Published online Oct 28, 2009. doi: 10.3748/wjg.15.5074
Revised: September 25, 2009
Accepted: October 2, 2009
Published online: October 28, 2009
AIM: To evaluate serum levels of N-terminal pro-brain natriuretic peptide (NTproBNP) and tumor necrosis factor α (TNF-α) in a large series of patients with hepatitis C associated with mixed cryoglobulinemia (MC+HCV).
METHODS: Serum NTproBNP and TNF-α levels were assayed in 50 patients with MC+HCV, and in 50 sex- and age-matched controls.
RESULTS: Cryoglobulinemic patients showed significantly higher mean NTproBNP and TNF-α levels than controls (P < 0.001; Mann-Whitney U test). By defining high NTproBNP level as a value higher than 125 pg/mL (the single cut-off point for outpatients under 75 years of age), 30% of MC+HCV and 6% of controls had high NTproBNP (χ2, P < 0.01). With a cut-off point of 300 pg/mL (used to rule out heart failure (HF) in patients under 75 years of age), 8% of MC+HCV and 0 controls had high NTproBNP (χ2, P < 0.04). With a cut-off point of 900 pg/mL (used for ruling in HF in patients aged 50-75 years; such as the patients of our study), 6% of MC+HCV and 0 controls had high NTproBNP (χ2, P = 0.08).
CONCLUSION: The study demonstrates high levels of circulating NTproBNP and TNF-α in MC+HCV patients. The increase of NTproBNP may indicate the presence of a subclinical cardiac dysfunction.
- Citation: Antonelli A, Ferri C, Ferrari SM, Galetta F, Franzoni F, Santoro G, Marco SD, Ghiri E, Fallahi P. High circulating N-terminal pro-brain natriuretic peptide and tumor necrosis factor-α in mixed cryoglobulinemia. World J Gastroenterol 2009; 15(40): 5074-5079
- URL: https://www.wjgnet.com/1007-9327/full/v15/i40/5074.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.5074
| Age (yr) | 57 ± 9 |
| Male/female | 13/37 |
| Disease duration with MC (yr) | 10 ± 11 |
| Purpura | 82% |
| Active vasculitis | 31% |
| Weakness | 91% |
| Arthralgias | 83% |
| Arthritis | 14% |
| Raynaud’s phenomenon | 51% |
| Sjogren’s syndrome | 45% |
| Peripheral neuropathy | 59% |
| Aminotransferase elevation and/or histologic activity1 | 71% |
| Cryocrit (%) | 4.2 ± 8.9 |
| CH50 (normal: 160-220 U) | 111 ± 36 |
| C3 (normal: 60-130 mg/dL) | 81 ± 36 |
| C4 (normal: 20-55 mg/dL) | 14 ± 18 |
| Autoantibodies2 | 25% |
The most common clinical features of hepatitis C associated with mixed cryoglobulinemia (MC+HCV) are correlated with vasculitis in the various organs and sometimes with increased viscosity of the plasma[1,2]. Signs and symptoms include purpura, ulcers of the extremities, arthralgia, proteinuria, hepatic damage, abdominal pain, mental confusion, oligo-anuria, hemorrhagic diathesis, and sometimes congestive heart failure (HF)[1-4]. Furthermore, HF has been described as heralding the clinical onset of essential mixed cryoglobulinemia (MC)[5]. Moreover, many MC+HCV patients experience symptoms such as fatigue, dyspnea and reduced physical activity. However, in many patients, these symptoms are not proportional to the liver involvement and resemble symptoms of HF.
Several studies have shown that plasma levels of brain natriuretic peptide (BNP) and N-terminal proBNP (NTproBNP) are reliable diagnostic and prognostic markers for cardiac disease[6,7] that correlate with symptoms of HF and the severity of systolic and diastolic dysfunction[8]. Some authors have recently stated that NTproBNP appears superior to BNP for the evaluation of suspected acute HF in patients with preserved left ventricular ejection fraction[9,10]. In the study by O’Donoghue et al[9], NTproBNP seems to correlate with HF severity better than BNP and is more sensitive.
Cytokines play an important role in chronic HF, and it has been shown that TNF-α and NTproBNP are independent predictors of long-term risk of death[11,12] for HF. Circulating TNF-α has been recently shown to be high in patients with MC[13].
However, to our knowledge, until now no study has evaluated circulating NTproBNP together with TNF-α levels, as possible markers of HF, in MC+HCV patients affected by cryoglobulinemic vasculitis.
The aim of this study was to evaluate serum levels of NTproBNP in a series of MC+HCV patients, and to correlate this parameter with the clinical features of the disease, and with the circulating levels of TNF-α.
Fifty MC+HCV patients (37 females and 13 males; mean age 57 ± 9 years; mean disease duration 10 ± 11 years), consecutively referred to our Rheumatology Unit, were recruited for the study between 2001 and 2006. The diagnosis of MC+HCV was based on the presence of serum mixed (IgG-IgM) cryoglobulins and the classical clinical triad, purpura, weakness, arthralgias, and on the exclusion of other well-known systemic disorders, such as immuno-rheumatic and neoplastic diseases[1,2,14-16].
The study included only patients with MC+HCV, without liver cirrhosis or hepatocellular carcinoma (assessed by histology, laboratory evidence of liver failure and/or ultrasound-proven portal hypertension)[17,18]. None of the patients had evident signs of HF, organic renal disease (patients with serum creatinine > 1.2 mg/dL and/or proteinuria > 0.5 g/24 h were excluded), thyroid disease, diabetes, cancer or any other major diseases. All patients had normal cardiac physical examinations and normal blood pressure.
Thirty eight out of 55 (76%) MC+HCV patients underwent liver biopsy for diagnostic purposes; liver histology activity index (grade) or stage of liver fibrosis were evaluated according to Ishak et al[19]. The mean activity index (grade) in MC+HCV patients was 5.0 ± 1.2, and the mean stage was 2.0 ± 0.9. Main demographic and clinico-serological features of MC+HCV patients are reported in Table 1.
Among the patients, 17 had been previously treated with interferon-alpha (IFN-α) for an average of 7 mo (range, 1-13 mo), at a mean dosage of 10.4 MU/week; the time elapsed from the last course of IFN-α treatment ranged from 6 to 69 mo (mean 40 ± 22 mo). No statistically significant differences were observed in the main demographic and clinico-serological features of MC+HCV patients treated or untreated with IFN-α.
At the time of study, 35 MC+HCV patients were taking low doses of corticosteroids, 8 had previously been on corticosteroids and 7 had never been treated with corticosteroids. No MC+HCV patient had had plasma exchange treatment in the last year before the study. In both patients and controls, a careful medical history was collected, in particular with regard to family history of thyroid disease, smoking habits, and drugs. The presence of Raynaud’s phenomenon, Sjogren’s syndrome, skin ulcers, peripheral neuropathy, and renal and liver involvement in MC+HCV patients was evaluated as previously described[16]. Routine blood chemistry was carried out by standard methods.
Each of the 50 MC+HCV patients eligible for the study was matched, by sex and age, one-to-one with a control group of healthy subjects of the general population from the same geographic area (North-West Tuscany). This control group was extracted from a larger sample of 1640 subjects taking part in a population-based survey of thyroid disorders; only HCV-negative subjects, without clinical and laboratory evidence of thyroid and liver disorders or autoimmune diseases and not treated with immunomodulators were included.
None of the controls had signs of HF, organic renal disease, thyroid disease, diabetes, cancer or any other major diseases. All patients had normal cardiac physical examination results and normal blood pressure.
Extraction of the control group from the original population was performed by finding the closest age match (± 2 years) to each case within either gender. When more than one age-match was available per case, the choice was made at random.
The study protocol was approved by the local Ethics Committee. All subjects gave their informed consent to enter the study.
Cryocrit was measured as the percentage of packed cryoglobulins after cold centrifugation of the serum; cryoglobulin composition was determined by the incorporation in cryoprecipitates of monoclonal or polyclonal IgM-rheumatoid factor (i.e. MC type II or MC type III); hemolytic complement C3-C4 fractions were measured as previously described[16], anti-nuclear, anti-smooth muscle, and anti-mitochondrial autoantibodies were detected by current techniques[16]. Sera with a titre > 1:40 were considered positive. Anti-extractable nuclear antigen antibodies, including anti-Scl70, -Sm, -RNP, -SSA/SSB, -PCNA, -SL and -Jo1 specificities, were detected by counter-immunoelectrophoresis according to the methods described by Bunn et al[20].
Antibodies against HCV (anti-HCV) and HCV-RNA were determined in serum clotted and centrifuged at 37°C and stored at -70°C. Anti-HCV and HCV-RNA levels [assayed by polymerase chain reaction (PCR) technique] in the serum were investigated as previously described[21,22].
Blood samples for analysis of plasma NTproBNP were collected, centrifuged and plasma was stored at -80°C until analysis. Plasma concentrations of NTproBNP were measured by a sandwich immunoassay on an Elecsys 2010 (Roche Diagnostics, Mannheim, Germany).
Serum TNF-α concentrations were measured using commercially available kits (R&D Systems, Minneapolis, MN). The mean minimum detectable dose was 0.12 pg/mL for TNF-α; the intra- and inter-assay coefficients of variation were 5.8% and 10.2%. Samples were assayed in duplicate. Quality control pools of low, normal, or high concentration for all parameters were included in each assay. Alanine aminotransferase (ALT) was assayed by conventional methods[18].
Values are given as mean ± SD for normally distributed variables, or as median ± IQR for not normally distributed variables (NTproBNP, TNF-α). Group values were compared by univariate ANOVA, for normally distributed variables; or by Kruskal-Wallis (≥ 3 groups) or Mann-Whitney U (2 groups) tests. Proportions were compared by the chi-square test. Post-hoc comparisons on normally distributed variables were carried out using the Bonferroni-Dunn test. Univariate analysis was performed by simple regression. A multivariate logistic regression analysis considering age, gender, ALT, and presence or absence of active vasculitis as independent variables and presence or absence of high levels of NTproBNP or TNF-α as dependent variables was performed in MC+HCV patients.
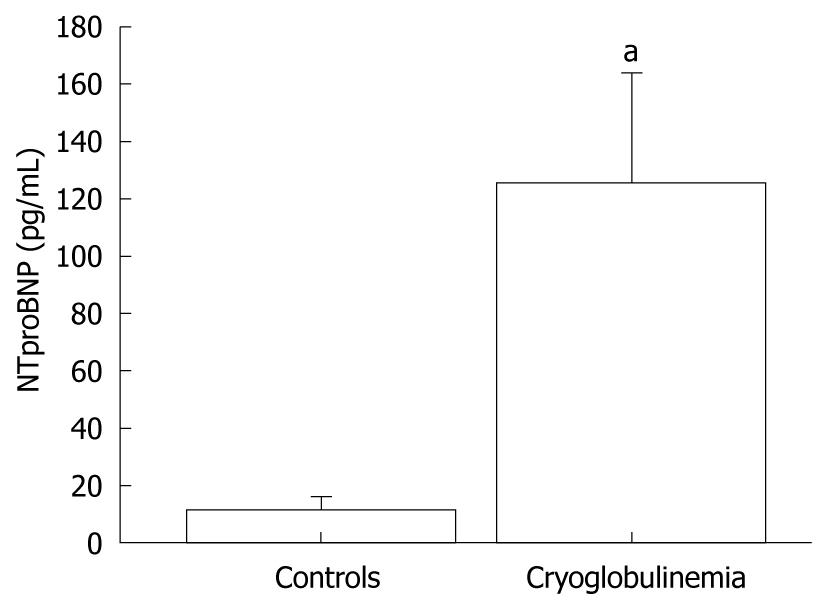
Plasma NTproBNP concentrations were significantly (P < 0.001; Mann-Whitney U test) higher in MC+HCV patients (mean 123 ± 112 pg/mL; median 36 pg/mL, range 8-1547 pg/mL) than in controls (mean 11 ± 12 pg/mL; median 3.1 pg/mL range 2-145 pg/mL) (Figure 1).
By defining high NTproBNP level as a value higher than 125 pg/mL (the single cut-off point for outpatients under 75 years of age[23]) 15/35 MC+HCV and 3/47 controls had high NTproBNP (χ2, P < 0.002).
With a cut-off point of 300 pg/mL (used to rule out HF in patients under 75 years of age[23]) 4/46 MC+HCV and 0/50 controls had high NTproBNP (χ2, P < 0.041).
With a cut-off point of 900 pg/mL (used for ruling in HF in patients with age 50-75; such as the patients of our study[23]) 3/47 MC+HCV and 0/50 controls had high NTproBNP (χ2, P = 0.08).
In order to better define the role of increased serum NTproBNP in MC+HCV, mean levels of this chemokine were separately evaluated (by Mann Whitney U test) among MC+HCV patient subgroups defined according to main demographic and clinical features (age > 55 years; gender; disease duration > 10 years; presence or absence of purpura, active vasculitis, weakness, arthralgias, arthritis, Raynaud’s phenomenon, Sjogren’s syndrome, peripheral neuropathy, aminotransferase elevation and/or histologic activity in the liver), but no significance was found. No significant correlations were observed between NTproBNP levels and serological findings of MC+HCV (levels of cryocrit and complement, presence/absence of autoantibodies) or previous/ongoing treatments.
Serum TNF-α was detectable in 85% of controls and in all MC+HCV patients; mean levels were significantly (P < 0.01; Mann Whitney U test) higher in MC+HCV patients (mean 26 ± 65 pg/mL; median 8.3 pg/mL, range 1.3-269 pg/mL) than in controls (mean 1.6 ± 1.1 pg/mL; median 1.2 pg/mL, range 0.6-3.4 pg/mL). No correlation was found between serum TNF-α and any of the following; ALT level, liver histology activity index, stage of liver fibrosis, the presence of active vasculitis, or the other demographic, serological and clinical features of MC.
MC+HCV patients had, obviously, raised ALT enzymes (P < 0.0001), in comparison with controls. No association was observed between NTproBNP or TNF-α levels and ALT levels in the MC+HCV patients.
Our study demonstrates the presence of significantly high serum levels of NTproBNP in patients with MC+HCV compared to healthy controls. Furthermore, our study confirms significantly high serum levels of TNF-α in patients with MC+HCV compared to healthy controls.
Some authors have recently stated that NTproBNP appears superior to BNP for the evaluation of suspected acute HF in patients with preserved left ventricular ejection fraction[9,10]. Furthermore[9], NTproBNP seems to correlate with HF severity better than BNP and appears more sensitive. The International Collaborative for NTproBNP Study (ICON) helped in defining appropriate cut-off points for NTproBNP in the emergency department[23]: 300 pg/mL should be used to rule out HF, while 450 pg/mL, 900 pg/mL, or 1800 pg/mL, depending on age (< 50, 50-75, or > 75 years; respectively), should be applied for ruling in HF. The age stratifications do offer significant positive predictive value. For outpatient evaluation the manufacturer suggested a single cut-off point of 125 pg/mL for patients under 75 years of age and 450 pg/mL for patients above 75 years of age[23].
The levels of NTproBNP found in MC+HCV are included in a gray zone (125-900 pg/mL) that is not necessarily associated with HF, however in 3/50 (6%) of MC+HCV patients the NTproBNP levels were higher than 900 pg/mL, which is the cut-off value for ruling in HF for patients of age 50-75 years, such as the patients of our study. Since NTproBNP level seems to correlate with HF severity, the values in the gray zone may be suggestive of a subclinical cardiac impairment. The exclusion of patients with renal failure from the study suggests that the NTproBNP increase is not related to any kidney involvement in the MC+HCV patients.
In a previous work, Matsumori et al[24] showed there was a significantly higher NTproBNP level in HCV patients and observed that the presence of anti-HCV antibodies in sera was more prevalent in patients with myocarditis and HF than in the general population. Future studies comparing NTproBNP levels between HCV and MC+HCV patients will be needed to evaluate the specific influence of the cryoglobulinemic vasculitis.
The findings of the present study may have important implications for patients with MC+HCV. Most patients complain about fatigue, dyspnea and reduced physical capacity. The pathogenesis of these symptoms is not well understood and sometimes attributed to the liver injury. However, it seems possible that these patients experience cardiac impairment which could at least contribute to these symptoms. Furthermore, among signs and symptoms of MC+HCV, sometimes congestive HF may be found, which in some cases has been described as heralding the clinical onset of essential MC[3-5].
Testing of NTproBNP level may serve as a screening marker for cardiac insufficiency in the differential diagnosis of fatigue and dyspnea and may aid the decision for further diagnostic testing of cardiac function as has been described for other groups of patients[25-28]. Besides diagnostic consequences, evaluation of NTproBNP may have therapeutic consequences for patients with MC+HCV. In patients with known congestive HF, elevated plasma BNP concentrations could be reduced by treatment with ACE inhibitors[29] or angiotensin II receptor antagonists[30] as well as treatment with diuretics and vasodilators[31]. As a consequence, plasma NTproBNP concentration may guide the intensity of pharmacotherapy as some interventional studies have suggested[32,33].
Cytokines play an important role in chronic HF, and it has been shown that TNF-α and NTproBNP are independent predictors of long-term risk of death[11,12] for patients with HF.
Our study confirms a high serum TNF-α level in HCV+ve patients, as previously demonstrated in other studies of HCV+ve patients[34-36]. The increase of TNF-α in MC+HCV patients is unlikely to be due to a more aggressive liver disease; in fact, no correlation was found between TNF-α levels and ALT levels, or with degree of liver inflammation in our study[13]. Other studies have shown an increased production of TNF-α by lymphocytes in MC+HCV patients[37,38], suggesting that the increase of TNF-α may be due to activation of lymphoid cells.
The fact that TNF-α and NTproBNP are independent predictors of long-term risk[11] is in agreement with the results of our study; in fact, no relationship has been observed between TNF-α and NTproBNP.
It has been shown that combining measurements of pro-inflammatory cytokine TNF-α and NTproBNP seems a promising tool in the prognostic assessment of HF patients[12]. However, even if we have shown that NTproBNP and TNF-α are both high in the circulation of MC+HCV patients, we cannot exclude that different pathogenetic pathways may be differentially implicated in the increase of each of these factors.
The interest in finding reliable markers of cardiac dysfunction is intensified by studies that strongly suggest an association between HCV chronic infection and atherosclerotic disease in the carotid or coronary artery[39-41].
In conclusion, our study shows elevated levels of NTproBNP in patients with MC+HCV, in association with TNF-α. This may indicate the presence of cardiac dysfunction and explain, at least in part, some of the clinical symptoms of patients with MC+HCV. Further larger, possibly multicenter prospective studies quantifying symptoms and correlating these with echocardiographic parameters are needed to confirm this association.
The most common clinical features of hepatitis C associated with mixed cryoglobulinemia (MC+HCV) are correlated with vasculitis in various organs and heart failure (HF) has been described as heralding the clinical onset of essential mixed cryoglobulinemia (MC). To our knowledge, until now no study has evaluated circulating NTproBNP together with TNF-α levels as possible markers of HF, in MC+HCV patients affected by cryoglobulinemic vasculitis.
This study demonstrates elevated serum levels of NTproBNP in patients with MC+HCV, in association with high TNF-α levels. This may indicate the presence of cardiac dysfunction and explain, at least in part, some of the clinical symptoms of patients with MC+HCV who have no evident signs of HF. Further larger, possibly multicenter prospective studies quantifying symptoms and correlating these with echocardiographic parameters are needed to confirm this association.
NTproBNP evaluation may serve as a screening marker for cardiac insufficiency in the differential diagnosis of fatigue and dyspnea, may aid the decision for further diagnostic testing of cardiac function and may have therapeutic and diagnostic consequences for patients with MC+HCV. In patients with known congestive HF, elevated plasma BNP concentrations could be reduced by treatment with ACE inhibitors, angiotensin II receptor antagonists and treatment with diuretics and vasodilators. As a consequence, plasma NTproBNP concentration may guide the intensity of pharmacotherapy as some interventional studies have suggested.
Plasma levels of BNP and NTproBNP are reliable diagnostic and prognostic markers for cardiac disease which correlate with symptoms of HF and the severity of systolic and diastolic dysfunction. TNF-α is a cytokine involved in systemic inflammation and is a member of a group of cytokines that stimulate the acute phase reaction. Circulating TNF-α has been recently shown to be high in patients with MC.
The study by authors has evaluated the circulating levels of NTproBNP and TNF-α in patients with HCV-related cryoglobulinemia. They found higher levels of both proteins in these patients as compared with normal controls and conclude that they may have a potential role in cardiac dysfunction of cryoglobulinemic patients. Though crude, these data are interesting.
| 1. | Ferri C, La Civita L, Longombardo G, Greco F, Bombardieri S. Hepatitis C virus and mixed cryoglobulinaemia. Eur J Clin Invest. 1993;23:399-405. |
| 2. | Ferri C, Mascia MT. Cryoglobulinemic vasculitis. Curr Opin Rheumatol. 2006;18:54-63. |
| 3. | Dammacco F, Scarpioni L, Antonaci S, Bonomo L. Cryoimmunoglobulinemia in four sisters. Acta Haematol. 1978;59:215-222. |
| 4. | Dammacco F, Miglietta A, Lobreglio G, Bonomo L. Cryoglobulins and pyroglobulins: an overview. Ric Clin Lab. 1986;16:247-267. |
| 5. | Bragagni G, Baldini A, Bianconcini M. [Heart failure as clinical onset of essential mixed cryoglobulinemia]. Minerva Med. 1998;89:283-286. |
| 6. | Doust JA, Glasziou PP, Pietrzak E, Dobson AJ. A systematic review of the diagnostic accuracy of natriuretic peptides for heart failure. Arch Intern Med. 2004;164:1978-1984. |
| 7. | Clerico A, Emdin M. Diagnostic accuracy and prognostic relevance of the measurement of cardiac natriuretic peptides: a review. Clin Chem. 2004;50:33-50. |
| 8. | Koglin J, Pehlivanli S, Schwaiblmair M, Vogeser M, Cremer P, vonScheidt W. Role of brain natriuretic peptide in risk stratification of patients with congestive heart failure. J Am Coll Cardiol. 2001;38:1934-1941. |
| 9. | O'Donoghue M, Chen A, Baggish AL, Anwaruddin S, Krauser DG, Tung R, Januzzi JL. The effects of ejection fraction on N-terminal ProBNP and BNP levels in patients with acute CHF: analysis from the ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) study. J Card Fail. 2005;11:S9-S14. |
| 10. | Jefic D, Lee JW, Jefic D, Savoy-Moore RT, Rosman HS. Utility of B-type natriuretic peptide and N-terminal pro B-type natriuretic peptide in evaluation of respiratory failure in critically ill patients. Chest. 2005;128:288-295. |
| 11. | Gardner RS, Chong V, Morton I, McDonagh TA. N-terminal brain natriuretic peptide is a more powerful predictor of mortality than endothelin-1, adrenomedullin and tumour necrosis factor-alpha in patients referred for consideration of cardiac transplantation. Eur J Heart Fail. 2005;7:253-260. |
| 12. | Miettinen KH, Lassus J, Harjola VP, Siirilä-Waris K, Melin J, Punnonen KR, Nieminen MS, Laakso M, Peuhkurinen KJ. Prognostic role of pro- and anti-inflammatory cytokines and their polymorphisms in acute decompensated heart failure. Eur J Heart Fail. 2008;10:396-403. |
| 13. | Antonelli A, Ferri C, Fallahi P, Ferrari SM, Sebastiani M, Ferrari D, Giunti M, Frascerra S, Tolari S, Franzoni F. High values of CXCL10 serum levels in mixed cryoglobulinemia associated with hepatitis C infection. Am J Gastroenterol. 2008;103:2488-2494. |
| 14. | Abel G, Zhang QX, Agnello V. Hepatitis C virus infection in type II mixed cryoglobulinemia. Arthritis Rheum. 1993;36:1341-1349. |
| 15. | Gorevic PD, Kassab HJ, Levo Y, Kohn R, Meltzer M, Prose P, Franklin EC. Mixed cryoglobulinemia: clinical aspects and long-term follow-up of 40 patients. Am J Med. 1980;69:287-308. |
| 16. | Ferri C, Sebastiani M, Giuggioli D, Cazzato M, Longombardo G, Antonelli A, Puccini R, Michelassi C, Zignego AL. Mixed cryoglobulinemia: demographic, clinical, and serologic features and survival in 231 patients. Semin Arthritis Rheum. 2004;33:355-374. |
| 17. | Mangia A, Schiavone G, Lezzi G, Marmo R, Bruno F, Villani MR, Cascavilla I, Fantasia L, Andriulli A. HCV and diabetes mellitus: evidence for a negative association. Am J Gastroenterol. 1998;93:2363-2367. |
| 18. | Antonelli A, Ferri C, Pampana A, Fallahi P, Nesti C, Pasquini M, Marchi S, Ferrannini E. Thyroid disorders in chronic hepatitis C. Am J Med. 2004;117:10-13. |
| 19. | Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696-699. |
| 20. | Bunn CC, Gharavi AE, Hughes GR. Antibodies to extractable nuclear antigens in 173 patients with DNA-binding positive SLE: an association between antibodies to ribonucleoprotein and Sm antigens observed by counterimmunoelectrophoresis. J Clin Lab Immunol. 1982;8:13-17. |
| 21. | Ferri C, Monti M, La Civita L, Longombardo G, Greco F, Pasero G, Gentilini P, Bombardieri S, Zignego AL. Infection of peripheral blood mononuclear cells by hepatitis C virus in mixed cryoglobulinemia. Blood. 1993;82:3701-3704. |
| 22. | Zignego AL, Deny P, Feray C, Ponzetto A, Gentilini P, Tiollais P, Bréchot C. Amplification of hepatitis delta virus RNA sequences by polymerase chain reaction: a tool for viral detection and cloning. Mol Cell Probes. 1990;4:43-51. |
| 23. | Januzzi JL Jr, Camargo CA, Anwaruddin S, Baggish AL, Chen AA, Krauser DG, Tung R, Cameron R, Nagurney JT, Chae CU. The N-terminal Pro-BNP investigation of dyspnea in the emergency department (PRIDE) study. Am J Cardiol. 2005;95:948-954. |
| 24. | Matsumori A, Shimada T, Chapman NM, Tracy SM, Mason JW. Myocarditis and heart failure associated with hepatitis C virus infection. J Card Fail. 2006;12:293-298. |
| 25. | Cabanes L, Richaud-Thiriez B, Fulla Y, Heloire F, Vuillemard C, Weber S, Dusser D. Brain natriuretic peptide blood levels in the differential diagnosis of dyspnea. Chest. 2001;120:2047-2050. |
| 26. | Morrison LK, Harrison A, Krishnaswamy P, Kazanegra R, Clopton P, Maisel A. Utility of a rapid B-natriuretic peptide assay in differentiating congestive heart failure from lung disease in patients presenting with dyspnea. J Am Coll Cardiol. 2002;39:202-209. |
| 27. | Nakamura M, Endo H, Nasu M, Arakawa N, Segawa T, Hiramori K. Value of plasma B type natriuretic peptide measurement for heart disease screening in a Japanese population. Heart. 2002;87:131-135. |
| 28. | Remme WJ, Swedberg K. Guidelines for the diagnosis and treatment of chronic heart failure. Eur Heart J. 2001;22:1527-1560. |
| 29. | Motwani JG, McAlpine H, Kennedy N, Struthers AD. Plasma brain natriuretic peptide as an indicator for angiotensin-converting-enzyme inhibition after myocardial infarction. Lancet. 1993;341:1109-1113. |
| 30. | Latini R, Masson S, Anand I, Judd D, Maggioni AP, Chiang YT, Bevilacqua M, Salio M, Cardano P, Dunselman PH. Effects of valsartan on circulating brain natriuretic peptide and norepinephrine in symptomatic chronic heart failure: the Valsartan Heart Failure Trial (Val-HeFT). Circulation. 2002;106:2454-2458. |
| 31. | Johnson W, Omland T, Hall C, Lucas C, Myking OL, Collins C, Pfeffer M, Rouleau JL, Stevenson LW. Neurohormonal activation rapidly decreases after intravenous therapy with diuretics and vasodilators for class IV heart failure. J Am Coll Cardiol. 2002;39:1623-1629. |
| 32. | Murdoch DR, McDonagh TA, Byrne J, Blue L, Farmer R, Morton JJ, Dargie HJ. Titration of vasodilator therapy in chronic heart failure according to plasma brain natriuretic peptide concentration: randomized comparison of the hemodynamic and neuroendocrine effects of tailored versus empirical therapy. Am Heart J. 1999;138:1126-1132. |
| 33. | Troughton RW, Frampton CM, Yandle TG, Espiner EA, Nicholls MG, Richards AM. Treatment of heart failure guided by plasma aminoterminal brain natriuretic peptide (N-BNP) concentrations. Lancet. 2000;355:1126-1130. |
| 34. | Lecube A, Hernández C, Genescà J, Simó R. Proinflammatory cytokines, insulin resistance, and insulin secretion in chronic hepatitis C patients: A case-control study. Diabetes Care. 2006;29:1096-1101. |
| 35. | Riordan SM, Skinner NA, Kurtovic J, Locarnini S, McIver CJ, Williams R, Visvanathan K. Toll-like receptor expression in chronic hepatitis C: correlation with pro-inflammatory cytokine levels and liver injury. Inflamm Res. 2006;55:279-285. |
| 36. | Neuman MG, Benhamou JP, Marcellin P, Valla D, Malkiewicz IM, Katz GG, Trepo C, Bourliere M, Cameron RG, Cohen L. Cytokine--chemokine and apoptotic signatures in patients with hepatitis C. Transl Res. 2007;149:126-136. |
| 37. | Loffreda S, Muratori P, Muratori L, Mele L, Bianchi FB, Lenzi M. Enhanced monocyte Th1 cytokine production in HCV-infected cryoglobulinemic patients. J Hepatol. 2003;38:230-236. |
| 38. | Saadoun D, Boyer O, Trébeden-Nègre H, Limal N, Bon-Durand V, Andreu M, Klatzmann D, Piette JC, Cacoub P. Predominance of type 1 (Th1) cytokine production in the liver of patients with HCV-associated mixed cryoglobulinemia vasculitis. J Hepatol. 2004;41:1031-1037. |
| 39. | Ishizaka Y, Ishizaka N, Takahashi E, Unuma T, Tooda E, Hashimoto H, Nagai R, Yamakado M. Association between hepatitis C virus core protein and carotid atherosclerosis. Circ J. 2003;67:26-30. |
| 40. | Boddi M, Abbate R, Chellini B, Giusti B, Solazzo V, Soft F, Pratesi G, Pratesi C, Gensini G, Zignego AL. HCV infection facilitates asymptomatic carotid atherosclerosis: preliminary report of HCV RNA localization in human carotid plaques. Dig Liver Dis. 2007;39 Suppl 1:S55-S60. |
Peer reviewer: Domenico Sansonno, Professor, Department of Internal Medicine and Clinical Oncology, University of Bari Medical School, Policlinico, Piazza Giulio Cesare 11, 70124 Bari, Italy
S- Editor Tian L L- Editor Logan S E- Editor Yin DH