CD and UC: Streptococcus in CD and Lactobacillus in UC. However, numbers of these bacteria decreased proportionally with the intensity of inflammation. Thus, our results also support the idea of a disruption of the balance between the different protective and harmful intestinal bacteria in the gastrointestinal tract. These results also provide evidence which suggest that the therapeutic use of probiotics, especially those containing Bifidobacterium and Lactobacillus may have some positive effects in patients with IBD.
The pathogenesis of inflammatory bowel disease (IBD) still remains unclear. Genetic, environmental and immunological factors seem to play a substantial role. However, an increasing number of both clinical and laboratory studies support the idea of the leading role of bacterial microflora in the onset and persistence of inflammation. It is well known, that the anatomic sites with the highest concentrations of luminal bacteria are most often involved in the inflammatory process. The intestinal flora of patients with active Crohn’s disease (CD) is different from that of quiescent subjects, ulcerative colitis (UC) patients and healthy controls. Transgenic mice kept under germ-free conditions did not develop inflammation until bacteria were introduced. In recent years several studies on gut microflora have suggested that inflammation may be due to a shift in the balance of healthy flora, which leads to dysbiosis and triggers or maintains inflammation.
The multiple interactions among bacterial microflora, mucus and host immunity in the children’s mucosa play a crucial role in IBD pathogenesis. We suggest that the local mucus layer in the intestine, especially in childhood, determines bacterial colonization and metabolism. The aim of our study was to assess mucosa-associated bacterial microflora and mucus layer thickness in relation to the inflammatory process in adolescents with newly diagnosed IBD in comparison to controls.
Up to now, this is the second study focused on the mucosa-associated bacterial microflora in children with IBD, used both culture and a PCR amplification method, and found significant differences between CD and UC children. The authors went a step further and assessed both the mucosa-associated bacterial microflora and intestinal mucosa.
Assessment of the multiple interactions among bacterial microflora and mucus in the very early stage of IBD, seen in newly diagnosed disease, is essential for a better understanding of disease pathogenesis and may help in suggesting therapeutic options such as the use of specific probiotics or antimicrobial treatment.
Mucosa-associated bacterial microflora consists of bacteria that are located very close to the mucus layer. This layer forms a specific environment in the gut and its disruption may play a crucial role in the development of IBD, promoting specific bacterial colonization and immunological response. The colonic mucus layer is an adherent, water insoluble gel that has several functions, including protection of the epithelium from mechanical trauma, toxins, allergens and from microbial invasion. The mucus consists of glycoproteins called mucins, which are encoded by many MUC genes - to date more than 20 types of MUC genes have been described. Thickness of the mucus layer is related to the dynamic equilibrium between mucus secretion and its subsequent removal into the lumen.
The authors demonstrated changes of mucosa-associated microbiota in adolescents with IBD, using classical culture techniques. They also showed depletion of the mucus layer in IBD patients. This paper is well documented.
| 1. | Hanauer SB. Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflamm Bowel Dis. 2006;12 Suppl 1:S3-S9. |
| 2. | Sokol H, Seksik P, Rigottier-Gois L, Lay C, Lepage P, Podglajen I, Marteau P, Doré J. Specificities of the fecal microbiota in inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:106-111. |
| 3. | Seksik P, Sokol H, Lepage P, Vasquez N, Manichanh C, Mangin I, Pochart P, Doré J, Marteau P. Review article: the role of bacteria in onset and perpetuation of inflammatory bowel disease. Aliment Pharmacol Ther. 2006;24 Suppl 3:11-18. |
| 4. | Giaffer MH, Holdsworth CD, Duerden BI. The assessment of faecal flora in patients with inflammatory bowel disease by a simplified bacteriological technique. J Med Microbiol. 1991;35:238-243. |
| 5. | Sellon RK, Tonkonogy S, Schultz M, Dieleman LA, Grenther W, Balish E, Rennick DM, Sartor RB. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun. 1998;66:5224-5231. |
| 6. | Sartor RB. Intestinal microflora in human and experimental inflammatory bowel disease. Curr Opin Gastroenterol. 2001;17:324-330. |
| 7. | Tamboli CP, Neut C, Desreumaux P, Colombel JF. Dysbiosis in inflammatory bowel disease. Gut. 2004;53:1-4. |
| 8. | Martin HM, Campbell BJ, Hart CA, Mpofu C, Nayar M, Singh R, Englyst H, Williams HF, Rhodes JM. Enhanced Escherichia coli adherence and invasion in Crohn's disease and colon cancer. Gastroenterology. 2004;127:80-93. |
| 9. | Conte MP, Schippa S, Zamboni I, Penta M, Chiarini F, Seganti L, Osborn J, Falconieri P, Borrelli O, Cucchiara S. Gut-associated bacterial microbiota in paediatric patients with inflammatory bowel disease. Gut. 2006;55:1760-1767. |
| 10. | Pullan RD, Thomas GA, Rhodes M, Newcombe RG, Williams GT, Allen A, Rhodes J. Thickness of adherent mucus gel on colonic mucosa in humans and its relevance to colitis. Gut. 1994;35:353-359. |
| 11. | Ryzko J, Woynarowski M. [Use of a scoring index for evaluating disease activity of Leśniowski-Crohn disease and ulcerative colitis in children]. Pediatr Pol. 1995;70:585-589. |
| 12. | Roth JLA. Ulcerative colitis. Gastroenterology. 3rd ed. Philadelphia: WB Saunders 1976; 645-749. |
| 13. | Clark PA, Cotton LN, Martin JH. Selection of bifidobacteria for use as dietary adjuncts in cultured dairy foods. II. Tolerance to simulated pH of human stomachs. Cult Dairy Prod J. 1993;28:11-14. |
| 14. | Freitas M, Axelsson LG, Cayuela C, Midtvedt T, Trugnan G. Microbial-host interactions specifically control the glycosylation pattern in intestinal mouse mucosa. Histochem Cell Biol. 2002;118:149-161. |
| 15. | Swidsinski A, Ladhoff A, Pernthaler A, Swidsinski S, Loening-Baucke V, Ortner M, Weber J, Hoffmann U, Schreiber S, Dietel M. Mucosal flora in inflammatory bowel disease. Gastroenterology. 2002;122:44-54. |
| 16. | Kudo T, Matsumoto T, Esaki M, Yao T, Iida M. Mucosal vascular pattern in ulcerative colitis: observations using narrow band imaging colonoscopy with special reference to histologic inflammation. Int J Colorectal Dis. 2009;24:495-501. |
| 17. | Martinez-Medina M, Aldeguer X, Gonzalez-Huix F, Acero D, Garcia-Gil LJ. Abnormal microbiota composition in the ileocolonic mucosa of Crohn's disease patients as revealed by polymerase chain reaction-denaturing gradient gel electrophoresis. Inflamm Bowel Dis. 2006;12:1136-1145. |
| 18. | Kleessen B, Kroesen AJ, Buhr HJ, Blaut M. Mucosal and invading bacteria in patients with inflammatory bowel disease compared with controls. Scand J Gastroenterol. 2002;37:1034-1041. |
| 20. | Schultsz C, Van Den Berg FM, Ten Kate FW, Tytgat GN, Dankert J. The intestinal mucus layer from patients with inflammatory bowel disease harbors high numbers of bacteria compared with controls. Gastroenterology. 1999;117:1089-1097. |
| 21. | Croucher SC, Houston AP, Bayliss CE, Turner RJ. Bacterial populations associated with different regions of the human colon wall. Appl Environ Microbiol. 1983;45:1025-1033. |
| 22. | Hartley CL, Neumann CS, Richmond MH. Adhesion of commensal bacteria to the large intestine wall in humans. Infect Immun. 1979;23:128-132. |
| 23. | Ott SJ, Musfeldt M, Wenderoth DF, Hampe J, Brant O, Fölsch UR, Timmis KN, Schreiber S. Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut. 2004;53:685-693. |
| 24. | Darfeuille-Michaud A, Neut C, Barnich N, Lederman E, Di Martino P, Desreumaux P, Gambiez L, Joly B, Cortot A, Colombel JF. Presence of adherent Escherichia coli strains in ileal mucosa of patients with Crohn's disease. Gastroenterology. 1998;115:1405-1413. |
| 25. | Gophna U, Sommerfeld K, Gophna S, Doolittle WF, Veldhuyzen van Zanten SJ. Differences between tissue-associated intestinal microfloras of patients with Crohn's disease and ulcerative colitis. J Clin Microbiol. 2006;44:4136-4141. |
| 26. | Seksik P, Lepage P, de la Cochetière MF, Bourreille A, Sutren M, Galmiche JP, Doré J, Marteau P. Search for localized dysbiosis in Crohn's disease ulcerations by temporal temperature gradient gel electrophoresis of 16S rRNA. J Clin Microbiol. 2005;43:4654-4658. |
| 27. | Sepehri S, Kotlowski R, Bernstein CN, Krause DO. Microbial diversity of inflamed and noninflamed gut biopsy tissues in inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:675-683. |
| 28. | Seksik P, Rigottier-Gois L, Gramet G, Sutren M, Pochart P, Marteau P, Jian R, Doré J. Alterations of the dominant faecal bacterial groups in patients with Crohn's disease of the colon. Gut. 2003;52:237-242. |
| 29. | Geier MS, Butler RN, Howarth GS. Inflammatory bowel disease: current insights into pathogenesis and new therapeutic options; probiotics, prebiotics and synbiotics. Int J Food Microbiol. 2007;115:1-11. |
| 30. | Andoh A, Benno Y, Kanauchi O, Fujiyama Y. Recent advances in molecular approaches to gut microbiota in inflammatory bowel disease. Curr Pharm Des. 2009;15:2066-2073. |
| 31. | Tannock GW. Molecular analysis of the intestinal microflora in IBD. Mucosal Immunol. 2008;1 Suppl 1:S15-S18. |
| 32. | Li M, Gao X, Guo CC, Wu KC, Zhang X, Hu PJ. OCTN and CARD15 gene polymorphism in Chinese patients with inflammatory bowel disease. World J Gastroenterol. 2008;14:4923-4927. |
| 33. | Achá SJ, Kühn I, Mbazima G, Colque-Navarro P, Möllby R. Changes of viability and composition of the Escherichia coli flora in faecal samples during long time storage. J Microbiol Methods. 2005;63:229-238. |
| 34. | Dan M, Richardson J, Miliotis MD, Koornhof HJ. Comparison of preservation media and freezing conditions for storage of specimens of faeces. J Med Microbiol. 1989;28:151-154. |
| 35. | Einerhand AW, Renes IB, Makkink MK, van der Sluis M, Büller HA, Dekker J. Role of mucins in inflammatory bowel disease: important lessons from experimental models. Eur J Gastroenterol Hepatol. 2002;14:757-765. |
| 36. | Hugot JP, Chamaillard M, Zouali H, Lesage S, Cézard JP, Belaiche J, Almer S, Tysk C, O'Morain CA, Gassull M. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411:599-603. |
| 37. | Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229-241. |
| 38. | Porchet N, Aubert JP. [MUC genes: mucin or not mucin? That is the question]. Med Sci (Paris). 2004;20:569-574. |
| 39. | Gersemann M, Becker S, Kübler I, Koslowski M, Wang G, Herrlinger KR, Griger J, Fritz P, Fellermann K, Schwab M. Differences in goblet cell differentiation between Crohn's disease and ulcerative colitis. Differentiation. 2009;77:84-94. |
| 40. | Ardesjö B, Portela-Gomes GM, Rorsman F, Gerdin E, Lööf L, Grimelius L, Kämpe O, Ekwall O. Immunoreactivity against Goblet cells in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:652-661. |
Peer reviewers: Akira Andoh, MD, Department of Internal Medicine, Shiga University of Medical Science, Seta Tukinowa, Otsu 520-2192, Japan; Dr. Roberto Berni Canani, Department of Pediatrics, University of Naples Federico II, Naples 80131, Italy
S- Editor Li LF L- Editor Webster JR E- Editor Lin YP