Published online Nov 7, 2009. doi: 10.3748/wjg.15.5176
Revised: September 21, 2009
Accepted: September 28, 2009
Published online: November 7, 2009
AIM: To analyze the polygraphic sleep patterns during cirrhosis progression in a rat model by repeated CCl4 administration.
METHODS: Male Wistar rats received three weekly injections of CCl4 for 11 wk, and were analyzed before and during the induction of cirrhosis. Rats were implanted with electrodes to record their sleep patterns. Polygraph recordings were made weekly over 11 wk for 8 h, during the light period. After a basal recording, rats received three weekly injections of CCl4. Histological confirmation of cirrhosis was performed after 11 wk.
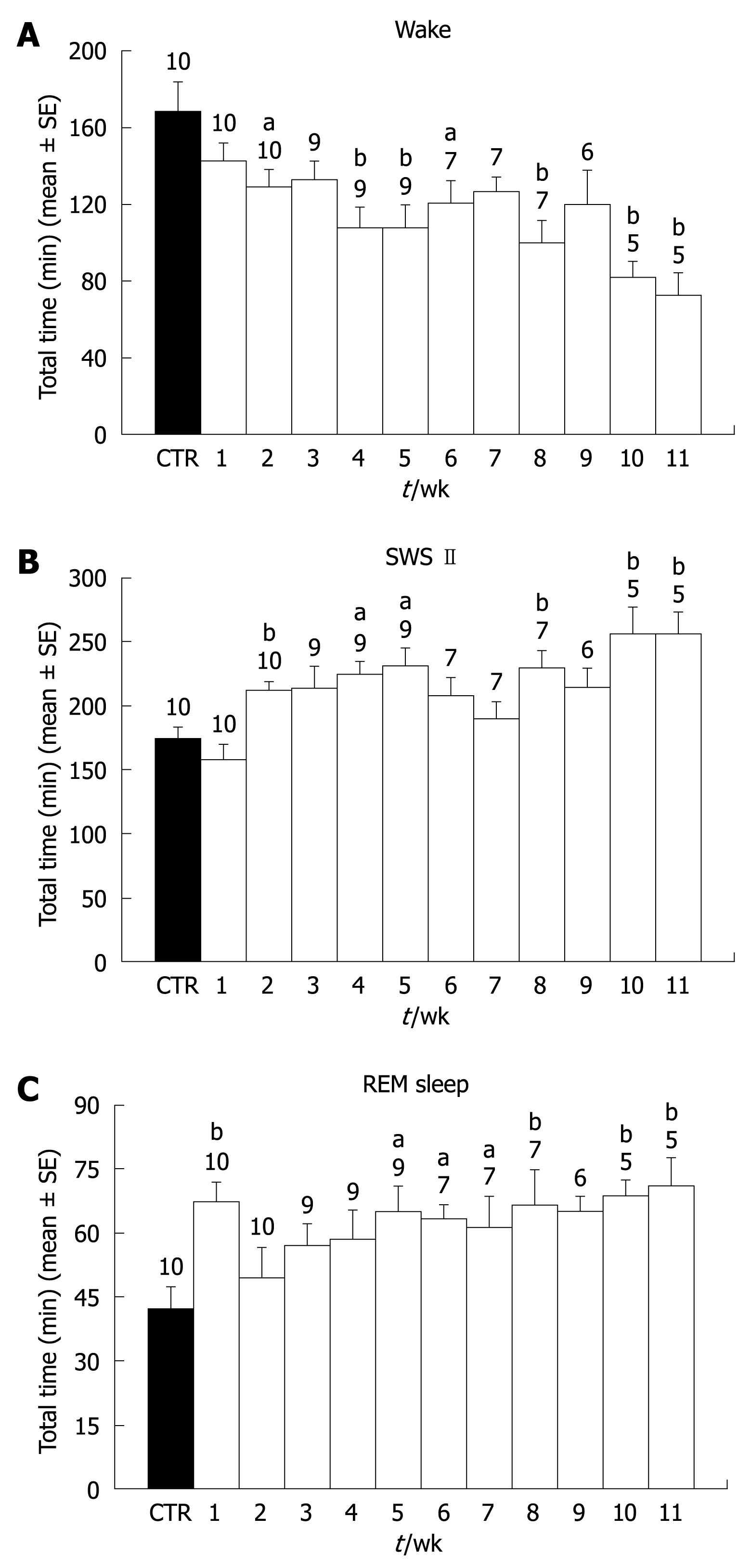
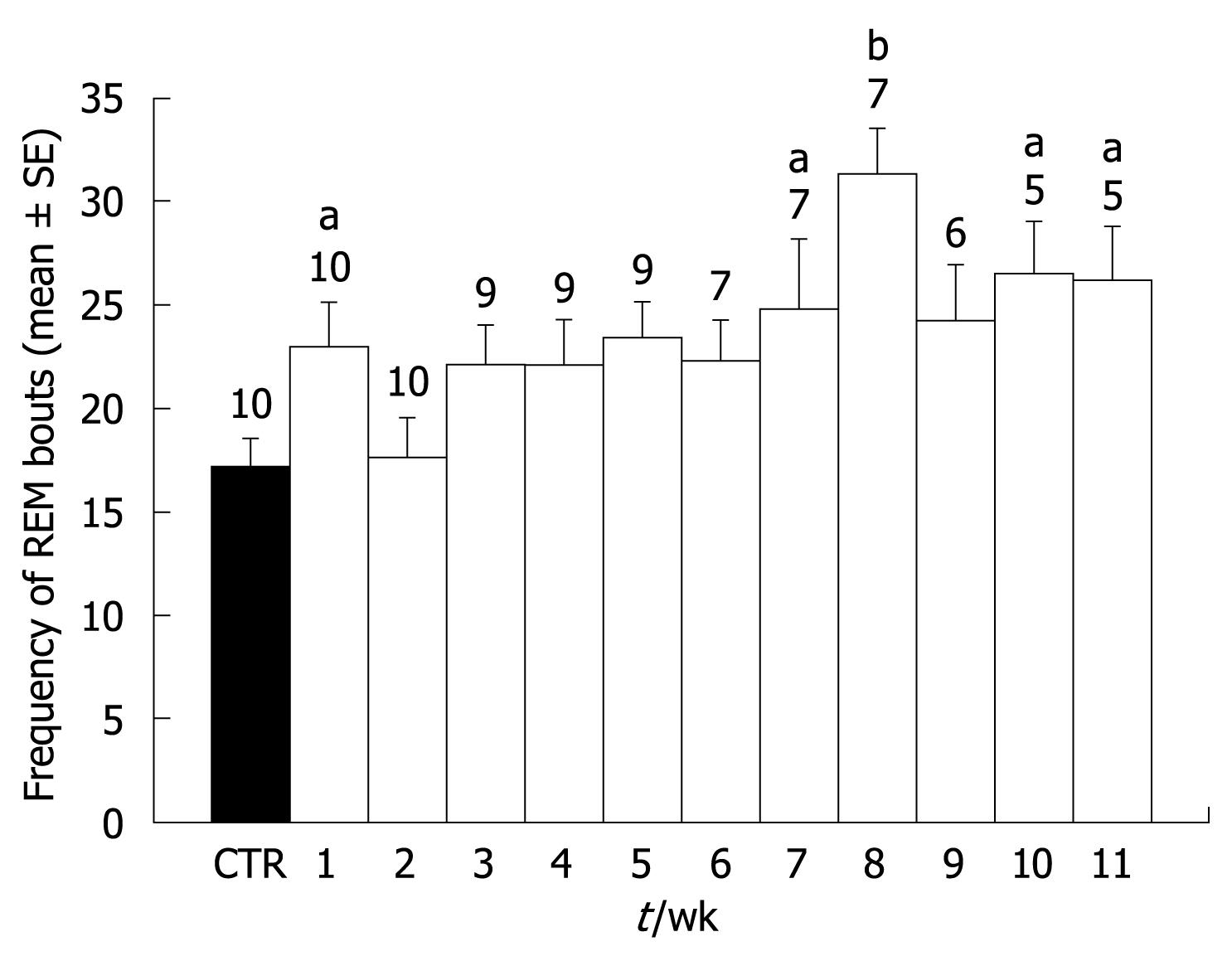
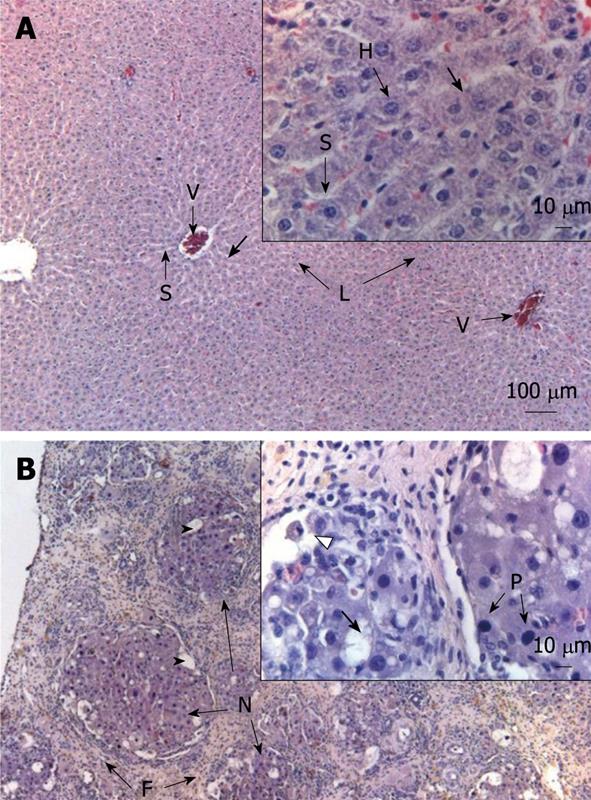
RESULTS: The results showed a progressive decrease in total wake time that reached statistical significance from the second week of treatment. In addition, there was an increase in total time of slow wave sleep (SWS) II and rapid eye movement sleep (REM sleep) in most of the 11 wk. SWS I showed no significant variations. During the final weeks, a significant increase in REM sleep frequency was also observed. Histological analyses of the livers showed unequivocal signs of cirrhosis.
CONCLUSION: These data suggest that hepatic failure produced by CCl4 administration is capable of modifying the sleep pattern even after only a few doses.
- Citation: Jiménez-Anguiano A, Díaz-Medina V, Farfán-Labonne BE, Giono-Chiang G, Kersenobich D, García-Lorenzana M, Gutiérrez-Ruiz MC, Velázquez-Moctezuma J. Modification of sleep architecture in an animal model of experimental cirrhosis. World J Gastroenterol 2009; 15(41): 5176-5180
- URL: https://www.wjgnet.com/1007-9327/full/v15/i41/5176.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.5176
| Treatment | Wake total time | SWS I total time | SWS II total time | REM sleep total time | Duration of REM sleep epochs | Frequency of REM sleep | Latency of REM sleep | Latency of SWS |
| Control (n = 10) | 169.03 ± 14.94 | 89.67 ± 9.21 | 174.82 ± 9.516 | 42.32 ± 5.47 | 2.16 ± 0.21 | 17.3 ± 1.315 | 48.84 ± 11.54 | 37.06 ± 6.78 |
| 1 wk (n = 10) | 142.93 ± 9.21 | 109.69 ± 12.89 | 158.40 ± 13.32 | 67.64 ± 4.89b | 2.95 ± 0.27 | 23 ± 2.27 | 69.7 ± 26.61 | 25.60 ± 6.05 |
| 2 wk (n = 10) | 128.85 ± 9.74b | 87.45 ± 8.45 | 213.62 ± 6.07a | 49.52 ± 7.39 | 2.56 ± 0.14 | 17.7 ± 1.93 | 80.27 ± 19.98 | 26.57 ± 5.58 |
| 3 wk (n = 9) | 133.40 ± 9.26a | 74.36 ± 14.90 | 214.38 ± 18.94a | 57.30 ± 5.44 | 2.44 ± 0.13 | 22.22 ± 1.98 | 51.45 ± 16.44 | 18.99 ± 4.43 |
| 4 wk (n = 9) | 107.77 ± 11.07b | 80.69 ± 10.27 | 225.89 ± 10.20b | 58.60 ± 7.94a | 2.44 ± 0.160 | 22.22 ± 2.17 | 63.80 ± 17.81 | 17.84 ± 3.41 |
| 5 wk (n = 9) | 107.52 ± 12.58b | 74.27 ± 13.55 | 232.54 ± 14.85b | 67.24 ± 5.45b | 2.70 ± 0.27 | 23.55 ± 1.74 | 73.67 ± 19.52 | 25.04 ± 4.98 |
| 6 wk (n = 7) | 121.19 ± 11.64b | 86.08 ± 8.86 | 208.17 ± 16.42 | 63.64 ± 3.37a | 2.72 ± 0.26 | 22.42 ± 2.01 | 79.99 ± 18.22 | 17.58 ± 2.30 |
| 7 wk (n = 7) | 126.87 ± 7.28b | 93.07 ± 10.69 | 191.5 ± 13.90 | 61.47 ± 8.03a | 2.31 ± 0.21 | 24.85 ± 3.47 | 71.49 ± 23.91 | 19.81 ± 4.54 |
| 8 wk (n = 7) | 99.64 ± 12.18b | 82.11 ± 8.89 | 230.48 ± 14.96b | 66.93 ± 9.00b | 1.96 ± 0.23 | 31.42 ± 2.17b | 75.81 ± 11.97 | 18.08 ± 4.95 |
| 9 wk (n = 6) | 120.06 ± 17.93b | 78.53 ± 7.85 | 215.29 ± 16.85a | 65.41 ± 3.90a | 2.56 ± 0.40 | 24.33 ± 2.78 | 85.26 ± 32.18 | 20.03 ± 6.91 |
| 10 wk (n = 5) | 82.03 ± 8.25b | 66.91 ± 13.22 | 256.29 ± 22.37b | 68.89 ± 3.72b | 2.60 ± 0.50 | 26.6 ± 2.56b | 60.46 ± 36.58 | 17.15 ± 7.64 |
| 11 wk (n = 5) | 72.79 ± 11.77b | 77.34 ± 17.61 | 257.84 ± 18.77b | 71.28 ± 7.67b | 2.63 ± 0.41 | 26.4 ± 2.56b | 39.64 ± 10.70 | 17.24 ± 5.22 |
The sleep-wakefulness cycle is an important circadian rhythm in mammals and is characterized by a reduction in the level of consciousness and by specific metabolic activities. Sleep is controlled by multiple areas of the brain and by several chemical factors, and can be readily modified by different activities, drugs, and pathological process, such as exercise, stress, alcoholism, depression, and metabolic diseases[1].
Cirrhosis, on the other hand, is an irreversible dysfunction of the liver characterized by damage of the parenchyma, alteration of the reticular structure and the connective tissue that sustains the lobules and sinusoids[2]. Cirrhosis can progress to hepatic encephalopathy and to coma[3], but even before these advanced stages, functional alterations of several brain nuclei have been detected during early stages by magnetic transfer ratio, a magnetic resonance imaging technique[4]. Cirrhosis could also impact pulmonary function and might be involved in the development of obstructive sleep apnea syndrome (OSAS) in patients with ascitis; however, the early stages have not been associated with OSAS[5]. In the advanced stages, cirrhotic patients show an increased frequency of moderate obstructive sleep apnea[6]. In addition, cirrhotic patients with metabolic alterations frequently show abnormalities in electroencephalographic recordings[7]. Furthermore, patients with severe cirrhosis show a significant increase in power potency associated with the theta frequency and a decrease associated with the α frequency in the electroencephalogram (EEG)[8,9]. Recently, Mostacci et al[10] reported significant sleep alterations in 178 patients with cirrhosis when compared to normal control subjects using questionnaires: the basic Nordic sleep and the Epworth Sleepiness Scale. They reported that patients with cirrhosis complained of more daytime sleepiness, because they had fragmented nocturnal sleep caused by frequent nocturnal waking and had difficulties for falling asleep.
Through questionnaires of quality of life, sleep disturbances have been recognized as one of the early signs in patients with cirrhosis and hepatic encephalopathy[11]. Sleep disturbances in cirrhosis has not been correlated with clinical parameters or with cognitive impairment. Cirrhotic subjects with unsatisfactory sleep show higher scores for depression and anxiety, raising the possibility that the effects of these chronic emotional alterations might underlie the pathogenesis of sleep disturbances. Moreover, cirrhotic patients show reduced sleep time, increased latencies to sleep and frequent wakening. These alterations are not due to previously prescribed medications, but are related to abnormalities of the circadian system[12].
From this evidence we can conclude that hepatic cirrhosis causes a series of cerebral changes, which could modify several behaviors, such as sleep. Alterations of the sleep pattern as hepatic disease develops have not been reported. In addition, chronic administration of CCl4 in rats induces reactive free radicals that attack membrane components, culminating in cell death[13] and promoting fibrosis[14]. This is a reliable procedure to induce experimental hepatic cirrhosis. The aim of this study was to analyze the sleep pattern in rats chronically treated with CCl4 as hepatic damage progresses.
Ten adult male Wistar rats (250-280 g) were used in this study. The animals were housed in a temperature-controlled room (22°C) and under a 12:12 normal light-dark cycle (light ON at 08:00 am). They were kept in individual clear polycarbonate cages with food and water available ad libitum. The experiments were performed following the guidelines of the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication No. 85-23, revised 1985).
Under deep general anesthesia induced with a cocktail (ketamine: 3.75 mg/100 g, xylazine: 0.19 mg/100 g, and acepromazine: 0.038 mg/100 g ip), rats were chronically implanted with a standard set of electrodes for sleep recording. Two stainless steel screw electrodes were implanted in the frontal and parietal cortex for the EEG and flexible wires were inserted in the neck muscles to record an electromyogram.
One week after surgery, and after 3 d of habituation to the recording conditions, rats (n = 10) polygraph recordings were made to obtain their basal sleep parameters. Thereafter, animals received an intraperitoneal (ip) injection, three times a week, containing different dilutions of CCl4 and mineral oil, always in a total volume of 0.25 mL. Treatment lasted for 11 wk under the following pattern of administration: in the first week the animals received a solution with 1 part of CCl4 and six parts of mineral oil (1:6). In the second week, the proportion of the solution changed to 1:5. In the third week the proportion of the solution was 1:4. From week four to week eleven the proportion of the solution was 1:3.
Polygraph recordings of the rats were obtained for 8 h within the light period, once every week, with the Nihon-Kohden model polygraph. Thus, sleep recordings were done before treatment and after every three injections. In addition to the polygraph recordings, rat behavior was observed during the recording period. The polygraph recordings were scored visually according to standard criteria[15]. The frequency and the duration of wakefulness, slow wave sleep (SWS) I, SWS II, and rapid eye movement (REM) sleep were quantified.
Half of the animals died during the treatment (Initial n = 10; final n = 5). After sleep recordings, animals were killed by an overdose of pentobarbital and their livers were removed for histological examination. Liver slices were stained with hematoxylin-eosin and their morphological characteristics were determined.
All data were expressed as mean ± SD and analyzed by SPSS 11.0 software. Data were analyzed using an ANOVA for repeated measurements, followed by Fisher post hoc comparisons to detect significant differences between groups. P < 0.05 was considered statistically significant.
Treatment with CCl4 induced behavioral changes in all rats, characterized mainly by a progressive decrease in locomotion. During the final 2 wk, four out of the five surviving animals showed ascitis. Five of the animals died before the end of the 11-wk period, so the size of the group decreased as the treatment progressed. Concerning sleep, CCl4 administration elicited a decrease of wake time throughout the 11 wk of treatment. The decrease was observed from the first week of treatment but only reached statistical significance from the second week. This decrease in wake time grew larger as the treatment progressed, and during weeks 10 and 11, wake time was less than 50% of pretreatment values (Figure 1A). Concerning SWS I, no significant modifications were observed. However, SWS II time showed a significant increase from the second week of treatment. The increase remained constant with only small variations during the 11 wk. Only during weeks six and seven did the increase did not reach statistical significance (Figure 1B). REM sleep showed a significant increase in the first week of treatment (Figure 1C). However, REM sleep duration returned to control levels during the second and third weeks of treatment and thereafter, there was a significant increase that lasted until week eleven. The increase of REM sleep time was due mainly to the duration of each period, because no significant increases in REM sleep frequency were observed during most of the weeks. Only during weeks eight, ten, and eleven were there significant increases in REM sleep frequency (Figure 2).
Table 1 summarizes the data obtained concerning all the parameters recorded during pretreatment and during the 11 wk of recording.
Histological examination revealed the effects of chronic CCl4 treatment on liver parenchyma. Figure 3 shows a sample of a liver treated with CCl4. A normal liver can be observed in Figure 3A. In Figure 3B, the effects of CCl4 treatment for 11 wk on liver histological features are shown. Clear indications of cirrhosis were observed (fibrosis, lipidic vacuoles, pycnotic nuclei, and necrosis).
The present results indicate that sleep-wake patterns changed as experimental cirrhosis progressed. REM sleep showed an acute response to the first administrations of CCl4. Wake and SWS II time were significantly modified after 2 wk of CCl4 treatment, and these effects showed a steady and slight increase during the following weeks. These results are consistent with previous reports showing that cirrhotic patients display a reduction in α activity and an increase in theta and delta activity[8].
Indirect tests, such as actigraphic recordings and questionnaires on sleep, have suggested that cirrhotic patients suffer from unsatisfactory sleep, mainly due to the reduction in the quality of sleep, produced by several awakenings throughout the night[12]. However, Steindl et al[16] did not find differences in polysomnographic recordings of cirrhotic patients compared to matched healthy controls. However, sleep diaries of these patients indicated more frequent nocturnal awakenings and daytime naps. Moreover, these researchers measured the levels of melatonin and found a significant increase during daytime, when melatonin is normally absent[16]. Recently, Velissaris et al[17] corroborated these results; they showed that melatonin circadian patterns were altered in cirrhosis patients without clinical encephalopathy. This disruption might reflect changes in the output of the circadian pacemaker located in the suprachiasmatic nucleus of the hypothalamus. It is possible that some of the metabolic disturbances generated by cirrhosis might also alter the function of this biological clock.
On the other hand, several diseases of sleep have been reported, such as the OSAS. OSAS is a frequent disease that has been extensively studied. In patients with advanced liver cirrhosis, OSAS has been reported to be associated with changes in autonomic nervous activities[18].
Recent molecular studies have shown an increased expression of genes associated with monoamine oxidase (MAO-A isoform) and nitric oxide synthase (nNOS isoform) in the brain of cirrhotic patients[3]. Moreover, oligodendroglial nodules have been observed in the white matter associated with CCl4-induced liver failure[19,20]. In addition, in rats submitted to portacaval anastomosis and in patients with cirrhosis, alterations of the circadian system have been noticed, especially in the rhythm of circadian locomotor activity and in the rhythm of pineal melatonin release[1,21,22]. All of these data can account for sleep effects observed in the present study.
Likewise, it has been shown that the activity of the EEG can be modified by several substances, such as ammonium, a potent neurotoxic produced by cirrhotic patients[3,11,23,24]. This increase of ammonium affects the homeostasis levels of neurotransmitters and neuropeptides in plasma and in the brain. Thus, the levels of dopamine, acetylcholine, glutamate, nitric oxide, and GABA are modified[24-26]. It is possible that, in the present study, the animals treated with CCl4 have several neurochemical alterations that could modify the levels of different neurotransmitters. These changes, in addition to the alterations in the biological clock, can produce disturbances in some nuclei involved in the regulation of sleep. Further research is needed to elucidate the precise mechanisms of the observed changes.
Sleep disturbances have been described in patients with advanced cirrhosis. However, the development and mechanisms of these alterations are not known. In this study, the sleep pattern of rats submitted to experimental cirrhosis was analyzed as liver damage progressed. The results showed an early decrease of wake time and sustained increases of slow wave sleep (SWS) and later, of REM sleep. This data suggest that sleep alterations could be the warning signs of liver disease.
This study adds information on the relationship between liver function and cerebral function. The mechanisms through which this reciprocal relationship works remain to be elucidated.
The animal model of liver cirrhosis induced by chronic administration of CCL4 is a suitable model to analyze the relationship between liver failure and brain function disturbances.
This study highlights the need for gastroenterologists to pay attention to sleep disturbances as early signs of liver failure.
SWS and rapid eye movement sleep and the two major sleep stages in most animal species.
The content of the article will be interesting not only for the gastroenterologists, but also for other specialists. Further investigations of mechanisms of sleep alterations might yield potentially efficacious approaches to its clinical management.
| 1. | Siegel J. Sleep as a circadian rhythm. The neural control of sleep & waking. New York: Springer-Verlag 2002; 93-101. |
| 3. | Butterworth RF. Complications of cirrhosis III. Hepatic encephalopathy. J Hepatol. 2000;32:171-180. |
| 4. | Iwasa M, Kinosada Y, Nakatsuka A, Watanabe S, Adachi Y. Magnetization transfer contrast of various regions of the brain in liver cirrhosis. AJNR Am J Neuroradiol. 1999;20:652-654. |
| 5. | Nikaina I, Pastaka C, Zachou K, Dalekos GN, Gourgoulianis K. Sleep apnoea syndrome and early stage cirrhosis: a pilot study. Eur J Gastroenterol Hepatol. 2006;18:31-35. |
| 6. | Tanné F, Gagnadoux F, Chazouillères O, Fleury B, Wendum D, Lasnier E, Lebeau B, Poupon R, Serfaty L. Chronic liver injury during obstructive sleep apnea. Hepatology. 2005;41:1290-1296. |
| 7. | Papenberg J, Lanzinger G, Kommerell B, Hoyer S. Comparative studies of the electroencephalogram and the cerebral oxidative metabolism in patients with liver cirrhosis. Klin Wochenschr. 1975;53:1107-1113. |
| 8. | Amodio P, Del Piccolo F, Pettenò E, Mapelli D, Angeli P, Iemmolo R, Muraca M, Musto C, Gerunda G, Rizzo C. Prevalence and prognostic value of quantified electroencephalogram (EEG) alterations in cirrhotic patients. J Hepatol. 2001;35:37-45. |
| 9. | Bahn E, Nolte W, Kurth C, Ramadori G, Rüther E, Wiltfang J. Quantification of the electroencephalographic theta/alpha ratio for the assessment of portal-systemic encephalopathy following implantation of transjugular intrahepatic portosystemic stent shunt (TIPSS). Metab Brain Dis. 2002;17:19-28. |
| 10. | Mostacci B, Ferlisi M, Baldi Antognini A, Sama C, Morelli C, Mondini S, Cirignotta F. Sleep disturbance and daytime sleepiness in patients with cirrhosis: a case control study. Neurol Sci. 2008;29:237-240. |
| 11. | Sherlock S, Summerskill WH, White LP, Phear EA. Portal-systemic encephalopathy; neurological complications of liver disease. Lancet. 1954;267:454-457. |
| 12. | Córdoba J, Cabrera J, Lataif L, Penev P, Zee P, Blei AT. High prevalence of sleep disturbance in cirrhosis. Hepatology. 1998;27:339-345. |
| 13. | Martínez M, Mourelle M, Muriel P. Protective effect of colchicine on acute liver damage induced by CCl4. Role of cytochrome P-450. J Appl Toxicol. 1995;15:49-52. |
| 14. | Desmoulière A, Xu G, Costa AM, Yousef IM, Gabbiani G, Tuchweber B. Effect of pentoxifylline on early proliferation and phenotypic modulation of fibrogenic cells in two rat models of liver fibrosis and on cultured hepatic stellate cells. J Hepatol. 1999;30:621-631. |
| 15. | Takeuchi E. [Polygraphical study on the wakefulness-sleep cycle of the rat]. Shinrigaku Kenkyu. 1970;41:248-256. |
| 16. | Steindl PE, Finn B, Bendok B, Rothke S, Zee PC, Blei AT. [Changes in the 24-hour rhythm of plasma melatonin in patients with liver cirrhosis--relation to sleep architecture]. Wien Klin Wochenschr. 1997;109:741-746. |
| 17. | Velissaris D, Karanikolas M, Kalogeropoulos A, Solomou E, Polychronopoulos P, Thomopoulos K, Labropoulou-Karatza C. Pituitary hormone circadian rhythm alterations in cirrhosis patients with subclinical hepatic encephalopathy. World J Gastroenterol. 2008;14:4190-4195. |
| 18. | Ogata T, Nomura M, Nakaya Y, Ito S. Evaluation of episodes of sleep apnea in patients with liver cirrhosis. J Med Invest. 2006;53:159-166. |
| 19. | Diemer NH. Size and density of oligodendroglial nuclei in rats with CCl4-induced liver disease. Neurobiology. 1975;5:197-206. |
| 20. | Luse SA, Wood WG. The brain in fatal carbon tetrachloride poisoning. Arch Neurol. 1967;17:304-312. |
| 21. | Steindl PE, Finn B, Bendok B, Rothke S, Zee PC, Blei AT. Disruption of the diurnal rhythm of plasma melatonin in cirrhosis. Ann Intern Med. 1995;123:274-277. |
| 22. | Zee PC, Mehta R, Turek FW, Blei AT. Portacaval anastomosis disrupts circadian locomotor activity and pineal melatonin rhythms in rats. Brain Res. 1991;560:17-22. |
| 23. | Del Piccolo F, Sacerdoti D, Amodio P, Bombonato G, Bolognesi M, Mapelli D, Gatta A. Central nervous system alterations in liver cirrhosis: the role of portal-systemic shunt and portal hypoperfusion. Metab Brain Dis. 2003;18:51-62. |
| 24. | Hazell AS, Butterworth RF. Hepatic encephalopathy: An update of pathophysiologic mechanisms. Proc Soc Exp Biol Med. 1999;222:99-112. |
| 25. | Bergeron M, Reader TA, Layrargues GP, Butterworth RF. Monoamines and metabolites in autopsied brain tissue from cirrhotic patients with hepatic encephalopathy. Neurochem Res. 1989;14:853-859. |
| 26. | Rose C, Felipo V. Limited capacity for ammonia removal by brain in chronic liver failure: potential role of nitric oxide. Metab Brain Dis. 2005;20:275-283. |
Peer reviewers: Vasiliy I Reshetnyak, MD, PhD, Professor, Scientist Secretary of the Scientific Research Institute of General Reanimatology, 25-2, Petrovka str., 107031, Moscow, Russia; Radha K Dhiman, Associate Professor, Department of Hepatology, Postgraduate Institute of Medical Education and Research, Chandigarh 160012, India
S- Editor Tian L L- Editor Stewart GJ E- Editor Zheng XM