Published online Sep 21, 2009. doi: 10.3748/wjg.15.4439
Revised: August 14, 2009
Accepted: August 21, 2009
Published online: September 21, 2009
AIM: To investigate the effect of Chai-Qin-Cheng-Qi Decoction (CQCQD) on cefotaxime (CTX) concentration in pancreas of rats with acute necrotizing pancreatitis (ANP).
METHODS: Sixty healthy male Sprague-Dawley rats were divided randomly into an ANP group (ANP model + CTX, n = 20), treatment group (ANP model + CTX + CQCQD, n = 20) and control group (normal rats + CTX, n = 20). ANP models were induced by retrograde intraductal injection of 3.5% sodium taurocholate (1 mL/kg), and the control group was injected intraductally with normal saline. All rats were injected introperitoneally with 0.42 g/kg CTX (at 12-h intervals for a continuous 72 h) at 6 h after intraductal injection. Meanwhile, the treatment group received CQCQD (20 mL/kg) intragastrically at 8-h intervals, and the ANP and control group were treated intragastrically with normal saline. At 15 min after the last CTX injection, blood and pancreas samples were collected for the determination of CTX concentration using validated high-performance liquid chromatography. Pathological changes and wet-to-dry-weight (W/D) ratio of pancreatic tissue were examined.
RESULTS: Serum CTX concentrations in three groups were not significantly different. Pancreatic CTX concentration and penetration ratio were lower in ANP group vs control group (4.4 ± 0.6 μg/mL vs 18.6 ± 1.7 μg/mL, P = 0.000; 5% vs 19%, P = 0.000), but significantly higher in treatment group vs ANP group (6.4 ± 1.7 μg/mL vs 4.4 ± 0.6 μg/mL, P = 0.020; 7% vs 5%, P = 0.048). The histological scores and W/D ratio were significantly decreased in treatment group vs ANP and control group.
CONCLUSION: CQCQD might have a promotive effect on CTX concentration in pancreatic tissues of rats with ANP.
- Citation: Deng LH, Xiang DK, Xue P, Zhang HY, Huang L, Xia Q. Effects of Chai-Qin-Cheng-Qi Decoction on cefotaxime in rats with acute necrotizing pancreatitis. World J Gastroenterol 2009; 15(35): 4439-4443
- URL: https://www.wjgnet.com/1007-9327/full/v15/i35/4439.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.4439
| Pathological grading | Scores |
| Edema | |
| Absent | 0 |
| Focal expansion of interlobular septae | 1 |
| Same as 1 + diffuse expansion of interlobar; | 2 |
| septae/diffuse expansion of interlobar septae | |
| Same as 2 + expansion of interacinar septae | 3 |
| Same as 3 + expansion of intercellular spaces | 4 |
| Inflammation and perivascular infiltrate (intralobular or perivascular leukocytes/HPF) | |
| 0-1 | 0 |
| 2-10 | 1 |
| 11-20 | 2 |
| 21-30 | 3 |
| > 30 or microabscesses | 4 |
| Acinar necrosis (necrotic cells/HPF) | |
| Absent | 0 |
| 1-4 | 1 |
| 5-10 | 2 |
| 11-16 | 3 |
| > 16 | 4 |
| Hemorrhage and fat necrosis (focus) | |
| Absent | 0 |
| 1-2 | 1 |
| 3-4 | 2 |
| 5-6 | 3 |
| > 7 | 4 |
Acute necrotizing pancreatitis (ANP) accounts for approximately 20% of patients with acute pancreatitis. When complicated by infection, pancreatic necrosis becomes a major contributor to late mortality[1-3]. Early broad-spectrum antibiotics in the initial stage of disease appear to be associated with significantly decreased mortality and therefore improve outcome[4]. The choice of the antibiotics must be driven by the flora as well as penetration in the pancreatic tissue[5-7]. Cefotaxime (CTX), a semisynthetic broad-spectrum cephalosporin antibiotic, has an active penetrability and sustains an effective anti-bactericidal concentration in human pancreatic tissue[8-10].
An important criterion in determining the most appropriate antibiotic treatment for pancreatitis is antibiotic tissue concentration in the pancreas, which varies during the progression of ANP and is mainly influenced by the changes in pancreatic tissue morphology and capillary blood flow. Previous studies have shown that the improvement of microcirculation and local infusion of the pancreas by regional arterial infusion can improve antibiotic concentrations in pancreatic tissue and reduce the pancreatic infection rate and mortality[11,12].
Chai-Qin-Cheng-Qi Decoction (CQCQD) is modified from Da-Cheng-Qi Decoction (Cortex Magnoliae Officinalis, Fructus Aurantii Immaturus, Radix et Rhizoma Rhei and Natrii Sulfas), which is a well-known and popular traditional Chinese medicine that is used as a purgative in China and East Asia. In recent decades, CQCQD has been used widely for pancreatitis[13]. Generally, CQCQD plays a multi-target and comprehensive role in ANP, but the exact therapeutic mechanism is still unclear.
In the present study, we performed validated high-pressure liquid chromatography (HPLC) to determinate the changes in CTX concentration and penetration ratio in pancreatic tissue of rats with ANP after oral administration of CQCQD. Our hypothesis was that CQCQD would increase CTX concentration and penetration rate in pancreatic tissue of ANP rats.
Sixty healthy male Sprague-Dawley rats weighting 250-300 g were purchased from the Experimental Animal Center of West China Center of Medical Sciences of Sichuan University [SCXK (Chuan-11-2006)]. The animals were kept at constant room temperature with a 12-h light-dark cycle, free access to water and standard laboratory chow, and adjusted to laboratory conditions for 1 wk with a 12-h fast but free access to drinking water before the experiments. Animal experiments and performance of this study were approved by the Animal Care Committee of Sichuan University.
CQCQD is composed of Chaihu (Radix Bupleuri) 15 g, Huangqin (Radix Scutellariae) 15 g, Houpo (Cortex Magnoliae Officinalis) 15 g, Zhishi (Fructus Aurantii Immaturus) 15 g, Yinchen (Herba Artemisiae Capillaris) 15 g, Zhizi (Fructus Gardeniae) 20 g, Dahuang (Radix et Rhizoma Rhei) 15 g, and Mangxiao (Natrii Sulfas) 10 g. All high-quality medicinal herbs were provided, prepared to decoction, vaporized and dried into lyophilized powder of CQCQD by Herbal Pharmacy of West China Hospital, Sichuan University, China. The lyophilized powder was stored at 4°C and freshly dissolved in drinking water (a raw herbal concentration of 2 g/mL) prior to use.
CTX sodium for injection (Claforan®) was a product of NCPC Create Pharma Co., Ltd., China. Sodium taurocholate powder was purchased from Sigma (T-0750; St. Louis, MO, USA). All other reagents were of the highest purity available.
Rats were randomized divided into an ANP group (ANP model + CTX, n = 20), treatment group (ANP model + CTX + CQCQD, n = 20) and control group (normal rats + CTX, n = 20). After anesthesia with intraperitoneal injection of 40 mg/kg sodium pentobarbital, ANP models were induced by retrograde intraductal injection of 3.5% sodium taurocholate (1 mL/kg) using a micropump, within 5 min. Rats in the control group were administered with the same volume of normal saline. Six hours after retrograde injection, all animals were injected introperitoneally with 0.42 g/kg CTX at 12-h intervals for a continuous 72 h; at the same time, rats in the treatment group were treated intragastrically with 20 mL/kg CQCQD at 8-h intervals, and the ANP group was given the same volume of normal saline as the positive control.
Fifteen minutes after the last CTX injection, blood samples were collected via the inferior vena cava and centrifuged at 3600 r/min for 5 min at 4°C. The serum was kept at -70°C. Pancreatic tissues were removed quickly and stored immediately in liquid nitrogen at -70°C. Histopathological examination was also performed.
Prior to HPLC, pancreatic tissues were thawed and centrifuged at 2500 r/min for 5 min. HPLC was performed as follows: 100 μL samples were mixed with 100 μL water and 50 μL internal standard, and then mixed with 500 μL methanol. After centrifugation at 13 000 r/min for 15 min at 16°C, the filtrate was evaporated under an air stream in a 40°C water bath. The residue was dissolved in 200 μL mobile phase with methanol/0.05 mol/L phosphate buffer (18/82: v/v), and 50 μL of the solution was injected into the HPLC system. The chromatographic separation was performed on a YMC-Pack ODS-A C18 column (5 μm, 150 mm × 4.6 mm). The flow rate was 0.8 mL/min. The retention time of CTX and the internal standard was 14.32 and 8.97 min, respectively. The detection was set at a wavelength of 254 nm.
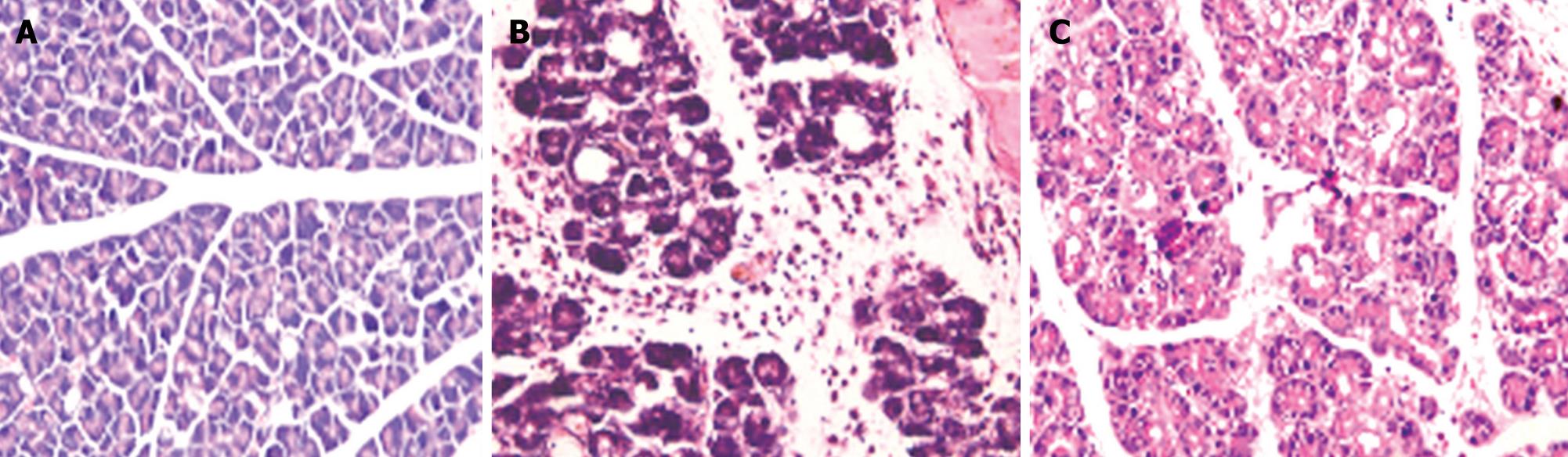
Pancreatic edema, inflammatory infiltration, hemorrhage and acinar cell necrosis were evaluated and scored according to the criteria shown in Table 1[14]. For each pathological section, 10 visual fields under a high-power microscope [hematoxylin and eosin (HE) stain, ×400] were selected randomly and scored by one single investigator (Xiang DK). The mean score of 10 visual fields from one pathological section was calculated as the pathological score.
We weighed the freshly collected pancreas samples in the wet state and incubated them at 210°C for 12 h, and the wet weight and dry weights were measured. The W/D ratio was used to represent water content in the pancreas.
Data were expressed as mean ± SD or percentage. P value was calculated using ANOVA test for multiple comparisons. P < 0.05 was considered statistically significant.
CTX concentration in serum (CS, μg/mL) and pancreatic tissue (CP, μg/g), as well as CTX penetration ratio (CP/CS × 100%, %) are shown in Table 2. Three groups had no significant differences in serum CTX concentration (P > 0.05). CTX concentration in pancreatic tissue (P < 0.001) and CTX penetration ratio (P < 0.001) were significantly lower in the ANP group versus the control group. Compared with the ANP group, the treatment group had a significantly higher CTX concentration in pancreatic tissue (P < 0.05) and CTX penetration ratio (P < 0.05).
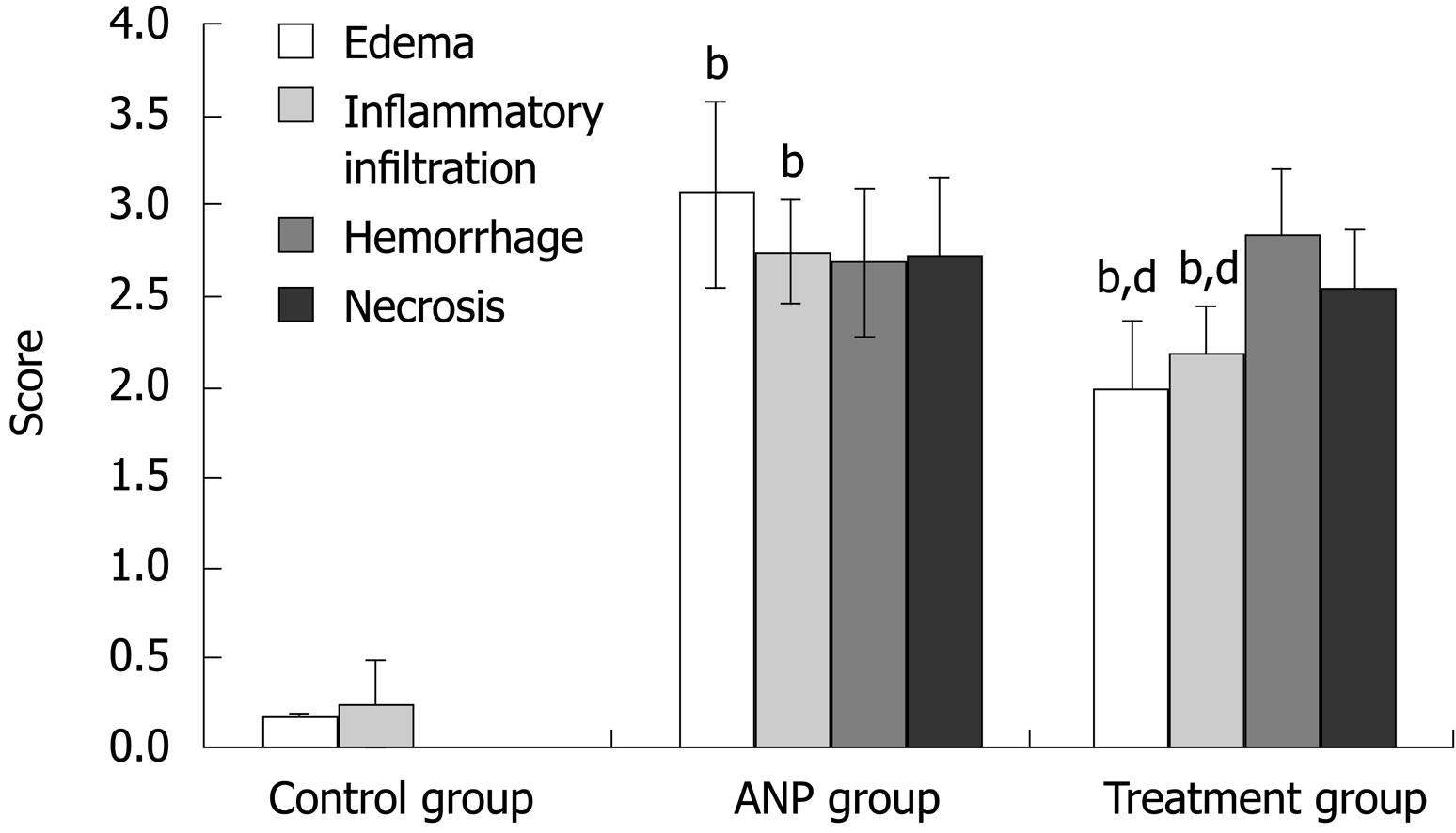
Histopathological changes in pancreatic tissues are shown in Figure 1. Comparison between the treatment and ANP groups indicated that scores for edema and inflammatory infiltration were significantly lower in the former (P < 0.001), although the differences in scores for hemorrhage and necrosis were not significant. Compared with the control group, pathological scores were significantly higher in the ANP and treatment groups (P < 0.001) (Figure 2).
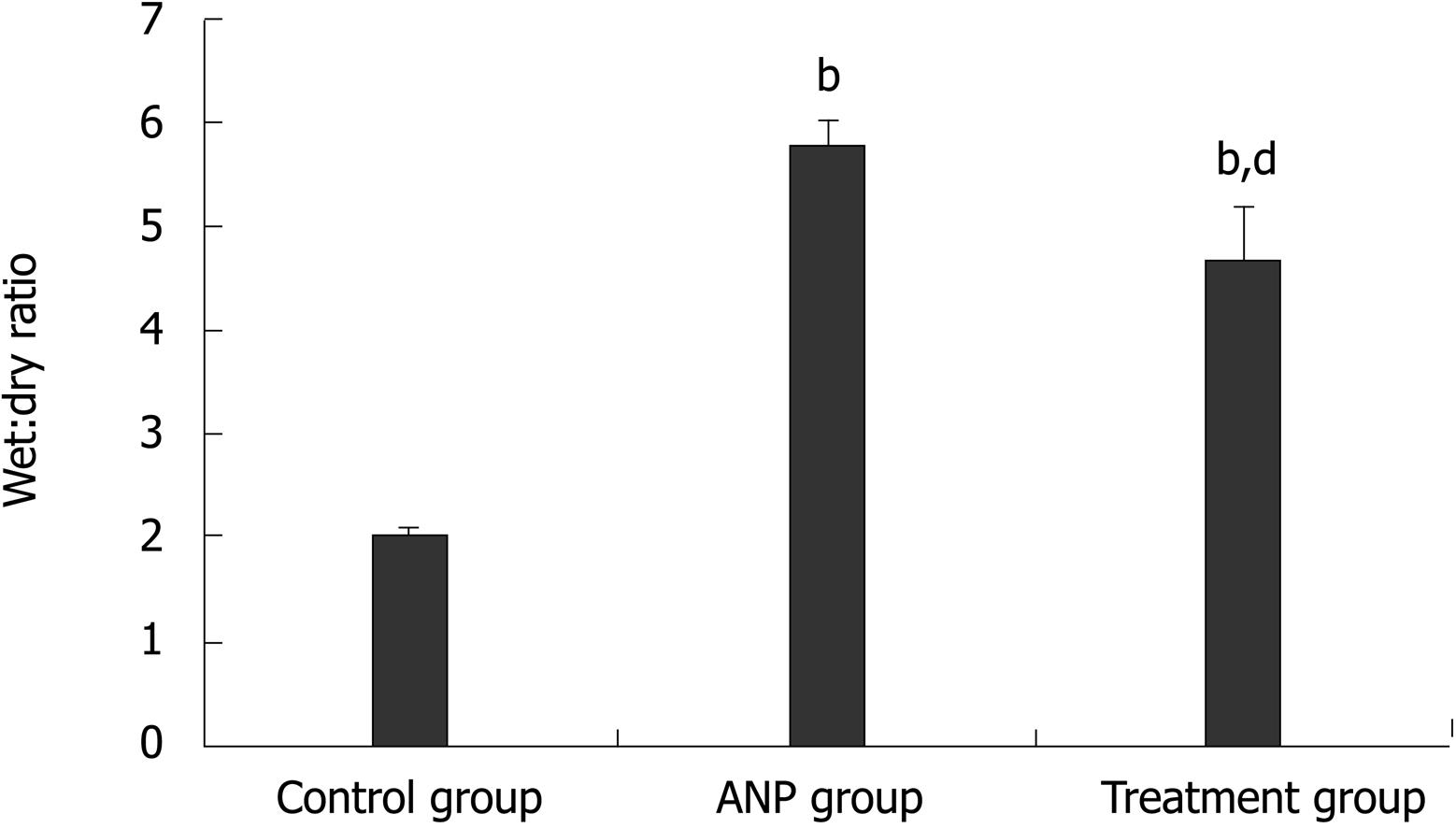
W/D ratio was higher in the ANP group than the control group (7.1 ± 1.7 vs 1.45 ± 0.1, P < 0.001). Although significantly higher than that in the control group (4.5 ± 1.5 vs 1.45 ± 0.1, P < 0.001), W/D ratio in the treatment group was significantly lower than that in the ANP group (4.5 ± 1.5 vs 7.1 ± 1.7, P < 0.01) (Figure 3).
Previous studies have reported that pancreatic perfusion decreases the progression of pancreatic necrosis[15,16]. Early antibiotic therapy may be favorable for infection of the pancreas with enteric microorganisms in the course of ANP[17,18]. Based on pharmacokinetic and bacteriological studies of the efficacy of different antibiotics in ANP, a consensus has emerged that antibiotics in ANP must not only cover the spectrum of bacteria usually found in necrotic pancreatic tissue (including anaerobes), but also penetrate into the pancreas with a sustainable anti-bactericidal concentration. The pancreatic concentration of antibiotic is determined by the pathophysiological condition of the pancreas, alkaline pH, high ion concentration, enzymatic and hormonal regulation, pathological changes, and infection[19]. Promoters of pancreatic perfusion and microcirculation might enhance the therapeutic effects against infection by increasing antibiotic tissue concentrations.
HPLC analysis showed that CTX concentration in healthy rats was as high as 98.9 ± 2.4 μg/mL in serum, but reduced to 18.6 ± 1.7 μg/mL in pancreatic tissue (CTX penetration ratio in normal rats was 19%). These results indicate that the distribution of antibiotics is restricted by the existence of a blood-pancreatic juice barrier[20]. Furthermore, the significantly reduced CTX concentration in pancreatic tissue and penetration ratio were detected in the ANP group more than in the control group (P < 0.001), as found by other investigators[11]. The results also showed significant higher pancreatic tissue concentration and penetration ratio of CTX in the treatment group, which confirmed our hypothesis that CQCQD treatment could improve antibiotic concentration and penetration ratio in pancreatic tissue of rats with ANP.
Meanwhile, pathological scoring confirmed that CQCQD improved the severity of interstitial edema, inflammatory infiltration, hemorrhage and acinar cell necrosis. The significantly reduced W/D ratio of pancreatic tissue in the treatment group confirmed that CQCQD alleviated the severity of parenchymal edema.
Although we found a promotive effect of CQCQD on CTX tissue concentration in rats with ANP, the exact mechanism remains unclear. Some previous studies have demonstrated that prescriptions composed of Dahuang, Mangxiao, Zhishi and Houpo increase the blood flow of most abdominal organs during peritonitis, reduce inflammatory exudate and lesions, inhibit the “waterfall effect” of cytokines, and therefore protect the target organ[21-23]. We speculate that the mechanism of action of CQCQD on antibiotics pancreatic concentrations in ANP rats may be related to promotion of pancreatic microcirculation, increasing local perfusion and blood flow of the pancreas, improving pancreatic tissue edema, and reducing anti-inflammatory responses[24-29].
There are some methodological aspects that should be taken into consideration when interpreting the results. (1) The ANP model was induced by intraductal retrograde injection of sodium taurocholate, which could closely mimic the etiology, pathogenesis and pathology of severe human pancreatitis, and is therefore the current standard and perfect modeling method with good reproducibility and comparability[30,31]; (2) The first CTX injection was at 6 h after induction of ANP, because it represented early antibiotic therapy in the initial stage of the disease, and the protocols for CTX administration were adopted from previous studies in small rodents[17], as well as from our clinical protocol; (3) Seventy-two hours after modeling was chosen as the measurement time-point when pancreatic necrosis was fully developed and when stable concentrations of CTX and CQCQD would be available in the blood.
In summary, the results of the present study validate the hypothesis that CQCQD increases pancreatic tissue concentration and penetration ratio of CTX in rats with ANP, through the possible mechanism of eliminating pancreatic inflammation and edema. Thus, the study might form the basis of advanced studies on the associated therapeutic mechanisms of Chinese medicine in ANP.
Infection in pancreatic necrosis is still regarded as the most serious complication of acute necrotizing pancreatitis (ANP), with a morbidity of 16%-47%, which has not substantially changed during the past two decades, despite of strenuous efforts and rapid progress in treatment. Early broad-spectrum antibiotics in the initial stage of the disease appear to decrease significantly mortality and improve outcome. Based on pharmacokinetic and bacteriological studies of the efficacy of different antibiotics, antibiotics used in patient with ANP must not only cover the spectrum of bacteria usually found in necrotic pancreatic tissue (including anaerobes), but also penetrate into the pancreas with a sustainable anti-bactericidal concentration.
Pancreatic perfusion and microcirculation are decreased in ANP, therefore, intravenous antibiotics might not reach an effective anti-bactericidal concentration in the pancreas. Promoters of pancreatic perfusion and microcirculation might enhance the therapeutic effects against infection by increasing antibiotic tissue concentrations.
The therapeutic mechanisms of Chinese medicines are characterized by multiple targets and complicated networks. The innovations and breakthroughs of the present study are the linkage between antibiotic tissue concentration and Chai-Qin-Cheng-Qi Decoction (CQCQD), which may explain the therapeutic mechanisms of Chinese medicine prescribed for ANP. The study methods and results might provide the basis for advanced studies in this field.
Acute pancreatitis is an acute inflammatory process of the pancreas that frequently involves peripancreatic tissues, and at times, remote organ systems. Pancreatic necrosis is defined as one or more areas of nonviable pancreatic parenchyma, and is associated usually with peripancreatic fat necrosis.
In this pilot study, the authors explored the effect of CQCQD on cefotaxime concentration in pancreatic tissue of rats with ANP, using validated high-performance liquid chromatography. The results indicated that CQCQD has a promotive effect on cefotaxime concentration in pancreatic tissues of rats with ANP. The study might elucidate the associated therapeutic mechanisms of Traditional Chinese Medicine in ANP.
Peer reviewer: Parimal Chowdhury, Professor, Department of Physiology and Biophysics, College of Medicine University of Arkansas for Medical Sciences, 4301 W Markham Street, Little Rock, Arkansas 72205, United States
S- Editor Tian L L- Editor Kerr C E- Editor Ma WH
| 1. | Schmid SW, Uhl W, Friess H, Malfertheiner P, Buchler MW. The role of infection in acute pancreatitis. Gut. 1999;45:311-316. |
| 2. | Buchler MW, Gloor B, Muller CA, Friess H, Seiler CA, Uhl W. Acute necrotizing pancreatitis: treatment strategy according to the status of infection. Ann Surg. 2000;232:619-626. |
| 3. | Isenmann R, Beger HG. Natural history of acute pancreatitis and the role of infection. Baillieres Best Pract Res Clin Gastroenterol. 1999;13:291-301. |
| 4. | Sainio V, Kemppainen E, Puolakkainen P, Taavitsainen M, Kivisaari L, Valtonen V, Haapiainen R, Schroder T, Kivilaakso E. Early antibiotic treatment in acute necrotising pancreatitis. Lancet. 1995;346:663-667. |
| 5. | Heinrich S, Schafer M, Rousson V, Clavien PA. Evidence-based treatment of acute pancreatitis: a look at established paradigms. Ann Surg. 2006;243:154-168. |
| 6. | Toouli J, Brooke-Smith M, Bassi C, Carr-Locke D, Telford J, Freeny P, Imrie C, Tandon R. Guidelines for the management of acute pancreatitis. J Gastroenterol Hepatol. 2002;17 Suppl:S15-S39. |
| 7. | Villatoro E, Bassi C, Larvin M. Antibiotic therapy for prophylaxis against infection of pancreatic necrosis in acute pancreatitis. Cochrane Database Syst Rev. 2006;17 Suppl:CD002941. |
| 8. | Foitzik T, Hotz HG, Kinzig M, Sorgel F, Buhr HJ. Influence of changes in pancreatic tissue morphology and capillary blood flow on antibiotic tissue concentrations in the pancreas during the progression of acute pancreatitis. Gut. 1997;40:526-530. |
| 9. | Lankisch PG, Klesel N, Seeger K, Seidel G, Winckler K. Penetration of cefotaxime into the pancreas. Z Gastroenterol. 1983;21:601-603. |
| 10. | Buchler M, Malfertheiner P, Friess H, Isenmann R, Vanek E, Grimm H, Schlegel P, Friess T, Beger HG. Human pancreatic tissue concentration of bactericidal antibiotics. Gastroenterology. 1992;103:1902-1908. |
| 11. | Donatini B. A systematic study of the vascularisation of the pancreas. Surg Radiol Anat. 1990;12:173-180. |
| 12. | Pederzoli P, Bassi C, Vesentini S, Campedelli A. A randomized multicenter clinical trial of antibiotic prophylaxis of septic complications in acute necrotizing pancreatitis with imipenem. Surg Gynecol Obstet. 1993;176:480-483. |
| 13. | Liu XB, Jiang JM, Huang ZW, Tian BL, Hu WM, Xia Q, Chen GY, Li QS, Yuan CX, Luo CX. [Clinical study on the treatment of severe acute pancreatitis by integrated traditional Chinese medicine and Western medicine]. Sichuan Daxue Xuebao Yixueban. 2004;35:204-208. |
| 14. | Rongione AJ, Kusske AM, Kwan K, Ashley SW, Reber HA, McFadden DW. Interleukin 10 reduces the severity of acute pancreatitis in rats. Gastroenterology. 1997;112:960-967. |
| 15. | Bassi D, Kollias N, Fernandez-del Castillo C, Foitzik T, Warshaw AL, Rattner DW. Impairment of pancreatic microcirculation correlates with the severity of acute experimental pancreatitis. J Am Coll Surg. 1994;179:257-263. |
| 16. | Klar E, Schratt W, Foitzik T, Buhr H, Herfarth C, Messmer K. Impact of microcirculatory flow pattern changes on the development of acute edematous and necrotizing pancreatitis in rabbit pancreas. Dig Dis Sci. 1994;39:2639-2644. |
| 17. | Foitzik T, Mithofer K, Ferraro MJ, Fernandez-del Castillo C, Lewandrowski KB, Rattner DW, Warshaw AL. Time course of bacterial infection of the pancreas and its relation to disease severity in a rodent model of acute necrotizing pancreatitis. Ann Surg. 1994;220:193-198. |
| 18. | Foitzik T, Fernandez-del Castillo C, Ferraro MJ, Mithofer K, Rattner DW, Warshaw AL. Pathogenesis and prevention of early pancreatic infection in experimental acute necrotizing pancreatitis. Ann Surg. 1995;222:179-185. |
| 19. | Beger HG, Bittner R, Block S, Buchler M. Bacterial contamination of pancreatic necrosis. A prospective clinical study. Gastroenterology. 1986;91:433-438. |
| 20. | Burns GP, Stein TA, Kabnick LS. Blood-pancreatic juice barrier to antibiotic excretion. Am J Surg. 1986;151:205-208. |
| 21. | Qi QH, Wang J, Liang GG, Wu XZ. Da-Cheng-Qi-Tang promotes the recovery of gastrointestinal motility after abdominal surgery in humans. Dig Dis Sci. 2007;52:1562-1570. |
| 22. | Zhang MJ, Zhang GL, Yuan WB, Ni J, Huang LF. Treatment of abdominal compartment syndrome in severe acute pancreatitis patients with traditional Chinese medicine. World J Gastroenterol. 2008;14:3574-3578. |
| 23. | Zhao YQ, Liu XH, Ito T, Qian JM. Protective effects of rhubarb on experimental severe acute pancreatitis. World J Gastroenterol. 2004;10:1005-1009. |
| 24. | Tang WF, Yu Q, Wan MH, Qin F, Wang YG, Chen GY, Liang MZ, Huang X. Simultaneous determination and pharmacokinetic studies of aloe emodin and chrysophanol in rats after oral administration of Da-Cheng-Qi decoction by high-performance liquid chromatography. Biomed Chromatogr. 2007;21:701-707. |
| 25. | Tang WF, Huang X, Yu Q, Qin F, Wan MH, Wang YG, Liang MZ. Determination and pharmacokinetic comparison of rhein in rats after oral dosed with Da-Cheng-Qi decoction and Xiao-Cheng-Qi decoction. Biomed Chromatogr. 2007;21:1186-1190. |
| 26. | Deng LH, Yang XN, Huang L, Xiang DK, Xia Q. [Effects of Chaiqinchengqi decoction on exocrine function of pancreatic acinar cells of acute pancreatitis rats]. Sichuan Daxue Xuebao Yixueban. 2008;39:555-557, 566. |
| 27. | Xue P, Deng LH, Zhang ZD, Yang XN, Xia Q, Xiang DK, Huang L, Wan MH. Effect of Chaiqinchengqi decoction on sarco/endoplasmic reticulum Ca2+-ATPase mRNA expression of pancreatic tissues in acute pancreatitis rats. World J Gastroenterol. 2008;14:2343-2348. |
| 28. | Zhang XP, Shi Y, Zhang L. Progress in the study of therapeutic effects of traditional Chinese medicine and extracts in treating severe acute pancreatitis. JOP. 2007;8:704-714. |
| 29. | Zhang XP, Liu DR, Shi Y. Study progress in therapeutic effects of traditional Chinese medicine monomer in severe acute pancreatitis. J Zhejiang Univ Sci B. 2007;8:147-152. |
| 30. | Schmidt J, Rattner DW, Lewandrowski K, Compton CC, Mandavilli U, Knoefel WT, Warshaw AL. A better model of acute pancreatitis for evaluating therapy. Ann Surg. 1992;215:44-56. |
| 31. | Chen CC, Wang SS, Tsay SH, Lee FY, Wu SL, Lu RH, Lee SD. A model of experimental acute necrotizing pancreatitis. Zhonghua Yixue Zazhi (Taipei). 1995;56:373-379. |