Published online Oct 28, 2009. doi: 10.3748/wjg.15.4993
Revised: September 5, 2009
Accepted: September 12, 2009
Published online: October 28, 2009
Several studies have demonstrated that the outcome of chronic hepatitis C (CHC) infection is profoundly influenced by a variety of comorbidities. Many of these comorbidities have a significant influence on the response to antiviral therapy. These comorbidities negatively affect the course and outcome of liver disease, often reducing the chance of achieving a sustained virological response with PEGylated interferon and ribavirin treatments. Comorbidities affecting response to antiviral therapy reduce compliance and adherence to inadequate doses of therapy. The most important comorbidities affecting the course of CHC include hepatitis B virus coinfection, metabolic syndrome, and intestinal bacterial overgrowth. Comorbidities affecting the course and response to therapy include schistosomiasis, iron overload, alcohol abuse, and excessive smoking. Comorbidities affecting response to antiviral therapy include depression, anemia, cardiovascular disease, and renal failure.
- Citation: El-Zayadi AR. Hepatitis C comorbidities affecting the course and response to therapy. World J Gastroenterol 2009; 15(40): 4993-4999
- URL: https://www.wjgnet.com/1007-9327/full/v15/i40/4993.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.4993
The prevalence of hepatitis C virus (HCV) infection varies throughout the world, with the highest number of infections reported in Egypt. The use of parenteral antischistosomal therapy in Egypt is thought to have contributed to a prevalence of antibodies against HCV in various regions ranging from 6% to 28% (mean, 22%)[1]. An estimated 70% to 85% of infected patients are likely to develop chronic hepatitis, and up to 30% of these cases might progress to cirrhosis[2].
When treating chronic hepatitis C (CHC), many clinicians do not take into consideration the presence of other comorbid conditions that lead to more progressive liver disease, such as cirrhosis and hepatocellular carcinoma (HCC)[3-5]. In recent studies, it has been proved that such comorbidities might reduce the response rate to PEGylated interferon (PEG-IFN)/ribavirin (RBV) therapy in HCV patients[6,7].
Eventually amelioration of these comorbidities before embarking on IFN-based therapy would improve the sustained virological response (SVR) and impair progression to cirrhosis and HCC.
Coexistent HCV infection has been estimated to be present in 10% to 15% of patients with chronic hepatitis B, and is more common among injecting drug users[8]. Acute coinfection with HBV and HCV can shorten the duration of HBs antigenemia and lower the peak serum aminotransferase concentrations compared with acute HBV infection alone[9,10]. However, acute coinfections of HCV and HBV, or acute HCV on preexisting chronic HBV, have also been reported to increase the risk of severe hepatitis and fulminant hepatic failure[11].
However, combined chronic hepatitis B and C leads to more severe liver disease, an increased risk of hepatocellular carcinoma[3,4] and lower response to IFN[6]. Furthermore, co-infected patients represent a treatment challenge. No standard recommendations exist for treatment of viral hepatitis due to dual HBV/HCV infection, and therefore treatment must be individualized[4].
Management: Treatment decisions should be based upon the determination of the "dominant" hepatitis virus. The more active virus should be treated using IFN plus RBV for hepatitis C and IFN plus Lamivudine for hepatitis B[3,4]. Caution must be exercised in treating coinfected patients, by observing reactivation of untreated virus as flares of the latter might occur.
MS is a cluster of abnormalities, including obesity, insulin resistance, type 2 diabetes mellitus, dyslipidemia, and hypertension. Moreover, patients with chronic HCV infection have increased prevalence of insulin resistance and of type 2 diabetes compared with age-, sex-, and liver disease-matched controls[12].
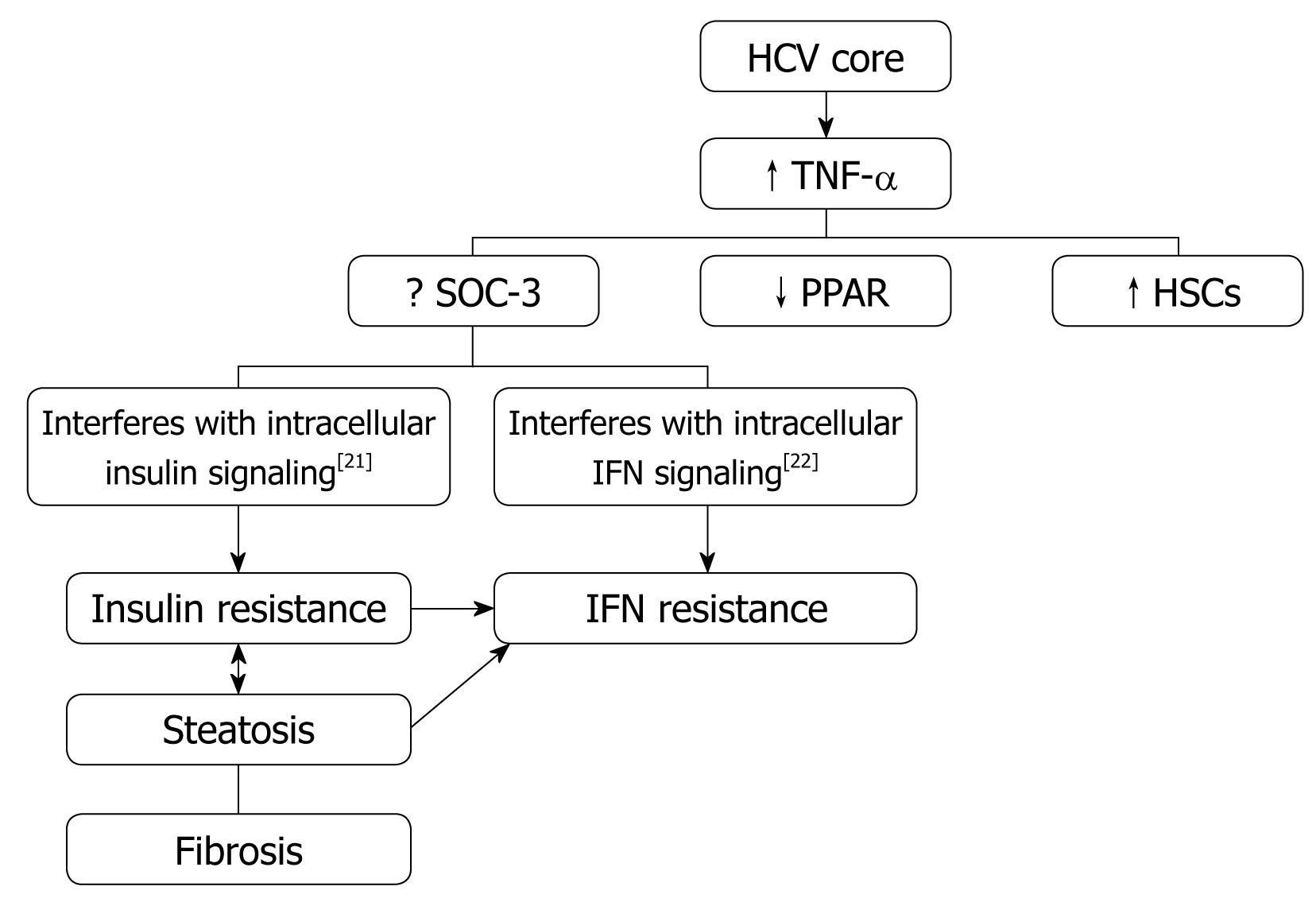
It has been observed that overweight, insulin resistance, and liver steatosis have a negative impact on the course of CHC, being associated with more severe and progressive liver fibrosis[5]. HCV proteins are associated with the dysfunction of mitochondria and endoplasmic reticulum that promote oxidative stress. The latter mediates signals that activate the expression of proinflammatory cytokines: tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-8, tumor growth factor-α, and the fas ligand.
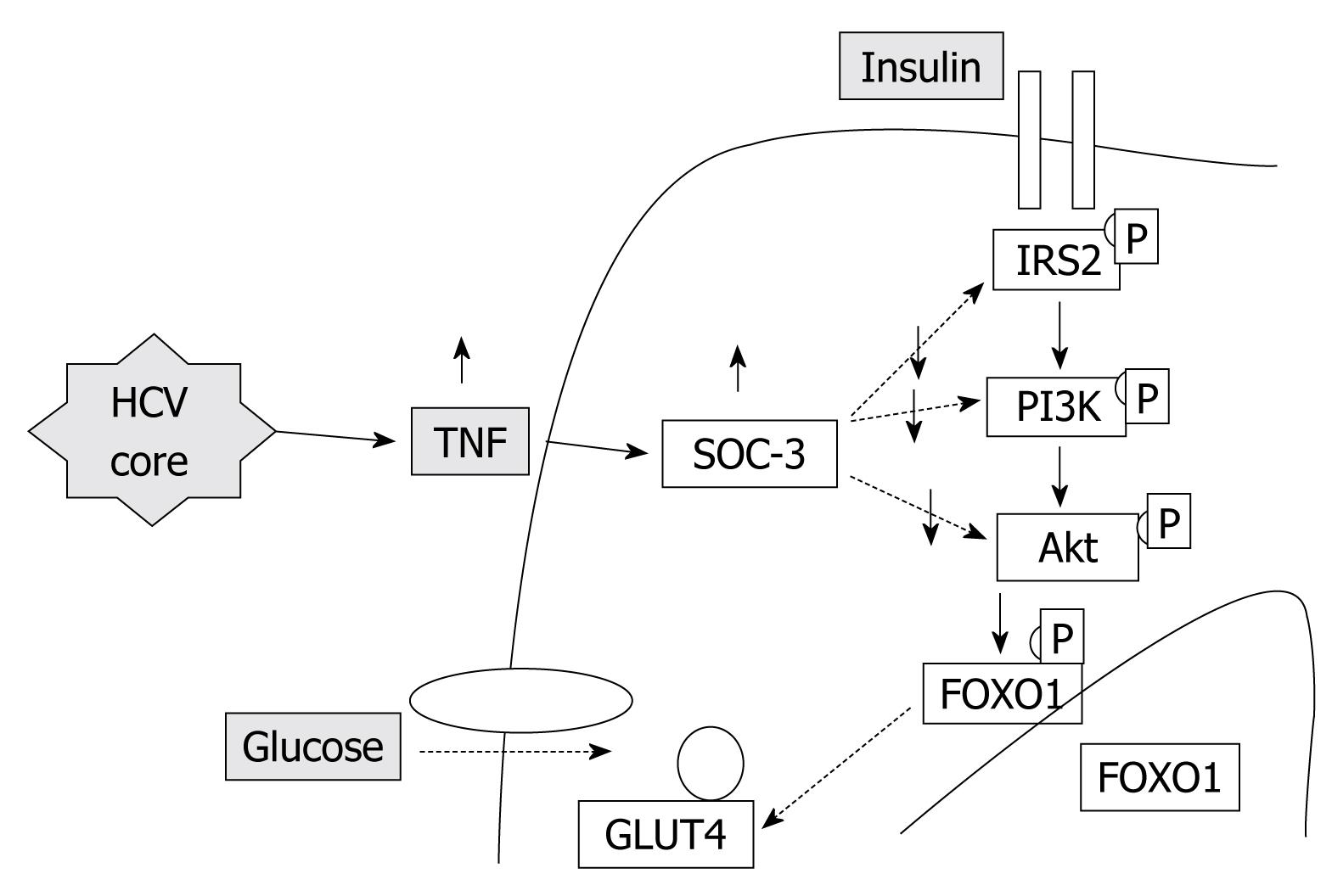
Insulin resistance: HCV proteins activate the expression of TNF-α which inhibits the function of insulin receptor substrates (IRS) and decreases the expression of glucose transporter-4 and lipoprotein lipase in peripheral tissues, which are responsible for the promotion of insulin resistance (Figure 1).
Furthermore, reduced adiponectin levels, loss of adiponectin receptors, and decreased anti-inflammatory peroxisome proliferator-activated receptor α (PPAR-α) in the liver of HCV patients might contribute to reduced fatty acid oxidation, inflammation, and eventually, lipotoxicity[12].
Insulin resistance has been clearly associated with steatosis, more severe and progressive fibrosis, and a reduced response to PEG-IFN and RBV therapy in HCV patients[13]. Several recent studies have confirmed that SVR is impaired in patients with a high homoeostasis model assessment index[14].
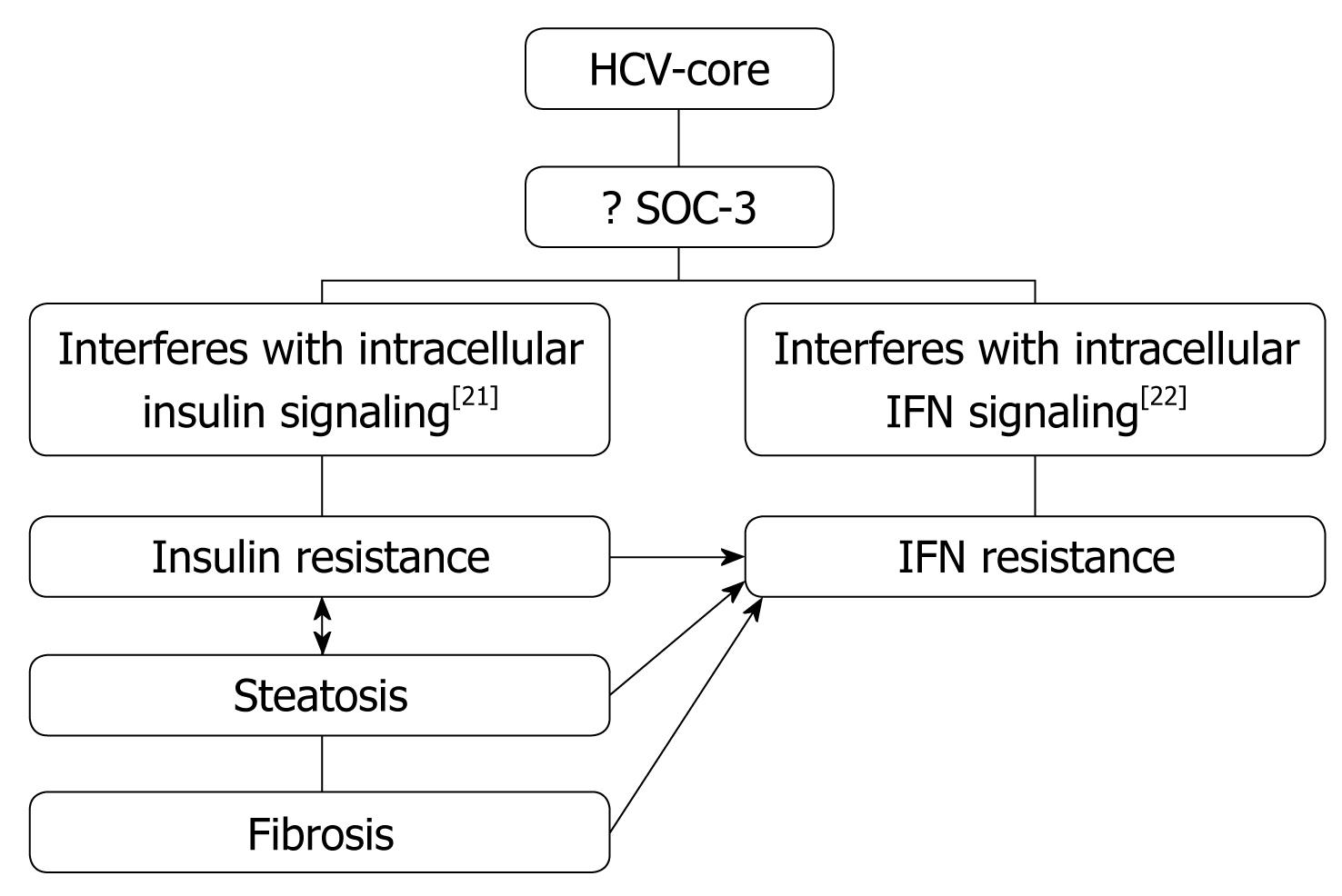
Obesity/Hepatic steatosis: In hepatitis C infection, hepatic steatosis might be either metabolic [overweight & obesity (BMI ≥ 25), diabetes mellitus] or cytopathic due to the effect of the virus, as in genotype 3. However, genotype-4, which is predominant in Egypt, has no direct relation with the development of steatosis[15]; however, it develops as a secondary metabolic effect as evidenced by HCV core protein promotes IR via TNF-α production[16] (Figures 2 and 3). In addition, HCV non-genotype-3 induces insulin resistance through downregulation of IRS[17-19]. HCV genotype-3 might induce a cytopathic effect and autoimmune aggression on β-cells of the pancreas[20]. Furthermore, HCV produces necroinflammation of hepatocyte membranes with consequent malfunction of insulin receptors.
Several studies have recently confirmed that the SVR[7] is impaired in patients with high body mass index and for those with hepatic steatosis. These data indicate that HCV carriers should avoid weight gain by diet and physical exercise before initiation of antiviral therapy, all efforts should be made to improve the metabolic steatosis of the patient.
Type 2 diabetes mellitus: Type 2 diabetes mellitus is frequently associated with hyperinsulinemia and fatty liver disease. The chronically elevated circulating insulin levels found in type 2 diabetic patients might be responsible for accumulation of fat in the liver by downregulating mitochondrial β-oxidation and blocking secretion of triglycerides from the liver. Diabetic individuals are at higher risk to develop non-alcoholic steatohepatitis, which might progress to cirrhosis in up to 5% of cases[24,25].
Intestinal bacterial overgrowth (IBOG): Chronic liver disease is associated with slow, transient, altered gut permeability and translocation of intestinal bacteria and toxins to portal and systemic circulation[26,27]. These toxins contribute to liver cell injury by induction of proinflammatory cytokines (TNF-α, IL-6, and IL-8) in the liver. Concomitant IBOG with HCV infection will aggravate the risk of severe hepatitis and progressive liver disease as a result of the immune response against HCV infection.
Management: Intestinal decontamination by metronidazole and probiotics to prevent endotoxin formation, open bowel by synthetic disaccharides (lactulose), and trigger peristalsis by prokinetics.
Schistosoma mansoni has been the major risk factor of liver diseases in Egypt, especially in rural areas. In many patients, HCV infection is associated with schistosomiasis because of iv anti-schistosomal therapy[28,29]. Schistosomiasis is an immunological disease, which suppress cellular immunity, triggering Th2 cytokine response favoring chronicity of hepatitis C infection.
Patients with CHC and concomitant schistosomiasis respond poorly to IFN therapy and have higher relapse rates compared to patients with HCV infection only. This might be due to HCV genotype 4 per se, or to the negative influence of schistosomiasis on the immune system, leading to higher HCV RNA titers, and more severe liver damage with a higher incidence of cirrhosis. The latter is promoted by the longer duration of both infections[30].
Diagnosis of schistosomiasis was based on a history of Schistosoma infection, detection of S. mansoni ova in stool, or a rectal biopsy[31]. At baseline, patients with CHC and schistosomiasis had higher HCV RNA titers compared to patients without schistosomiasis[30].
Management: Praziquantel 40 mg/kg single dose (can be repeated up to three weekly consecutive doses) before initiation of antiviral therapy.
Insulin resistance has been clearly associated with steatosis, a more severe and progressive fibrosis, and a reduced response to PEG-IFN and RBV therapy in HCV patients[13].
How does concomitant MS with HCV infection impair the response to interferon?
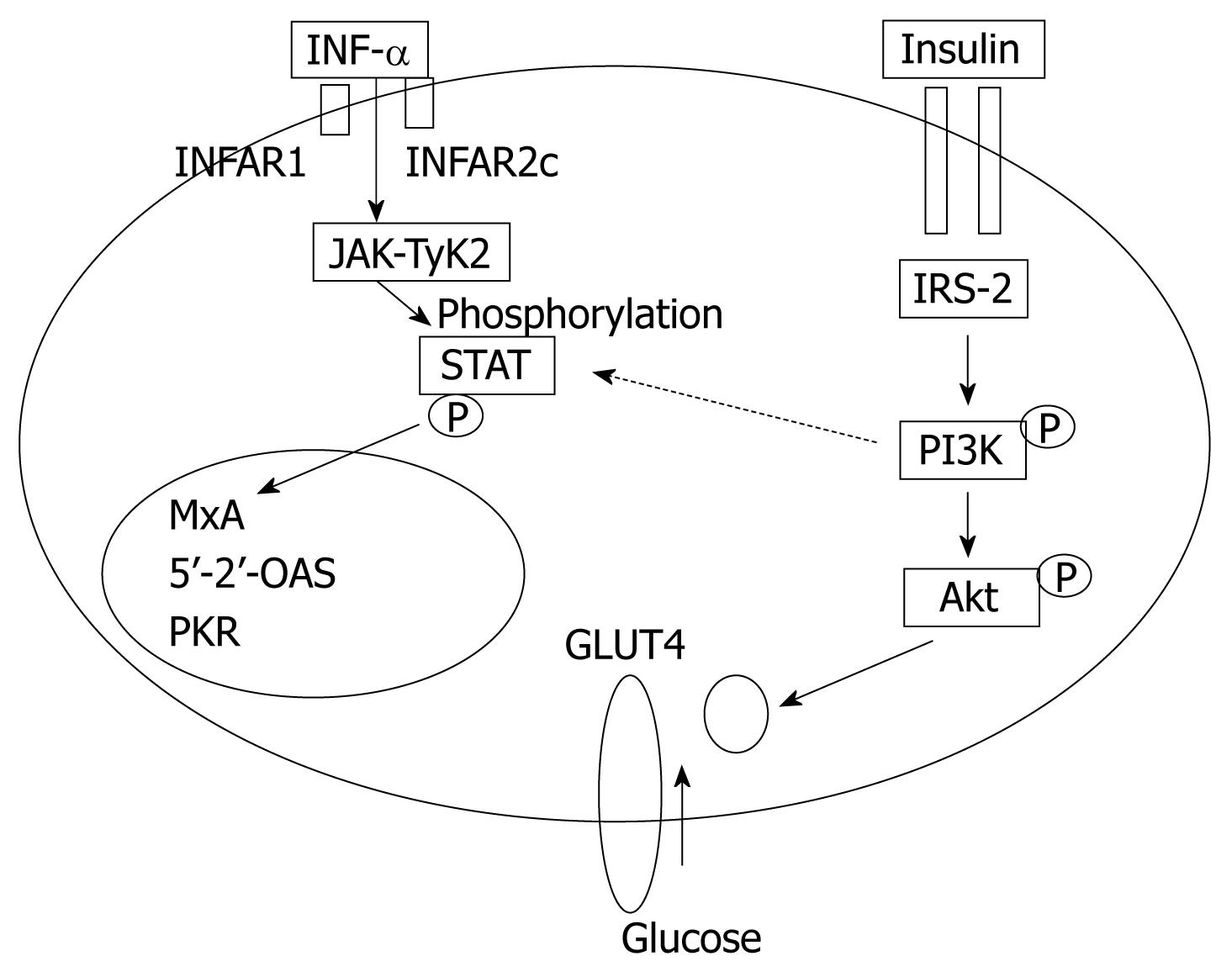
In obese patients, subcutaneous fat impairs absorption of interferon at the site of injection. In addition, hepatic steatosis decreases contact between interferon and hepatocytes receptors[16]. Steatosis interferes with the signaling cascade of interferon [Janus Kinase (JAK) activate signal transduction and activator of transcription (STAT) which express IFN genes][32]. On the other hand, the HCV core protein promotes insulin resistance via TNF-production and insulin resistance induces steatosis, fibrogenesis, and IFN-resistance[16,33,34]. In addition, HCV core protein and TNF-α upregulate suppressor of cytokines-3 which binds to JAK, inhibiting phosphorylation of STAT1[35] and eventually interfering with IFN signaling[33]. Furthermore, obesity in general is associated with a suppressed immune response[36,37] (Figures 3 and 4).
Management of MS: Amelioration of metabolic factors before starting interferon therapy favors a good response to interferon therapy. HCV patients should avoid weight gain by life style modification (hypocaloric diet/exercise), use of insulin sensitizers, in the form of Metformin, to reduce hepatic gluconeogenesis, and pioglitazone to sensitize insulin receptors and mobilize visceral fat to subcutaneous tissues. Antioxidants (vitamin E, betaine, silymarin, and β-carotine), hepatoprotective drugs (UDCA) might add therapeutic benefit to inhibit the toxic effects of free radicals. Gut decontamination with metronidazole and probiotics to prevent gut endotoxin formation should be considered; the later induce proinflammatory cytokines in the liver that promote steatosis and steatohepatitis.
In hepatitis C patients, increased iron absorption in cirrhosis and difficulty in excreting iron from the body contribute to development of iron overload[38]. There is growing evidence that iron overload enhances the amount of liver injury and progression to cirrhosis and HCC. In addition, it decreases the SVR to IFN/RBV treatment[39,40].
Management: Venesection, restrict diets rich in iron, give antioxidants such as silymarin, betaine and vitamin E to nullify the toxic effects of free radicals.
Alcohol abuse favors the development of alcoholic steatosis, steatohepatitis, and subsequently cirrhosis. It plays a role in resistance to interferon therapy through immunosuppression of CD4+ and NK cells[41], by increased hepatic iron load, and by inhibiting the IFN-α-activated signals[42,43].
Most studies found that alcohol decreased the response to interferon-based therapy and this effect is alcohol dose-dependent[42-44]. Median daily alcohol use > 30 g/d is associated with failure to respond to PEG-IFN and RBV for treatment of hepatitis C. Past alcohol use should be evaluated when considering treatment for hepatitis C[42].
Management: Stop alcohol abuse, intestinal antibiotics and probiotics to prevent gut endotoxin formation; and antioxidants as vitamin E, betaine and silymarin to block lipid peroxidation. Pentoxifylline is an oral phosphodiesterase inhibitor which decreases expression of TNF-α (and other proinflammatory cytokines) and which may inhibit apoptosis. When the full course is completed, the SVR is similar, regardless of alcohol intake[45].
Heavy smoking increases the severity of hepatic inflammation and fibrosis when associated with hepatitis C infection[46]. Heavy smoking induces resistance to interferon therapy by suppression of CD4+ and NK activity[41] inducing apoptosis of T-cells[47], and increasing hepatic iron load[48].
Management: Stop smoking, venesection to reduce iron level, limit diet rich in iron and use of antioxidants as silymarin, vitamin E, betaine, β-carotine, lecithin and selenium.
These comorbidities reduce compliance and adherence to inadequate PEG-IFN or RBV doses.
Depression is significantly more prevalent in chronically HCV-infected patients than in the general population[49], which negatively affect patients’ functional health, ability to work, self-perceived health, health-related quality of life (HRQL) and well being[50].
The presence of mild/moderate depression at baseline is not considered an absolute contraindication to initiate antiviral therapy with PEG-IFN and RBV. However, this condition is certainly associated with a higher risk of developing severe depression during therapy that might lead to higher rates of treatment discontinuation in the absence of adequate antidepressant therapy[51].
It has been reported that IFN-α downregulated glucocorticoid receptor (GR) and serotonin receptor 1A (5-HTR1A) levels in cell lines. These levels of GR and 5-HTR1A, following IFN-α-induced downregulation, recovered after withdrawal of IFN-α or addition of desipramine or fluoxetine. These data provide insights regarding the pathogenesis of IFN-α-induced depression[52].
The pharmacokinetics of PEGylated IFN are different from those of standard IFN[53] and the time of depression occurrence also differs between PEGylated and standard IFN[54]; therefore, it will be important to determine if the mechanisms described by Cai et al[52] on GR and 5-HTR1A receptors also apply to PEGylated IFN. In addition, anxiety and depression impair the level and activity of B cells, T cells, and NK cells[55]. No differences in depression rates were observed by Neri et al[56] comparing PEGylated-α 2a and PEGylated-α 2b, and this finding has recently been confirmed in the large randomized comparison trial ‘IDEAL’[57].
Depression and anxiety of significant severity can adversely effect compliance and tolerance to medication. Although anemia and depression were associated with HRQL impairment, depression was the most consistent predictor[50]. Several studies concluded that individuals who experience significant worsening of depressive symptoms during IFN therapy are less likely to achieve a virological response during therapy and an SVR after therapy withdrawal[13].
Management: Therapeutic intervention has been shown to be effective in the management of IFN-induced depression in a controlled study[58]. The most effective and well-studied antidepressants in this setting are those of the selective serotonin reuptake inhibitor class, in particular citalopram 25 mg/d[59]. Importantly, all patients who received antidepressant treatment were able to complete the full course of IFN therapy; while discontinuation was necessitated in patients in the placebo arm[60].
Anemia that develops in a patient receiving HCV therapy often has multiple potential contributing factors, including RBV, interferon or PEG-interferon, underlying liver disease caused by HCV infection, and co-morbid conditions, such as HIV infection or chronic renal failure[61]. The anemia associated with RBV most often occurs as dose-dependent hemolytic anemia, typically developing within the first 4 wk of therapy[62,63]. At higher doses of RBV (1000-1200 mg/d), hemoglobin levels frequently decline by 2-3 g/dL. In addition to causing hemolysis, RBV can also downregulate the number of erythropoietin receptors[64]. Interferon can also contribute to the development of anemia by suppressing bone marrow production of erythrocytes, but this process is generally slower and might account for the continued decline in hemoglobin concentration during the second and third months of treatment. Finally, patients developing anemia during HCV therapy often have inappropriately poor serum erythropoietin responses[65], probably related to their underlying liver disease. It is often not possible to pinpoint one particular drug as the primary cause of the anemia, because of the mixed nature of HCV treatment-associated anemia.
Management of anemia associated with HCV Therapy: The conventional standard of care for managing anemia during HCV antiviral therapy has consisted of reducing the RBV dose by half if the hemoglobin level decreases to less than 10 g/dL, and to completely stop the RBV if the hemoglobin level drops below 8.5 g/dL. A decrease in RBV dose, especially in the first several months of treatment, can diminish response rates considerably. Furthermore, the strategy of decreasing the RBV dose will only partially correct the anemia. On average, the hemoglobin level will increase 1 g/dL with RBV dose reduction[66]. Finally, some patients who have co-morbid conditions, such as diabetes mellitus, coronary artery disease, and chronic obstructive pulmonary disease, might poorly tolerate even mild levels of anemia.
The recombinant erythropoietin hormone, epoetin α, has emerged as an excellent option for improving HCV treatment-related anemia while supporting optimal treatment doses of RBV and interferon. Recombinant erythropoietin hormone acts by increasing the number of erythroid progenitor cells, and has demonstrated efficacy and safety in patients with chronic renal disease, those with malignancies receiving chemotherapy. Patients receiving once weekly epoetin α (4000 unites) had significantly higher hemoglobin levels at week 16[67]. The major drawbacks of using any of the recombinant erythropoietin medications are high cost and slightly increased risk of thrombotic events.
HCV RNAs were found in the hearts of patients with cardiomyopathies, and negative strands of HCV RNA were also detected in the hearts, suggesting that HCV replicates in myocardial tissues[68].
A major subset of CHC patients currently considered ineligible for PEG-IFN/RBV is represented by those with co-existing clinically significant heart disease. Durante-Mangoni et al[69] prospectively evaluated safety and efficacy of PEG-IFN/RBV treatment in CHC patients with heart disease. They concluded that treatment with PEG-IFN/RBV might be safely offered to CHC patients with co-existing, clinically significant, heart disease. In qualified centers, CHC patients with overt hear disease should not be denied treatment, whenever indicated[69].
However, some patients with coronary artery disease poorly tolerate even mild levels of anemia. Hence, doses should be reduced in more than 25% of patients for both PEG-IFN/RBV to avoid the serious adverse events on the sick heart.
CHC and chronic renal failure might occur together because of the association of HCV infection with cryoglobulinemia and membranoproliferative glomerulonephritis, or by infection of chronic renal failure patients from exposure to HCV-contaminated blood or hemodialysis equipment.
Those with renal failure and chronic HCV infection might have significant liver disease. Although patients on dialysis tend to have milder liver disease and normal liver enzymes compared with patients with normal renal function[70], patients with end-stage renal disease (ESRD) and CHC might have severe chronic hepatitis on liver biopsy[71].
A liver biopsy should be performed in patients with CHC who are receiving hemodialysis[72] and do not have major comorbidities. Among dialysis patients who are not candidates for renal transplantation, antiviral therapy is recommended in those with fibrotic disease for viral eradication and potential reduction of the stage of fibrosis.
In candidates for renal transplantation, cirrhosis is a contraindication to renal transplantation[73]; a combined liver and kidney transplantation might be indicated in patients who progress to decompensated cirrhosis. In candidates for renal transplantation, treatment is appropriate in the pre-transplant setting[73]. Interferon therapy is ineffective and has an unacceptably high risk of precipitating rejection after transplantation. Even in patients with mild liver disease, antiviral treatment is recommended to obtain a SVR before transplantation[73], which will avoid the risk of progressive liver disease after transplantation.
Management of HCV infection associated with renal failure: RBV is cleared by the kidneys and thus contraindicated in patients with renal failure[74,75]. There is a risk of enhancement of the RBV-related hemolytic anemia, with a marked fall in hemoglobin levels. Interferon monotherapy at the standard dosing schedule of 3 million units subcutaneously three times weekly, or PEG-interferon monotherapy (α-2a or α-2b) injected once weekly, is used in patients with renal disease. The pharmacokinetics are similar to patients with renal disease down to a creatinine clearance of 20 mL/min. Trials of PEG-interferon in patients with ESRD have used either 135 mcg of PEG-interferon α-2a or 0.5-1.0 mcg/kg of PEG-interferon α-2b. In dialysis patients, the sustained virologic response achieved with interferon monotherapy is at least as good as in the general population[76]. Adverse events leading to drug discontinuation, in decreasing order of frequency, include flu-like symptoms, neutropenia, depression, and neurological symptoms. Drop-out rates seen with interferon given at 3 million units three times weekly are between 20% and 30%. PEG-interferon monotherapy trials in patients with ESRD are currently under way.
In chronic hepatitis C, control or amelioration of comorbidities before embarking on antiviral therapy represents the milestone for higher post-antiviral therapy response.
| 1. | Frank C, Mohamed MK, Strickland GT, Lavanchy D, Arthur RR, Magder LS, El Khoby T, Abdel-Wahab Y, Aly Ohn ES, Anwar W. The role of parenteral antischistosomal therapy in the spread of hepatitis C virus in Egypt. Lancet. 2000;355:887-891. |
| 2. | Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med. 2001;345:41-52. |
| 3. | Benvegnù L, Fattovich G, Noventa F, Tremolada F, Chemello L, Cecchetto A, Alberti A. Concurrent hepatitis B and C virus infection and risk of hepatocellular carcinoma in cirrhosis. A prospective study. Cancer. 1994;74:2442-2448. |
| 4. | Crockett SD, Keeffe EB. Natural history and treatment of hepatitis B virus and hepatitis C virus coinfection. Ann Clin Microbiol Antimicrob. 2005;4:13. |
| 5. | Contos MJ, Sanyal AJ. The clinicopathologic spectrum and management of nonalcoholic fatty liver disease. Adv Anat Pathol. 2002;9:37-51. |
| 6. | Weltman MD, Brotodihardjo A, Crewe EB, Farrell GC, Bilous M, Grierson JM, Liddle C. Coinfection with hepatitis B and C or B, C and delta viruses results in severe chronic liver disease and responds poorly to interferon-alpha treatment. J Viral Hepat. 1995;2:39-45. |
| 7. | Kaserer K, Fiedler R, Steindl P, Müller CH, Wrba F, Ferenci P. Liver biopsy is a useful predictor of response to interferon therapy in chronic hepatitis C. Histopathology. 1998;32:454-461. |
| 8. | Strader DB. Understudied populations with hepatitis C. Hepatology. 2002;36:S226-S236. |
| 9. | Liaw YF, Tsai SL, Chang JJ, Sheen IS, Chien RN, Lin DY, Chu CM. Displacement of hepatitis B virus by hepatitis C virus as the cause of continuing chronic hepatitis. Gastroenterology. 1994;106:1048-1053. |
| 10. | Mimms LT, Mosley JW, Hollinger FB, Aach RD, Stevens CE, Cunningham M, Vallari DV, Barbosa LH, Nemo GJ. Effect of concurrent acute infection with hepatitis C virus on acute hepatitis B virus infection. BMJ. 1993;307:1095-1097. |
| 11. | Chu CM, Yeh CT, Liaw YF. Fulminant hepatic failure in acute hepatitis C: increased risk in chronic carriers of hepatitis B virus. Gut. 1999;45:613-617. |
| 12. | Sheikh MY, Choi J, Qadri I, Friedman JE, Sanyal AJ. Hepatitis C virus infection: molecular pathways to metabolic syndrome. Hepatology. 2008;47:2127-2133. |
| 13. | Alberti A. What are the comorbidities influencing the management of patients and the response to therapy in chronic hepatitis C? Liver Int. 2009;29 Suppl 1:15-18. |
| 14. | Chu CJ, Lee SD, Hung TH, Lin HC, Hwang SJ, Lee FY, Lu RH, Yu MI, Chang CY, Yang PL. Insulin resistance is a major determinant of sustained virological response in genotype 1 chronic hepatitis C patients receiving peginterferon alpha-2b plus ribavirin. Aliment Pharmacol Ther. 2009;29:46-54. |
| 15. | El-Zayadi A, Attia M, Barakat EMF, Zalata K, Saeid A, Hamdy H, El-Nakeeb A. Hepatic steatosis in hepatitis C genotype 4 infected patients. Arab J Gastroenterol. 2007;8:5-9. |
| 16. | Romero-Gómez M, Del Mar Viloria M, Andrade RJ, Salmerón J, Diago M, Fernández-Rodríguez CM, Corpas R, Cruz M, Grande L, Vázquez L. Insulin resistance impairs sustained response rate to peginterferon plus ribavirin in chronic hepatitis C patients. Gastroenterology. 2005;128:636-641. |
| 17. | Cavallo-Perin P, Cassader M, Bozzo C, Bruno A, Nuccio P, Dall'Omo AM, Marucci M, Pagano G. Mechanism of insulin resistance in human liver cirrhosis. Evidence of a combined receptor and postreceptor defect. J Clin Invest. 1985;75:1659-1665. |
| 18. | Cavallo-Perin P, Bruno A, Nuccio P, Goria M, Pagano G, Lenti G. Feedback inhibition of insulin secretion is altered in cirrhosis. J Clin Endocrinol Metab. 1986;63:1023-1027. |
| 19. | Nygren A, Adner N, Sundblad L, Wiechel KL. Insulin uptake by the human alcoholic cirrhotic liver. Metabolism. 1985;34:48-52. |
| 20. | el-Zayadi AR, Selim OE, Hamdy H, Dabbous H, Ahdy A, Moniem SA. Association of chronic hepatitis C infection and diabetes mellitus. Trop Gastroenterol. 1998;19:141-144. |
| 21. | Kawaguchi T, Yoshida T, Harada M, Hisamoto T, Nagao Y, Ide T, Taniguchi E, Kumemura H, Hanada S, Maeyama M. Hepatitis C virus down-regulates insulin receptor substrates 1 and 2 through up-regulation of suppressor of cytokine signaling 3. Am J Pathol. 2004;165:1499-1508. |
| 22. | Gadina M, Hilton D, Johnston JA, Morinobu A, Lighvani A, Zhou YJ, Visconti R, O'Shea JJ. Signaling by type I and II cytokine receptors: ten years after. Curr Opin Immunol. 2001;13:363-373. |
| 23. | Leandro G, Mangia A, Hui J, Fabris P, Rubbia-Brandt L, Colloredo G, Adinolfi LE, Asselah T, Jonsson JR, Smedile A. Relationship between steatosis, inflammation, and fibrosis in chronic hepatitis C: a meta-analysis of individual patient data. Gastroenterology. 2006;130:1636-1642. |
| 24. | Caldwell SH, Oelsner DH, Iezzoni JC, Hespenheide EE, Battle EH, Driscoll CJ. Cryptogenic cirrhosis: clinical characterization and risk factors for underlying disease. Hepatology. 1999;29:664-669. |
| 25. | Silverman JF, O'Brien KF, Long S, Leggett N, Khazanie PG, Pories WJ, Norris HT, Caro JF. Liver pathology in morbidly obese patients with and without diabetes. Am J Gastroenterol. 1990;85:1349-1355. |
| 26. | González Alonso R, González García M, Albillos Martínez A. [Physiopathology of bacterial translocation and spontaneous bacterial peritonitis in cirrhosis]. Gastroenterol Hepatol. 2007;30:78-84. |
| 27. | Guarner C, Soriano G. Bacterial translocation and its consequences in patients with cirrhosis. Eur J Gastroenterol Hepatol. 2005;17:27-31. |
| 28. | Abdel-Wahab MF, Mahmoud SS. Schistosomiasis mansoni in Egypt. Clin Trop Med Commun Dis. 1987;2:371-395. |
| 29. | Angelico M, Renganathan E, Gandin C, Fathy M, Profili MC, Refai W, De Santis A, Nagi A, Amin G, Capocaccia L. Chronic liver disease in the Alexandria governorate, Egypt: contribution of schistosomiasis and hepatitis virus infections. J Hepatol. 1997;26:236-243. |
| 30. | Kamal SM, Madwar MA, Peters T, Fawzy R, Rasenack J. Interferon therapy in patients with chronic hepatitis C and schistosomiasis. J Hepatol. 2000;32:172-174. |
| 31. | Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 1972;14:397-400. |
| 32. | McCullough AJ. Obesity and its nurturing effect on hepatitis C. Hepatology. 2003;38:557-559. |
| 33. | Romero-Gómez M. Insulin resistance and hepatitis C. World J Gastroenterol. 2006;12:7075-7080. |
| 34. | Yoneda M, Saito S, Ikeda T, Fujita K, Mawatari H, Kirikoshi H, Inamori M, Nozaki Y, Akiyama T, Takahashi H. Hepatitis C virus directly associates with insulin resistance independent of the visceral fat area in nonobese and nondiabetic patients. J Viral Hepat. 2007;14:600-607. |
| 35. | Bloomgarden ZT. The 1st World Congress on the Insulin Resistance Syndrome. Diabetes Care. 2004;27:602-609. |
| 36. | Samartin S, Chandra RK. Obesity, overnutrition and the immune system. Nutr Res. 2001;21:243-262. |
| 37. | Tanaka S, Inoue S, Isoda F, Waseda M, Ishihara M, Yamakawa T, Sugiyama A, Takamura Y, Okuda K. Impaired immunity in obesity: suppressed but reversible lymphocyte responsiveness. Int J Obes Relat Metab Disord. 1993;17:631-636. |
| 39. | Bonkovsky HL, Banner BF, Rothman AL. Iron and chronic viral hepatitis. Hepatology. 1997;25:759-768. |
| 40. | Pietrangelo A. Hemochromatosis gene modifies course of hepatitis C viral infection. Gastroenterology. 2003;124:1509-1523. |
| 41. | Zeidel A, Beilin B, Yardeni I, Mayburd E, Smirnov G, Bessler H. Immune response in asymptomatic smokers. Acta Anaesthesiol Scand. 2002;46:959-964. |
| 42. | Chang A, Skole K, Gautam M, Schmutz J, Black M, Thomas R, Horwitz B, Friedenberg FK. The impact of past alcohol use on treatment response rates in patients with chronic hepatitis C. Aliment Pharmacol Ther. 2005;22:701-706. |
| 43. | Tabone M, Sidoli L, Laudi C, Pellegrino S, Rocca G, Della Monica P, Fracchia M, Galatola G, Molinaro GC, Aricò S. Alcohol abstinence does not offset the strong negative effect of lifetime alcohol consumption on the outcome of interferon therapy. J Viral Hepat. 2002;9:288-294. |
| 44. | Okazaki T, Yoshihara H, Suzuki K, Yamada Y, Tsujimura T, Kawano K, Yamada Y, Abe H. Efficacy of interferon therapy in patients with chronic hepatitis C. Comparison between non-drinkers and drinkers. Scand J Gastroenterol. 1994;29:1039-1043. |
| 45. | Anand BS, Currie S, Dieperink E, Bini EJ, Shen H, Ho SB, Wright T. Alcohol use and treatment of hepatitis C virus: results of a national multicenter study. Gastroenterology. 2006;130:1607-1616. |
| 46. | Pessione F, Ramond MJ, Njapoum C, Duchatelle V, Degott C, Erlinger S, Rueff B, Valla DC, Degos F. Cigarette smoking and hepatic lesions in patients with chronic hepatitis C. Hepatology. 2001;34:121-125. |
| 47. | Suzuki N, Wakisaka S, Takeba Y, Mihara S, Sakane T. Effects of cigarette smoking on Fas/Fas ligand expression of human lymphocytes. Cell Immunol. 1999;192:48-53. |
| 48. | El-Zayadi AR, Selim O, Hamdy H, El-Tawil A, Moustafa H. Heavy cigarette smoking induces hypoxic polycythemia (erythrocytosis) and hyperuricemia in chronic hepatitis C patients with reversal of clinical symptoms and laboratory parameters with therapeutic phlebotomy. Am J Gastroenterol. 2002;97:1264-1265. |
| 49. | Coughlan B, Sheehan J, Hickey A, Crowe J. Psychological well-being and quality of life in women with an iatrogenic hepatitis C virus infection. Br J Health Psychol. 2002;7:105-116. |
| 50. | Dan AA, Martin LM, Crone C, Ong JP, Farmer DW, Wise T, Robbins SC, Younossi ZM. Depression, anemia and health-related quality of life in chronic hepatitis C. J Hepatol. 2006;44:491-498. |
| 51. | Younossi Z, Kallman J, Kincaid J. The effects of HCV infection and management on health-related quality of life. Hepatology. 2007;45:806-816. |
| 52. | Cai W, Khaoustov VI, Xie Q, Pan T, Le W, Yoffe B. Interferon-alpha-induced modulation of glucocorticoid and serotonin receptors as a mechanism of depression. J Hepatol. 2005;42:880-887. |
| 53. | Hadziyannis SJ, Papatheodoridis GV. Peginterferon-alpha2a (40 kDa) for chronic hepatitis C. Expert Opin Pharmacother. 2003;4:541-551. |
| 54. | Schaefer M, Schwaiger M, Garkisch AS, Pich M, Hinzpeter A, Uebelhack R, Heinz A, van Boemmel F, Berg T. Prevention of interferon-alpha associated depression in psychiatric risk patients with chronic hepatitis C. J Hepatol. 2005;42:793-798. |
| 55. | Morimoto K, Takeshita T, Inoue-Sakurai C, Maruyama S. Lifestyles and mental health status are associated with natural killer cell and lymphokine-activated killer cell activities. Sci Total Environ. 2001;270:3-11. |
| 56. | Neri S, Pulvirenti D, Bertino G. Psychiatric symptoms induced by antiviral therapy in chronic hepatitis C: comparison between interferon-alpha-2a and interferon-alpha-2b. Clin Drug Investig. 2006;26:655-662. |
| 57. | Sulkowski M, Lawitz E, Shiffman ML, Muir AJ, Galler G, McCone J. Final results of the IDEAL (Individualized dosing efficacy versus flat dosing to assess optimal Pegylated Interferon Therapy) Phase IIIb study. 43rd Annual Meeting of the European Association for the Study of the Liver (EASL 2008); 2008 April 23-27; Milan, Italy. . |
| 58. | Raison CL, Borisov AS, Broadwell SD, Capuron L, Woolwine BJ, Jacobson IM, Nemeroff CB, Miller AH. Depression during pegylated interferon-alpha plus ribavirin therapy: prevalence and prediction. J Clin Psychiatry. 2005;66:41-48. |
| 59. | Hauser P, Khosla J, Aurora H, Laurin J, Kling MA, Hill J, Gulati M, Thornton AJ, Schultz RL, Valentine AD. A prospective study of the incidence and open-label treatment of interferon-induced major depressive disorder in patients with hepatitis C. Mol Psychiatry. 2002;7:942-947. |
| 60. | Kraus MR, Schäfer A, Schöttker K, Keicher C, Weissbrich B, Hofbauer I, Scheurlen M. Therapy of interferon-induced depression in chronic hepatitis C with citalopram: a randomised, double-blind, placebo-controlled study. Gut. 2008;57:531-536. |
| 61. | Bräu N. Epoetin alfa treatment for acute anaemia during interferon plus ribavirin combination therapy for chronic hepatitis C. J Viral Hepat. 2004;11:191-197. |
| 62. | Kowdley KV. Hematologic side effects of interferon and ribavirin therapy. J Clin Gastroenterol. 2005;39:S3-S8. |
| 63. | Tran TT, Martin P. Chronic Hepatitis C. Curr Treat Options Gastroenterol. 2001;4:503-510. |
| 64. | Mamus SW, Oken MM, Zanjani ED. Suppression of normal human erythropoiesis by human recombinant DNA-produced alpha-2-interferon in vitro. Exp Hematol. 1986;14:1015-1022. |
| 65. | Gogu SR, Beckman BS, Wilson RB, Agrawal KC. Inhibitory effects of zidovudine in erythroid progenitor cells. Reversal with a combination of erythropoietin and interleukin-3. Biochem Pharmacol. 1995;50:413-419. |
| 66. | Sulkowski MS, Wasserman R, Brooks L, Ball L, Gish R. Changes in haemoglobin during interferon alpha-2b plus ribavirin combination therapy for chronic hepatitis C virus infection. J Viral Hepat. 2004;11:243-250. |
| 67. | Dieterich DT, Wasserman R, Bräu N, Hassanein TI, Bini EJ, Bowers PJ, Sulkowski MS. Once-weekly epoetin alfa improves anemia and facilitates maintenance of ribavirin dosing in hepatitis C virus-infected patients receiving ribavirin plus interferon alfa. Am J Gastroenterol. 2003;98:2491-2499. |
| 68. | Matsumori A. [Hepatitis C virus infection and cardiomyopathy]. Nippon Rinsho. 1999;57:455-463. |
| 69. | Durante-Mangoni E, Iossa D, Pinto D, Utili R. Safety and efficacy of peginterferon-ribavirin treatment in hepatitis C patients with heart disease. Dig Liver Dis. 2009;41:A41-A45. |
| 70. | Trevizoli JE, de Paula Menezes R, Ribeiro Velasco LF, Amorim R, de Carvalho MB, Mendes LS, Neto CJ, de Deus Macedo JR, de Assis F, Neves R. Hepatitis C is less aggressive in hemodialysis patients than in nonuremic patients. Clin J Am Soc Nephrol. 2008;3:1385-1390. |
| 71. | Sterling RK, Sanyal AJ, Luketic VA, Stravitz RT, King AL, Post AB, Mills AS, Contos MJ, Shiffman ML. Chronic hepatitis C infection in patients with end stage renal disease: characterization of liver histology and viral load in patients awaiting renal transplantation. Am J Gastroenterol. 1999;94:3576-3582. |
| 72. | Pawa S, Ehrinpreis M, Mutchnick M, Janisse J, Dhar R, Siddiqui FA. Percutaneous liver biopsy is safe in chronic hepatitis C patients with end-stage renal disease. Clin Gastroenterol Hepatol. 2007;5:1316-1320. |
| 73. | Epidemiological data concerning end-stage renal failure. Evaluation, selection and preparation of the potential transplant recipient. Nephrol Dial Transplant. 2000;15:3-38. |
| 74. | Fabrizi F, Locatelli F. Combination of interferon alpha and ribavirin in the treatment of hepatitis C: implications for the clinical nephrologist. Nephrol Dial Transplant. 1999;14:2079-2081. |
| 75. | Glue P. The clinical pharmacology of ribavirin. Semin Liver Dis. 1999;19 Suppl 1:17-24. |
| 76. | Fabrizi F, Poordad FF, Martin P. Hepatitis C infection and the patient with end-stage renal disease. Hepatology. 2002;36:3-10. |
Peer reviewer: Frank J Burczynski, Professor, Faculty of Pharmacy, University of Manitoba, 50 Sifton Road, Winnipeg, Manitoba, R3T 2N2, Canada
S- Editor Li LF L- Editor Stewart GJ E- Editor Zheng XM