Published online Oct 7, 2009. doi: 10.3748/wjg.15.4720
Revised: August 22, 2009
Accepted: August 29, 2009
Published online: October 7, 2009
AIM: To investigate the correlation between the antifibrotic effect of baicalin and serum cytokine production in rat hepatic fibrosis.
METHODS: Forty male Sprague-Dawley rats were divided randomly into four groups: normal control group, model group, baicalin-treated group, and colchicine-treated group. Except for the normal control group, all rats in the other groups were administered with carbon tetrachloride to induce hepatic fibrosis. At the same time, the last two groups were also treated with baicalin or colchicine. At the end of the 8 wk, all animals were sacrificed. Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), transforming growth factor (TGF)-β1, tumor necrosis factor (TNF)-α, interleukin (IL)-6 and IL-10 were measured. Liver index, hepatic hydroxyproline content and the degree of liver fibrosis were also evaluated.
RESULTS: The levels of ALT, AST and liver index in the baicalin-treated group were markedly lower than those in the model group (ALT: 143.88 ± 14.55 U/L vs 193.58 ± 24.35 U/L; AST: 263.66 ± 44.23 U/L vs 404.37 ± 68.29 U/L; liver index: 0.033 ± 0.005 vs 0.049 ± 0.009, P < 0.01). Baicalin therapy also significantly attenuated the degree of hepatic fibrosis, collagen area and collagen area percentage in liver tissue (P < 0.01). Furthermore, the levels of serum TGFβ1, TNF-α and IL-6 were strikingly reduced in the baicalin-treated group compared with the model group, while the production of IL-10 was up-regulated: (TGF-β1: 260.21 ± 31.01 pg/mL vs 375.49 ± 57.47 pg/mL; TNF-α: 193.40 ± 15.18 pg/mL vs 260.04 ± 37.70 pg/mL; IL-6: 339.87 ± 72.95 pg/mL vs 606.47 ± 130.73 pg/mL; IL-10: 506.22 ± 112.07 pg/mL vs 316.95 ± 62.74 pg/mL, P < 0.01).
CONCLUSION: Baicalin shows certain therapeutic effects on hepatic fibrosis, probably by immunoregulating the imbalance between profibrotic and antifibrotic cytokines.
- Citation: Peng XD, Dai LL, Huang CQ, He CM, Chen LJ. Correlation between anti-fibrotic effect of baicalin and serum cytokines in rat hepatic fibrosis. World J Gastroenterol 2009; 15(37): 4720-4725
- URL: https://www.wjgnet.com/1007-9327/full/v15/i37/4720.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.4720
Hepatic fibrosis is a common pathological process of chronic liver injury, regardless of etiology, and its progression leads to cirrhosis and liver cancer[1]. Despite extensive efforts, its etiology and pathogenesis remain unclear, and effective therapy with limited side effects is still lacking[2]. Baicalin is a major bioactive flavonoid contained in dried roots of Scutellaria baicalensis Georgi (common name: Huangqin in China, a traditional Chinese herbal medicine) and it possesses a multitude of pharmacological activities. For instance, Baicalin exerts the inhibitory effects against several virus including influenza virus, human T cell leukemia virus and acquired human immunodeficiency virus type I[3-5]. It can act as potent anti-inflammatory, anti-allergic and anti-bacterial agent in a variety of inflammatory diseases[6-8]. It may also be potentially useful in the treatment of prostate and bladder cancers as well as hepatoma via multiple cellular mechanisms[9-11]. Most importantly, previous studies show that baicalin has significant scavenging effects on oxygen free radicals and protective effects on liver injury induced by iron overload and CCl4, suggesting that it is a potent free radical scavenger and hepatoprotective drug[12,13]. However, the exact mechanisms of anti-fibrotic effect of baicalin remain unclear. Therefore, it is necessary to be further elucidated.
CCl4-induced hepatic fibrosis is a well-established animal model to study the pathogenesis and therapy of chronic liver injury. Zhang et al[14] have reported that several profibrotic cytokines, including transforming growth factor (TGF)-β1, tumor necrosis factor (TNF)-α and interleukin (IL)-6, play an important role in the initiation and perpetuation of CCl4-induced liver fibrosis, whereas IL-10 plays an antifibrogenic role by counterbalancing the former effects .This study aimed to further investigate the effect of baicalin on hepatic fibrosis induced by CCl4 and its relationship with the expression of TGF-β1, TNF-α, IL-6 and IL-10.
Baicalin (7-glucuronic acid, 5,6-dihydroxyflavone, CAS No.: 21967-41-9) was provided by Sichuan Guanghan Bencao Plant Chemical Co., Ltd (Sichuan, China), and purity was assessed by HPLC (> 98%). Colchicine was purchased from Xiamen Sanland Chemical Co., Ltd (Fujian, China). CCl4 was obtained from Chongqing Chemical Reagent Co., Ltd (Chongqing, China). Male Sprague-Dawley (SD) rats weighing 150-180 g were purchased from the Experimental Animal Center of Third Military Medical University. All studies involving rats were approved by the Institutional Animal Care and Use Committee.
Forty male SD rats were divided randomly into four groups: normal (n = 9); model (n = 11); baicalin-treated (n = 10); and colchicine-treated (n = 10). Except for the normal control group, all rats in the other groups were treated with subcutaneous injection of 40% CCl4 (initial dose of 0.5 mL/kg, followed by 0.3 mL/kg), mixed with vegetable oil, twice weekly for 8 wk. The latter two groups were also treated with baicalin (70 mg/kg, dissolved in sterile saline water, intraperitoneal injection, once daily), or colchicine (50 μg/kg, dissolved in sterile saline water, intraperitoneal injection, once daily) on the same day as CCl4 administration and continued for the 8-wk experimental period. The two drug doses were selected based on a previous study[13]. Simultaneously, normal control and model groups were intraperitoneally administered with the same volume of vehicle (sterile saline water) once daily. At the end of the 8-wk experimental period, all animals were anesthetized with 3% chloral hydrate and dissected. Blood and liver were obtained for further analysis.
Serum AST and alanine aminotransferase (ALT) levels were measured using on an automated analyzer of biochemistry (Hitachi 7170, Tokyo, Japan) according to the manufacturer’s instructions.
Liver index was measured according to the formula: (rat liver weight/rat weight) × 100%[15].
Samples were obtained from the same liver lobe in all animals and fixed in 10% buffered formalin, embedded in paraffin, sectioned, and stained with hematoxylin-eosin (HE) or van gieson (VG) stain.
The degree of liver fibrosis was evaluated on HE-stained sections as described previously[16,17]. The collagen content of the sections was also determined on VG-stained sections by a computer image analysis system (CM2000B, Beijing University of Aeronautics & Astronautics, China). Five random fields were chosen in each section and the amount of total collagen was detected in the area stained by VG, and expressed as percentage relative to the total area[18].
Cytokine levels in the serum samples were measured by a commercially available ELISA kit (Biosources, San Jose, CA, USA) according to the manufacturer’s instructions.
Statistical analysis was performed with SPSS for Windows, version 13.0 (Chicago, IL, USA). Parametric data were analyzed statistically by one-way ANOVA followed by post-hoc tests when appropriate. Degree of hepatic fibrosis was analyzed by Kruskal-Wallis nonparametric test. Data were expressed as the means ± SD. A significant difference was defined as P < 0.05.
Irritability, aggression and weight loss were present predominantly in the model group. At the end of the 8-wk experimental period, no death was found in the normal control group. There were four deaths in the model group, one in the colchicine-treated group, and one in the baicalin-treated group.
Liver index in the normal control group was 0.026 ± 0.004. However, 8 wk after CCl4 injection, the liver index increased markedly. The increase was significantly attenuated by baicalin or colchicine treatment (P < 0.01, Table 1).
We then measured serum aminotransferase activity in different experimental groups. The levels of serum AST and ALT were significantly increased in the model group compared with those in the normal control group. In contrast, baicalin or colchicine treatment significantly suppressed upregulation of these parameters induced by CCl4 (P < 0.05 or P < 0.01, Table 1).
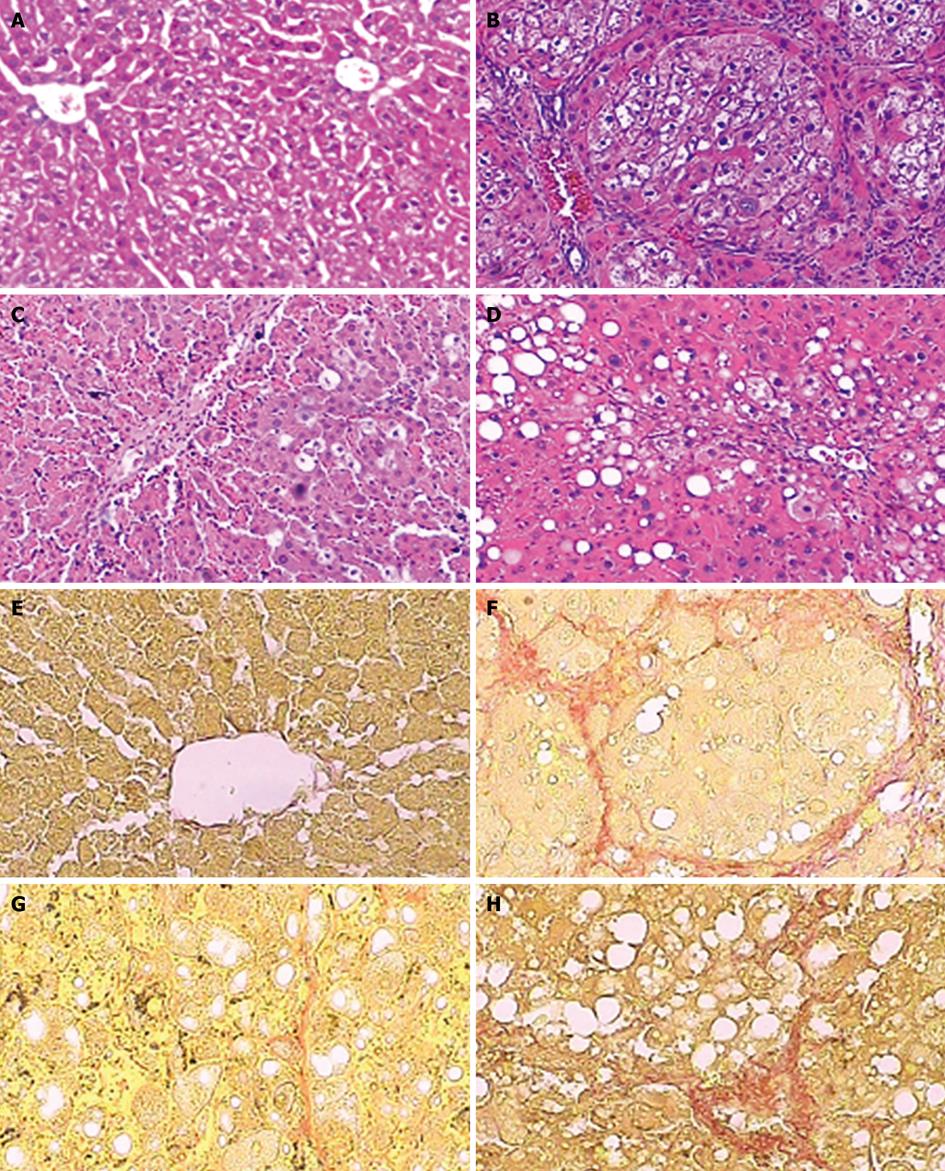
Using HE staining, we observed that the liver tissue in normal control rats showed normal lobular architecture with central veins and radiating hepatic cords. However, liver sections taken from rats in the model group exhibited more inflammatory infiltration, steatosis, hepatocyte coagulative necrosis and fibrous septa compared with the normal control rats after 8 wk of CCl4 treatment. In contrast, baicalin or colchicine treatment markedly ameliorated these histopathological changes (Figure 1A-D). The results were further supported by a significantly decreased staging score of hepatic fibrosis after baicalin or colchicine therapy (P < 0.01, Table 2).
We evaluated by VG staining the collagen level in the liver tissue from different treatment groups. Compared with the normal control group, both collagen area and collagen area percentage were significantly increased in the model group. The increases were reduced by baicalin or colchicine treatment, similar to the changes in hepatic histological examination (P < 0.01; Figure 1E-H, Table 3). Therefore, the above findings show that baicalin can prevent CCl4-induced hepatic fibrosis in rats.
As shown in Table 4, the levels of serum TGF-β1, TNF-α and IL-6 in the model group were significantly higher than those in the normal control group (P < 0.01). Upregulation was markedly inhibited by treatment with 70 mg/kg baicalin (P < 0.01). On the other hand, IL-10 production in the model group was sharply decreased compared with that in the normal control group (55% reduction, P < 0.01). However, baicalin therapy significantly recovered the decrease induced by CCl4 (P < 0.01).
In the present study, baicalin significantly lowered the levels of serum ALT, AST and liver index, reduced histological changes of liver fibrosis, suppressed the expression of cytokines, including TGF-β1, TNF-α and IL-6, and improved significantly the serum level of IL-10. Furthermore, our previous study showed that the increases of several fibrosis indices including serum hyaluronic acid, type IV collagen and hepatic hydroxyproline content after the CCl4 injection can be notablely inhibited by baicalin treatment[13]. The above findings demonstrated that baicalin can effectively prevent CCl4-induced hepatic fibrosis in rats and regulate the production of cytokines correlated with fibrosis.
Carbon tetrachloride (CCl4), is a known hepatotoxin that can cause liver necrosis, fibrosis and cirrhosis when administered repeatedly. Hepatotoxicity is thought to involve two phases[19,20]. The initial phase involves bioactivation by a microsomal cytochrome-P450-dependent monooxygenase system, which results in the formation of free radicals and oxidative stress/lipid peroxidation which exhibits the increase of malondialdehyde (MDA) amounts and decrease of superoxide dismutase (SOD) levels[19,21]. The second step involves the activation of Kupffer cells, which is accompanied by the production of profibrotic mediators such as TGF-β1, TNF-α and IL-6[22]. Hepatic stellate cells (HSC), activated by pro-fibrotic factors, lose vitamin A and transform into myofibroblasts (MFB), expressing α-smooth muscle actin (α-SMA) and thus gaining the function of contractibility, proliferation and fibrogenesis[23,24]. In this study, we observed that baicalin significantly reduced the increase in profibrotic cytokines such as TGF-β1, TNF-α and IL-6 induced by CCl4. The reduction in profibrotic cytokines may be correlated closely with previous results that baicalin has good radical scavenging action (lessening the MDA level and activating the SOD activity) and can thus reduce the production of activated Kupffer cells[12,13]. The down-regulation of pro-fibrotic cytokines induced by baicalin treatment then significantly inhibits the activation and proliferation of HSC and enhances HSC apoptosis in vitro or vivo studies[13], which results in the extenuation of hepatic fibrosis. Thus, the reduction of pro-fibrotic cytokines such as TGF-β1, TNF-α and IL-6 levels is one important mechanism associated with anti-fibrotic effect of baicalin.
IL-10 is a pluripotent cytokine produced by many activated immune cell types, including T-helper cells, B cells, macrophages, monocytes and keratinocytes[25]. Recent studies have indicated that IL-10 might play an important role in antifibrogenesis during CCl4-induced hepatic fibrogenesis[26-28]. Our study showed that the level of circulating IL-10 in the model group was lower than that in the normal control group, which was consistent with a previous study[14]. In contrast, baicalin significantly restored the decrease in IL-10 content induced by CCl4, probably contributing to the antifibrotic effect of baicalin.
In conclusion, baicalin has significant antifibrogenic effects on CCl4-induced liver fibrosis in rats. In addition to the inhibition of HSC activation and lipid peroxidation, as previously reported, immunoregulation of the imbalance between profibrotic and antifibrotic cytokines is one of the most important factors involved in the preventive effect of baicalin on CCl4-induced liver fibrosis. The exact molecular mechanisms remain to be explored.
Hepatic fibrosis is a common pathological process of chronic liver injuries, regardless of etiology, and its progression leads to cirrhosis and liver cancer. Despite extensive efforts, its etiology and pathogenesis remain unclear and effective therapies with limited side effects are still deficient. Baicalin is a flavonoid purified from the medicinal plant Scutellaria baicalensis Georgi, a well known Traditional Chinese Medicine. The previous studies show that baicalin has significant scavenging effects on oxygen free radicals and protective effects on liver injuries induced by iron overload and CCl4, however, its exact mechanisms remain unclear.
Baicalin is a flavonoid purified from the medicinal plant Scutellaria baicalensis Georgi, a well known Traditional Chinese Medicine. In the area of prevention of hepatic fibrosis with baicalin, one of research hotspots is that how is the actual mechanism of anti-fibrotic effect of baicalin.
The previous studies have shown that baicalin has significant scavenging effects on oxygen free radicals and protective effects on liver injuries induced by CCl4. In this study, the effect of baicalin on hepatic fibrosis induced by CCl4 and its relationship with the expression of pro-fibrotic and anti-fibrotic cytokines were first investigated. The levels of liver index and serum aminotransferases in baicalin-treated group were markedly lower than those in model group. Baicalin therapy also significantly attenuated the degree of hepatic fibrosis, collagen area and collagen area percent in liver tissue. Furthermore, the levels of serum transforming growth factor-β1, tumor necrosis factor-α and interleukin (IL)-6 were strikingly reduced in baicalin-treated group compared with model group while the production of IL-10 was up-regulated .The above results show that baicalin has certain therapeutic effects on hepatic fibrosis probably by immunoregulating the imbalance between pro-fibrotic and anti-fibrotic cytokines.
The study demonstrates that baicalin is a good hepatoprotective drug for preventing and treating human liver fibrosis probably by immunoregulating the imbalance between pro-fibrotic and anti-fibrotic cytokines.
Hepatic fibrosis is characterized by elevated deposition and altered composition of extracellular matrix, which is a common stage in most chronic liver injuries. Baicalin is a bioactive anti-inflammatory flavone purified from the medicinal plant Scutellaria baicalensis Georgi, a well known Traditional Chinese Medicine.
The authors analyzed the preventative effects and mechanism of baicalin in the treatment of liver fibrosis in rats. The results are interesting and suggest that baicalin may be a clinical useful agent for preventing and treating human liver fibrosis.
| 1. | Alcolado R, Arthur MJ, Iredale JP. Pathogenesis of liver fibrosis. Clin Sci (Lond). 1997;92:103-112. |
| 2. | Lotersztajn S, Julien B, Teixeira-Clerc F, Grenard P, Mallat A. Hepatic fibrosis: molecular mechanisms and drug targets. Annu Rev Pharmacol Toxicol. 2005;45:605-628. |
| 3. | Baylor NW, Fu T, Yan YD, Ruscetti FW. Inhibition of human T cell leukemia virus by the plant flavonoid baicalin (7-glucuronic acid, 5,6-dihydroxyflavone). J Infect Dis. 1992;165:433-437. |
| 4. | Li BQ, Fu T, Dongyan Y, Mikovits JA, Ruscetti FW, Wang JM. Flavonoid baicalin inhibits HIV-1 infection at the level of viral entry. Biochem Biophys Res Commun. 2000;276:534-538. |
| 5. | Zeng Y, Song C, Ding X, Ji X, Yi L, Zhu K. Baicalin reduces the severity of experimental autoimmune encephalomyelitis. Braz J Med Biol Res. 2007;40:1003-1010. |
| 6. | Lin CC, Shieh DE. The anti-inflammatory activity of Scutellaria rivularis extracts and its active components, baicalin, baicalein and wogonin. Am J Chin Med. 1996;24:31-36. |
| 7. | Kubo M, Matsuda H, Tanaka M, Kimura Y, Okuda H, Higashino M, Tani T, Namba K, Arichi S. Studies on Scutellariae radix. VII. Anti-arthritic and anti-inflammatory actions of methanolic extract and flavonoid components from Scutellariae radix. Chem Pharm Bull (Tokyo). 1984;32:2724-2729. |
| 8. | Zhang XP, Tian H, Lai YH, Chen L, Zhang L, Cheng QH, Yan W, Li Y, Li QY, He Q. Protective effects and mechanisms of Baicalin and octreotide on renal injury of rats with severe acute pancreatitis. World J Gastroenterol. 2007;13:5079-5089. |
| 9. | Ikezoe T, Chen SS, Heber D, Taguchi H, Koeffler HP. Baicalin is a major component of PC-SPES which inhibits the proliferation of human cancer cells via apoptosis and cell cycle arrest. Prostate. 2001;49:285-292. |
| 10. | Ikemoto S, Sugimura K, Yoshida N, Yasumoto R, Wada S, Yamamoto K, Kishimoto T. Antitumor effects of Scutellariae radix and its components baicalein, baicalin, and wogonin on bladder cancer cell lines. Urology. 2000;55:951-955. |
| 11. | Motoo Y, Sawabu N. Antitumor effects of saikosaponins, baicalin and baicalein on human hepatoma cell lines. Cancer Lett. 1994;86:91-95. |
| 12. | Zhang Y, Li H, Zhao Y, Gao Z. Dietary supplementation of baicalin and quercetin attenuates iron overload induced mouse liver injury. Eur J Pharmacol. 2006;535:263-269. |
| 13. | Li X, Peng XD, Zhang WL, Dai LL. [Inhibiting effects of denshensu, baicalin, astragalus and Panax notoginseng saponins on hepatic fibrosis and their possible mechanisms]. Zhonghua Ganzangbing Zazhi. 2008;16:193-197. |
| 14. | Zhang LJ, Yu JP, Li D, Huang YH, Chen ZX, Wang XZ. Effects of cytokines on carbon tetrachloride-induced hepatic fibrogenesis in rats. World J Gastroenterol. 2004;10:77-81. |
| 15. | Yang Q, Xie RJ, Luo XH, Han B, Yang T, Fang L, Cheng ML. [Expression of PKC in rat hepatic fibrosis and the effect of Dan-shao-hua-xian Capsule on its expression pattern]. Zhonghua Ganzangbing Zazhi. 2005;13:707-708. |
| 16. | Brunt EM. Grading and staging the histopathological lesions of chronic hepatitis: the Knodell histology activity index and beyond. Hepatology. 2000;31:241-246. |
| 17. | The infectious and parasitic diseases branchs of China Medical Association. A viral hepatitis prevention and control program. Zhonghua Ganzangbing Zazhi. 2000;8:324-329. |
| 18. | Cheung PY, Zhang Q, Zhang YO, Bai GR, Lin MC, Chan B, Fong CC, Shi L, Shi YF, Chun J. Effect of WeiJia on carbon tetrachloride induced chronic liver injury. World J Gastroenterol. 2006;12:1912-1917. |
| 19. | Kamalakkannan N, Rukkumani R, Varma PS, Viswanathan P, Rajasekharan KN, Menon VP. Comparative effects of curcumin and an analogue of curcumin in carbon tetrachloride-induced hepatotoxicity in rats. Basic Clin Pharmacol Toxicol. 2005;97:15-21. |
| 20. | Armendariz-Borunda J, Seyer JM, Kang AH, Raghow R. Regulation of TGF beta gene expression in rat liver intoxicated with carbon tetrachloride. FASEB J. 1990;4:215-221. |
| 21. | Koch RR, Glende EA Jr, Recknagel RO. Hepatotoxicity of bromotrichloromethane--bond dissociation energy and lipoperoxidation. Biochem Pharmacol. 1974;23:2907-2915. |
| 22. | Edwards MJ, Keller BJ, Kauffman FC, Thurman RG. The involvement of Kupffer cells in carbon tetrachloride toxicity. Toxicol Appl Pharmacol. 1993;119:275-279. |
| 23. | Gressner AM. Cytokines and cellular crosstalk involved in the activation of fat-storing cells. J Hepatol. 1995;22:28-36. |
| 24. | Hellemans K, Rombouts K, Quartier E, Dittié AS, Knorr A, Michalik L, Rogiers V, Schuit F, Wahli W, Geerts A. PPARbeta regulates vitamin A metabolism-related gene expression in hepatic stellate cells undergoing activation. J Lipid Res. 2003;44:280-295. |
| 25. | Goldman M, Velu T. Interleukin-10 and its implications for immunopathology. Adv Nephrol Necker Hosp. 1995;24:79-90. |
| 26. | Thompson K, Maltby J, Fallowfield J, McAulay M, Millward-Sadler H, Sheron N. Interleukin-10 expression and function in experimental murine liver inflammation and fibrosis. Hepatology. 1998;28:1597-1606. |
| 27. | Louis H, Van Laethem JL, Wu W, Quertinmont E, Degraef C, Van den Berg K, Demols A, Goldman M, Le Moine O, Geerts A. Interleukin-10 controls neutrophilic infiltration, hepatocyte proliferation, and liver fibrosis induced by carbon tetrachloride in mice. Hepatology. 1998;28:1607-1615. |
| 28. | Nelson DR, Lauwers GY, Lau JY, Davis GL. Interleukin 10 treatment reduces fibrosis in patients with chronic hepatitis C: a pilot trial of interferon nonresponders. Gastroenterology. 2000;118:655-660. |
Peer reviewer: Sun-Lung Tsai, MD, PhD, Professor, Director, Hepatogastroenterology Section, Department of Internal Medicine and Liver Research Unit, Department of Medical Research, Chi Mei Medical Center, 901 Chung Hwa Road, Young-Kang City, Tainan County 710, Taiwan, China
S- Editor Li LF L- Editor Kerr C E- Editor Yin DH