Published online Nov 7, 2009. doi: 10.3748/wjg.15.5181
Revised: September 12, 2009
Accepted: September 19, 2009
Published online: November 7, 2009
AIM: To evaluate the therapeutic role of caffeic acid phenethyl ester (CAPE) in a rat model of cerulean-induced acute pancreatitis (AP).
METHODS: Seventy male Wistar albino rats were divided into seven groups. Acute edematous pancreatitis was induced by subcutaneous cerulein injection (20 μg/kg) four times at 1-h intervals. CAPE (30 mg/kg) was given by subcutaneous injection at the beginning (CAPE 1 group) and 12 h after the last cerulein injection (CAPE 2 group). Serum amylase, lipase, white blood cell count, and tumor necrosis factor (TNF)-α levels were measured, and pancreatic histopathology was assessed.
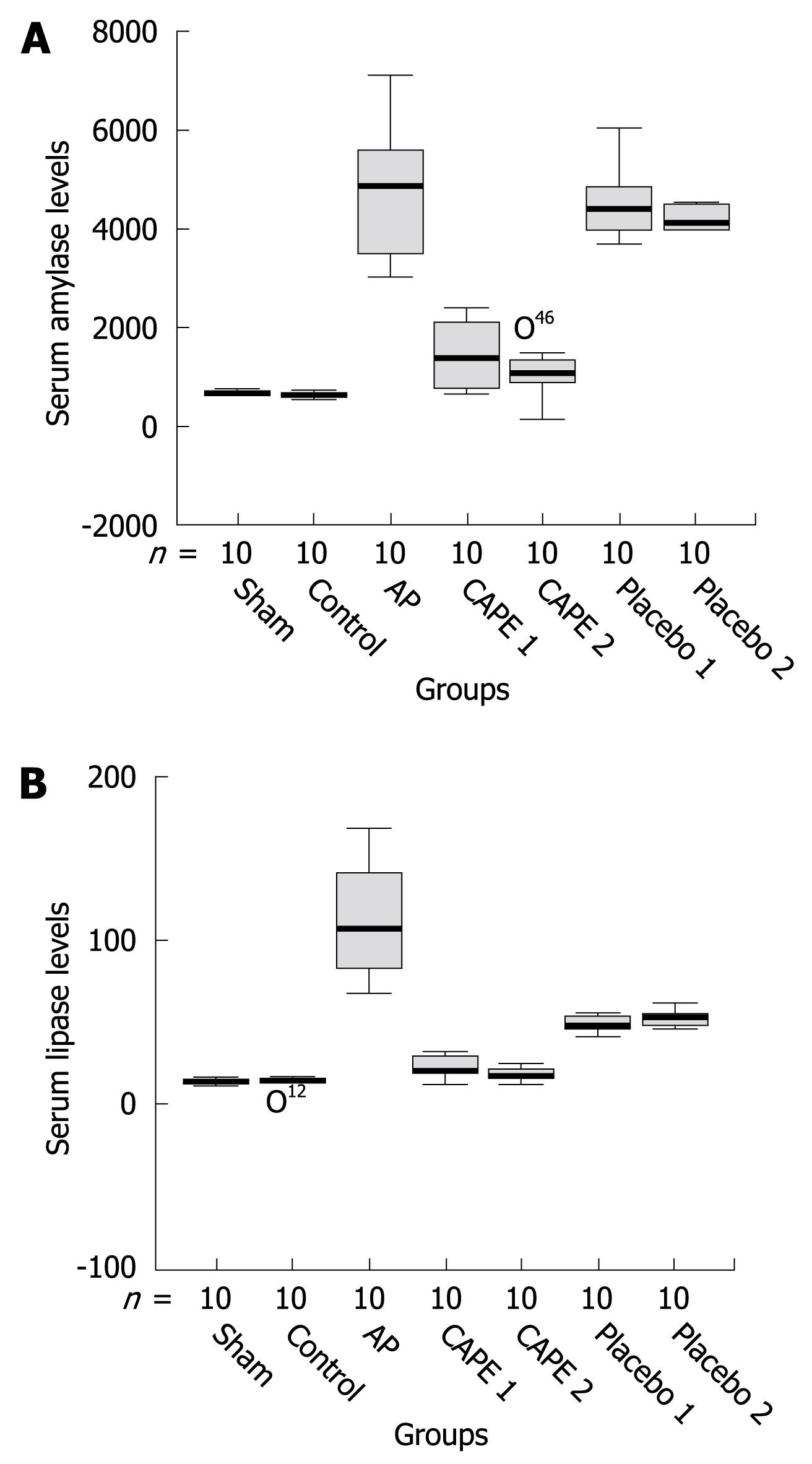
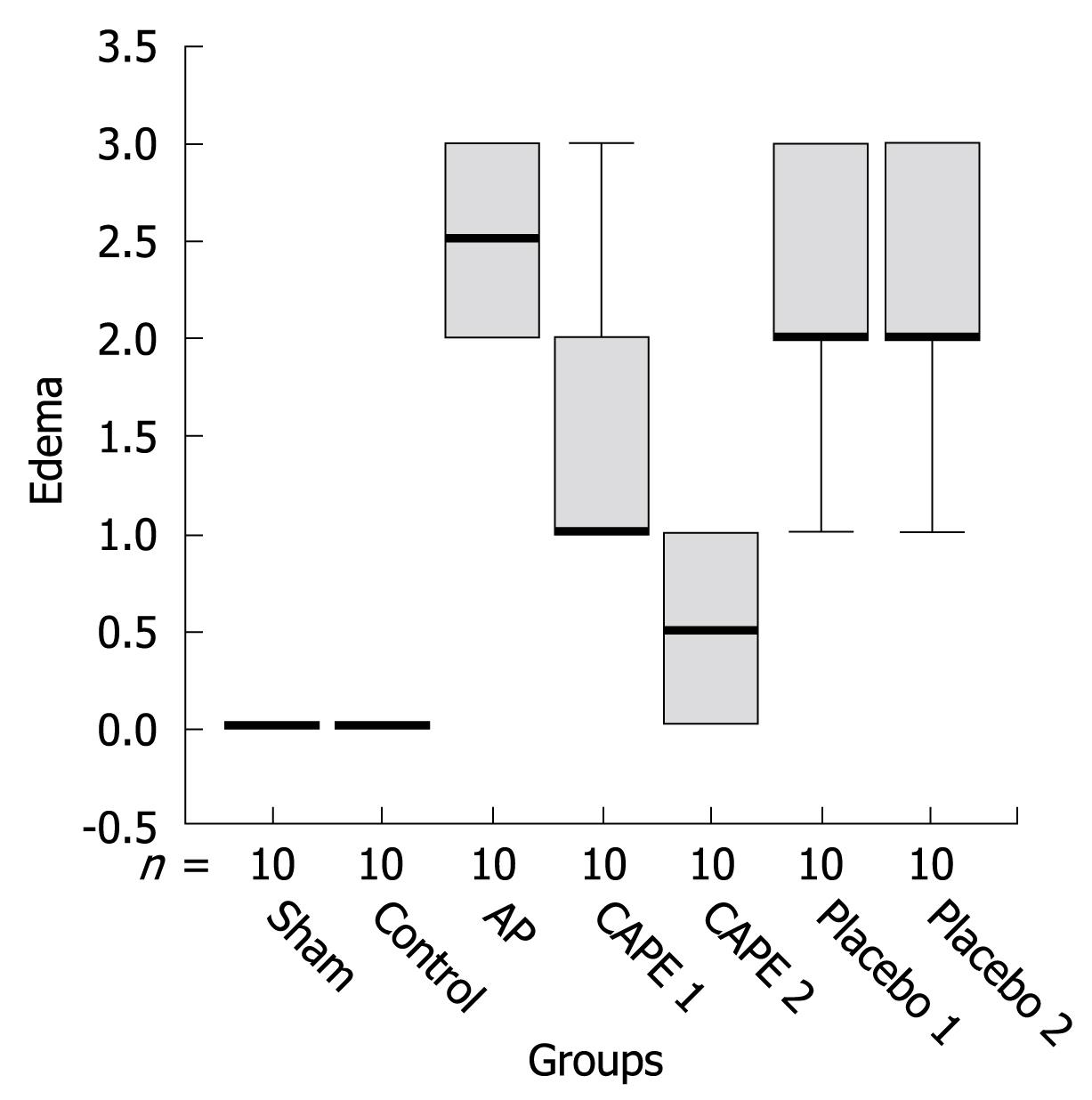
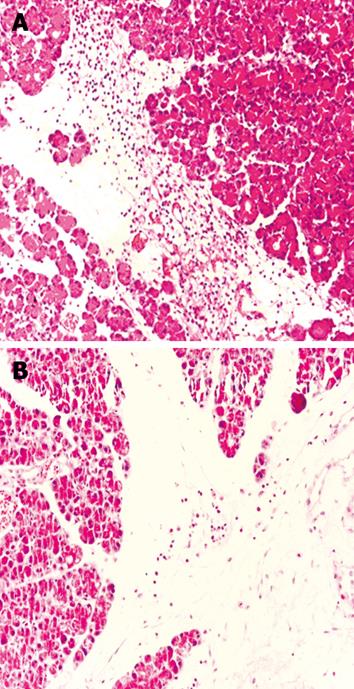
RESULTS: In the AP group, amylase and lipase levels were found to be elevated and the histopathological evaluation showed massive edema and inflammation of the pancreas, with less fatty necrosis when compared with sham and control groups. Amylase and lipase levels and edema formation decreased significantly in the CAPE therapy groups (P < 0001); especially in the CAPE 2 group, edema was improved nearly completely (P = 0001). Inflammation and fatty necrosis were partially recovered by CAPE treatment. The pathological results and amylase level in the placebo groups were similar to those in the AP group. White blood cell count and TNF-α concentration was nearly the same in the CAPE and placebo groups.
CONCLUSION: CAPE may be useful agent in treatment of AP but more experimental and clinical studies are needed to support our observation of beneficial effects of CAPE before clinical usage of this agent.
- Citation: Buyukberber M, Savaş MC, Bagci C, Koruk M, Gulsen MT, Tutar E, Bilgic T, Ceylan N&. Therapeutic effect of caffeic acid phenethyl ester on cerulein-induced acute pancreatitis. World J Gastroenterol 2009; 15(41): 5181-5185
- URL: https://www.wjgnet.com/1007-9327/full/v15/i41/5181.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.5181
| Edema | 0 | No edema |
| 1 | Interlobular edema | |
| 2 | Moderate interlobular edema + intra-acinar edema | |
| 3 | Severe interlobular and intra-acinar edema | |
| Inflammatory infiltration | 0 | No infiltration |
| 1 | Intravascular margination of granulocytes | |
| 2 | Granulocytes present in the perivascular tissue | |
| 3 | Diffuse infiltration of entire pancreatic gland | |
| Fat necrosis | 0 | No necrosis |
| 1 | 1-4 necrotic cells ( each microscopic area) | |
| 2 | 5-10 necrotic cells | |
| 3 | 11-16 necrotic cells |
| Groups | TNF-α(pg/mL) | Amylase (U/L) | Lipase (U/L) | White blood cells | Edema | Leukocytic infiltration | Total pathological score | Fat necrosis |
| Sham | 65.14 ± 1.7 | 665.14 ± 54 | 14.41 ± 1.7 | 9279 ± 1867 | 0.00 | 0.00 | 0.00 | 0.00 |
| Control | 63.29 ± 3.8 | 630.20 ± 64 | 14.92 ± 1.7 | 8755 ± 1098 | 0.00 | 0.00 | 0.00 | 0.00 |
| AP | 63.83 ± 3.8 | 4752 ± 1328b | 112.3 ± 34.8b | 8574 ± 1437 | 2.50 ± 0.5 | 2.80 ± 0.42 | 8.00 | 0.30 ± 0.48 |
| CAPE 1 | 61.55 ± 8.0 | 1400 ± 680d | 22.92 ± 6.9d | 8407 ± 418 | 1.50 ± 0.7 | 2.50 ± 0.52 | 4.00c | 0.00 |
| CAPE 2 | 60.52 ± 6.5 | 1084 ± 533d | 18.65 ± 3.7d | 8940 ± 2746 | 0.50 ± 0.5d | 2.40 ± 0.51 | 3.00c | 0.00 |
| Placebo 1 | 62.38 ± 9.5 | 4516 ± 749 | 49.2 ± 5.3 | 9267 ± 927 | 2.30 ± 0.6 | 2.70 ± 0.48 | 8.00 | 0.30 ± 0.48 |
| Placebo 2 | 61.56 ± 2.5 | 4219 ± 235 | 52.54 ± 4.8 | 8544 ± 895 | 2.20 ± 0.6 | 2.70 ± 0.48 | 8.00 | 0.30 ± 0.48 |
Acute pancreatitis (AP) is a process of acute inflammation in the pancreas, with variable involvement of regional tissues or organ systems. In most patients, acute necrotizing pancreatitis leads to remote organ failure, sepsis and a high death rate[1]. Pathophysiology of AP is poorly understood, but interleukin (IL)-1, IL-6 and tumor necrosis factor (TNF)-α as pro-inflammatory cytokines, oxidative stress and microvascular ischemia are important factors[2-4]. In recent years, pathogenesis-oriented treatments of AP have gained importance. Therefore, new experimental studies have focused on pathophysiological mechanisms such as oxidative stress and inflammatory cytokines[5,6]. Propolis is a natural substance that is produced by honeybees from the gum of various plants. It contains several chemical compounds such as polyphenolic compounds like flavonoids, cinnamic acid derivates, various steroids, and amino acids[7,8]. Caffeic acid phenethyl ester (CAPE) is also a phenolic compound and an active substrate of propolis. Several investigators have shown that CAPE has anti-inflammatory activity by inhibiting the release of arachidonic acid from cell membranes, and suppressing cyclooxygenase (COX)-1 and COX-2 enzyme activity[9], antioxidant activity by lipoxygenase inhibition[10,11], and anti-proliferative, antimutagenic and antitumoral effects by inducing apoptosis in tumor cell lines[12]. In addition, CAPE is a potent and specific inhibitor of nuclear factor (NF)-κB and inhibits the activation of NF-κB that is induced by TNF-α and other inflammatory agents[13].
The aim of this study was to investigate the therapeutic efficacy of CAPE in the cerulean-induced acute edematous pancreatitis in rats.
Seventy male Wistar albino rats, weighing 250-320 g were used from the Physiology Laboratory of Gaziantep University Medical School. The animals were housed under a 12-h light-dark cycle at a temperature of 24°C. Food was withdrawn 12 h before the experiment. All experiments were performed in accordance with the recommendations of the national guidelines for the care and handling of laboratory animals, and followed a protocol approved by the local animal ethics committee.
Acute edematous pancreatitis was induced by subcutaneous cerulein (Sigma, St Louis, MO, USA) injection (20 g/kg) four times at 1-h intervals[14]. Seventy male rats were divided into seven groups of 10.
Group 1 (sham): nothing was applied to the sham group. Group 2 (control): 1 mL saline was given by subcutaneous injecting four times at 1-h intervals, but no medication was applied. Animals were killed 12 h after the last injection. Group 3 (AP group): AP was induced by subcutaneous cerulein injection (20 g/kg dissolved in 1 mL saline) four times at 1-h intervals, but no medication was applied. Animals were killed 12 h after the last injection. Group 4 (CAPE 1) 30 mg/kg CAPE (Sigma) was given by subcutaneous injection at the beginning of the procedure, and at the same time, AP was induced by subcutaneous cerulein injection as described before. Group 5 (CAPE 2): AP was induced in the same way as described above, and CAPE (30 mg/kg) was given at 12 h after the last cerulein injection. Animals were killed 6 h after the CAPE injection. Group 6 (placebo 1): AP was induced by subcutaneous cerulein injection (20 μg/kg) four times at 1-h intervals, and 1 mL saline was given at the beginning of the studies. Animals were killed 12 h after the last injection. Group 7 (placebo 2): AP was induced in the same way as described above, and 1 mL saline was given at 12 h after the last cerulein injection. Animals were killed 6 h after the saline injection.
Under ketamine anesthesia, midline laparotomy was performed on all rats, except Groups 5 and 7, at 15 h (12 h after the last cerulein or saline injection). Groups 5 and 7 were killed 6 h after CAPE injection (Group 5) or saline injection (Group 7). Shortly after the blood specimens were taken from the inferior vena cava, the whole pancreas was extracted quickly and the animals were sacrificed. Blood samples were centrifuged at 3000 rpm for 10 min and the plasma was stored at -70°C until assayed. White blood cell count, amylase, lipase and TNF-α concentrations were measured. Plasma TNF-α concentration was measured by immunoassay kit (Rat TNF-α immunoassay; R&D Systems Inc., Minneapolis, MN, USA), plasma amylase and lipase were measured by commercially available kits from Roche Diagnostics (Mannheim, Germany), using an enzymatic photometric method based on cleavage of the substrate ethylidene-4-nitrophenyl maltoheptaose. Results are expressed as U/L.
Histopathological evaluation of the pancreas was made in order to understand the extent of the injury. Pancreatic tissue was fixed in formaldehyde solution and embedded in paraffin. Sections were stained with hematoxylin and eosin and were evaluated by light microscopy by two experienced pathologists who were blinded to the experimental treatment groups, according to the Schoenberg grading system[15] (Table 1). The tissues were scored using a scale for edema, neutrophil infiltration and fatty necrosis .
Results were given as mean ± SD. Comparisons between and among the groups were made using non-parametric test (Mann-Whitney U test) and one-way ANOVA. Data were evaluated statistically using SPSS for Windows version 10.0 (Chicago, IL, USA). P < 0.05 was taken as significant.
Serum biochemical analysis of amylase, lipase and TNF-α levels and pathological examination results are shown in Table 2. Serum amylase and lipase levels were significantly increased in the cerulean-induced AP group when compared to the control and sham groups (P < 0.001). Amylase and lipase levels decreased significantly in the CAPE treatment groups (P < 0001) but the levels were higher than those of the control and sham groups. The levels of amylase and lipase in the placebo groups were similar to those in the AP group (Figure 1A and B). There were no statistically significant differences in serum TNF-α and white blood cell count between the study groups (P > 0.05, Table 2).
In the AP group, histopathological evaluation showed massive edema and inflammation of the pancreas, with less fatty necrosis when compared with the control and sham groups. CAPE treatment significantly decreased edema formation, and the most striking finding was that edema was improved nearly completely in the CAPE 2 group (P = 0001, Figure 2). Polymorphonuclear leukocytic infiltration was increased in the AP groups (P < 0.05, Figure 3A). In the therapy groups, inflammation was partially recovered. In the AP groups, fatty necrosis score was 0.30 ± 0.48. We observed grade 1 fatty necrosis in only three rats in the AP groups. Fatty necrosis was ameliorated in the CAPE treatment groups but this improvement was not statistically significantly (P > 0.05). The pathological results of the placebo groups were similar to those in the AP groups. After CAPE treatment, the total pathological mean score was decreased significantly (P < 005) after CAPE treatment (Figure 3B).
Current therapeutic methods are usually insufficient for the treatment of severe AP, despite the development of new diagnostic and therapeutic procedures. Therefore, recently, several experimental studies have focused on the pathogenesis of AP. Several mechanisms, such as oxidative stress, COX-2 and inflammatory cytokines play an important role in the pathogenesis of the disease[2-4,16]. CAPE is a phenolic antioxidant, which is an active component of propolis. Previous investigators have demonstrated that CAPE has anti-inflammatory, antioxidant, anti-proliferative and antitumoral effects in vitro and in vivo[12]. In the light of previous findings, we investigated the therapeutic role of CAPE as a new agent for the treatment of AP.
TNF-α is a cytokine that plays a central role in the pathogenesis of the disease[2]. TNF receptor antagonist observed a reduction in the severity and mortality of experimental pancreatitis[17]. Plasma half-life of TNF-α is very short (14-18 min)[18], therefore, we studied TNF-α serum levels in rats, despite this kind of measurement being difficult. We obtained serum at 15 h, and that is probably why the results were low in all groups.
Norman et al[19] have shown marked amelioration of pancreatic tissue damage and decreased serum amylase and lipase levels after treatment with IL-1 antagonist. Oxidative stress plays an important role in the pathophysiology of AP. For this reason, several studies have reported the therapeutic effect of antioxidant agents. A previous study has disclosed that various antioxidant agents improve pancreatic edema in cerulean-induced pancreatitis, however antioxidants showed no improvement in a sodium-taurocholate model of pancreatitis in rats[20]. On the contrary, one recent study in a sodium-taurocholate model of pancreatitis in rats has shown that serum amylase and lipase, edema, leukocytic infiltration, parenchymal necrosis and hemorrhage were significantly decreased by N-acetylcysteine (NAC) treatment. In addition, in the NAC-treated rats, while serum nitrite/nitrate levels were significantly increased, serum concentration of the lipid peroxidation product was significantly decreased. The beneficial effect of NAC may result from its antioxidant activity and the production of and/or inhibition of degradation of nitric oxide[5]. In a similar study, Vaquero et al[21] have demonstrated that treatment with NAC reduces neutrophil infiltration and mRNA expression for IL-6, cytokines and inducible nitric oxide synthase in pancreatic tissue, by inhibition of NF-κB activity. In conclusion, NF-κB is a key regulator cytokine in induction and oxidative stress in AP. In experimental pancreatitis, the beneficial effect of antioxidants can be explained by inhibition of NF-κB activation. CAPE is a specific and potent inhibitor of NF-κB and causes inhibition of pro-inflammatory cytokine production[13]. Likewise, Fitzpatrick et al[22] have shown that CAPE (30 mg/kg) treatment significantly inhibits NF-κB, and colonic cytokines (TNF-α and IL-1β) are reduced in experimental colitis in rats.
AP is associated with induction of COX-2 expression. In cerulean-induced pancreatitis, Ethridge et al[16] have found that COX-2 gene expression is increased in pancreatic tissue. Although serum amylase and lipase are not reduced, the severity of pancreatic necrosis and leukocytic inflammation are significantly decreased by treatment with NS-398 (a COX-2 inhibitor). It has been demonstrated that COX-2 gene expression, activity of COX-1 and COX-2 enzymes, and release of arachidonic acid from cell membranes are inhibited by CAPE[9]. In light of this, we investigated the beneficial efficacy of CAPE (which is an antioxidant and anti-inflammatory agent) on the experimental model of cerulean-induced acute edematous pancreatitis in rats. As far as we know, there are no published data on the treatment effect of CAPE in experimental pancreatitis models. In the present study, we showed that CAPE ameliorated the harmful effects in a rat model of cerulein-induced pancreatitis. Serum amylase and lipase levels were decreased by CAPE treatment. In addition, CAPE treatment significantly reduced edema and total pathological mean score. Inflammation and fatty necrosis score were improved but the improvement was not statistically significantly.
There are several models of experimental pancreatitis, such as the cerulein-induced and sodium-taurocholate models. Pancreatic injury is evenly distributed throughout the pancreas in the cerulein-induced models. The reason why we chose the cerulein-induced AP model was that this form of pancreatitis is very similar to that in humans and it occurs within a short time[23]. This model is used widely to study potential agents for the treatment of AP[24]. In this model, pancreatic inflammation reaches the most severe stage at 12 h, which is why we ended the first part of the study at 12 h after cerulein injection[25]. Secondly, we formed a CAPE 2 group to study the effect of CAPE on the most severe stage of pancreatitis at 12 h. Here, our concern was to study the efficacy of the treatment in severe pancreatitis, especially in the full-blown situation. In fact, patients with AP often attend the hospital at an advanced stage, even sometimes with systemic complications.
In conclusion, in the cerulein-induced model of experimental AP, an improvement in the biochemical and histopathological findings were observed in the CAPE treatment groups. CAPE decreased pancreatic tissue injury and this supports the hypothesis that antioxidant and anti-inflammatory treatment is effective in AP. It is important that CAPE was effective in the CAPE 2 group when AP had already occurred. This will enlighten the following phase 3 and phase 4 studies. Another important step will be to study the efficacy of CAPE in experimental necrotizing pancreatitis. Nevertheless, more experimental and clinical studies are needed to support our observation of the beneficial effects of CAPE before clinical usage of this agent.
Pathophysiology of acute pancreatitis (AP) is poorly understood. Therefore, new experimental therapeutic studies have focused on the pathophysiological mechanisms. The present experimental study investigated the therapeutic role of caffeic acid phenethyl ester (CAPE) as a new agent for the treatment of AP.
CAPE is a specific and potent inhibitor of nuclear factor (NF)-κB and causes inhibition of pro-inflammatory cytokine production. CAPE (30 mg/kg) treatment significantly inhibited NF-κB, and colonic cytokines tumor necrosis factor-α and interleukin-1β were reduced in experimental colitis in rats.
There are no published data on the treatment effect of CAPE in experimental pancreatitis models. In the cerulein-induced model of experimental AP, improvement in biochemical and histopathological findings was observed in the CAPE treatment groups.
CAPE may be a useful agent in the treatment of AP but more experimental and clinical studies are needed to support our observation of its beneficial effects.
CAPE is a phenolic compound and an active substrate of propolis. CAPE has anti-inflammatory, antioxidant, anti-proliferative and antitumoral effects in vitro and in vivo.
This work provides experimental evidence for a protective function of CAPE in a rat model of acute pancreatitis. The study is generally well-designed and controlled. The results show that administration of CAPE reduces serum amylase and lipase levels in cerulein-treated rats and improves pathological scores in pancreatic tissue specimens.
| 1. | Whitcomb DC. Clinical practice. Acute pancreatitis. N Engl J Med. 2006;354:2142-2150. |
| 2. | Kusske AM, Rongione AJ, Reber HA. Cytokines and acute pancreatitis. Gastroenterology. 1996;110:639-642. |
| 3. | Gukovskaya AS, Gukovsky I, Zaninovic V, Song M, Sandoval D, Gukovsky S, Pandol SJ. Pancreatic acinar cells produce, release, and respond to tumor necrosis factor-alpha. Role in regulating cell death and pancreatitis. J Clin Invest. 1997;100:1853-1862. |
| 4. | Sweiry JH, Mann GE. Role of oxidative stress in the pathogenesis of acute pancreatitis. Scand J Gastroenterol Suppl. 1996;219:10-15. |
| 5. | Yagci G, Gul H, Simsek A, Buyukdogan V, Onguru O, Zeybek N, Aydin A, Balkan M, Yildiz O, Sen D. Beneficial effects of N-acetylcysteine on sodium taurocholate-induced pancreatitis in rats. J Gastroenterol. 2004;39:268-276. |
| 6. | Oruc N, Ozutemiz AO, Yukselen V, Nart D, Celik HA, Yuce G, Batur Y. Infliximab: a new therapeutic agent in acute pancreatitis? Pancreas. 2004;28:e1-e8. |
| 7. | Garcia-Viguera C, Ferreres F, Tomas-Barberan FA. Study of Canadian propolis by GC-MS and HPLC. Z Naturforsch. 1993;48:731-735. |
| 8. | Gabrys J, Konecki J, Krol W, Scheller S, Shani J. Free amino acids in bee hive product (propolis) as identified and quantified by gas-liquid chromatography. Pharmacol Res Commun. 1986;18:513-518. |
| 9. | Michaluart P, Masferrer JL, Carothers AM, Subbaramaiah K, Zweifel BS, Koboldt C, Mestre JR, Grunberger D, Sacks PG, Tanabe T. Inhibitory effects of caffeic acid phenethyl ester on the activity and expression of cyclooxygenase-2 in human oral epithelial cells and in a rat model of inflammation. Cancer Res. 1999;59:2347-2352. |
| 10. | Sud'ina GF, Mirzoeva OK, Pushkareva MA, Korshunova GA, Sumbatyan NV, Varfolomeev SD. Caffeic acid phenethyl ester as a lipoxygenase inhibitor with antioxidant properties. FEBS Lett. 1993;329:21-24. |
| 11. | Mirzoeva OK, Sud'ina GF, Pushkareva MA, Korshunova GA, Sumbatian NV, Varfolomeev SD. [Lipophilic derivatives of caffeic acid as lipoxygenase inhibitors with antioxidant properties]. Bioorg Khim. 1995;21:143-151. |
| 12. | Banskota AH, Tezuka Y, Kadota S. Recent progress in pharmacological research of propolis. Phytother Res. 2001;15:561-571. |
| 13. | Natarajan K, Singh S, Burke TR Jr, Grunberger D, Aggarwal BB. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-kappa B. Proc Natl Acad Sci USA. 1996;93:9090-9095. |
| 14. | Lampel M, Kern HF. Acute interstitial pancreatitis in the rat induced by excessive doses of a pancreatic secretagogue. Virchows Arch A Pathol Anat Histol. 1977;373:97-117. |
| 15. | Schoenberg MH, Büchler M, Gaspar M, Stinner A, Younes M, Melzner I, Bültmann B, Beger HG. Oxygen free radicals in acute pancreatitis of the rat. Gut. 1990;31:1138-1143. |
| 16. | Ethridge RT, Chung DH, Slogoff M, Ehlers RA, Hellmich MR, Rajaraman S, Saito H, Uchida T, Evers BM. Cyclooxygenase-2 gene disruption attenuates the severity of acute pancreatitis and pancreatitis-associated lung injury. Gastroenterology. 2002;123:1311-1322. |
| 17. | Norman JG, Fink GW, Franz MG. Acute pancreatitis induces intrapancreatic tumor necrosis factor gene expression. Arch Surg. 1995;130:966-970. |
| 18. | Makhija R, Kingsnorth AN. Cytokine storm in acute pancreatitis. J Hepatobiliary Pancreat Surg. 2002;9:401-410. |
| 19. | Norman JG, Franz MG, Fink GS, Messina J, Fabri PJ, Gower WR, Carey LC. Decreased mortality of severe acute pancreatitis after proximal cytokine blockade. Ann Surg. 1995;221:625-631; discussion 631-634. |
| 20. | Niederau C, Niederau M, Borchard F, Ude K, Lüthen R, Strohmeyer G, Ferrell LD, Grendell JH. Effects of antioxidants and free radical scavengers in three different models of acute pancreatitis. Pancreas. 1992;7:486-496. |
| 21. | Vaquero E, Gukovsky I, Zaninovic V, Gukovskaya AS, Pandol SJ. Localized pancreatic NF-kappaB activation and inflammatory response in taurocholate-induced pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1197-G1208. |
| 22. | Fitzpatrick LR, Wang J, Le T. Caffeic acid phenethyl ester, an inhibitor of nuclear factor-kappaB, attenuates bacterial peptidoglycan polysaccharide-induced colitis in rats. J Pharmacol Exp Ther. 2001;299:915-920. |
| 23. | de Waal Malefyt R, Haanen J, Spits H, Roncarolo MG, te Velde A, Figdor C, Johnson K, Kastelein R, Yssel H, de Vries JE. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991;174:915-924. |
| 24. | Adler G, Rohr G, Kern HF. Alteration of membrane fusion as a cause of acute pancreatitis in the rat. Dig Dis Sci. 1982;27:993-1002. |
| 25. | Rongione AJ, Kusske AM, Kwan K, Ashley SW, Reber HA, McFadden DW. Interleukin 10 reduces the severity of acute pancreatitis in rats. Gastroenterology. 1997;112:960-967. |
Peer reviewers: Parimal Chowdhury, Professor, Department of Physiology and Biophysics, College of Medicine University of Arkansas for Medical Sciences, 4301 W Markham Street Little Rock, Arkansas 72205, United States; Kostas Pantopoulos, Associate Professor, Department of Medicine, McGill University, Lady Davis Institute for Medical Research, 3755 Cote Ste-Catherine Road, Montreal, Quebec, H3T 1E2, Canada
S- Editor Tian L L- Editor Kerr C E- Editor Zheng XM