Published online Sep 21, 2009. doi: 10.3748/wjg.15.4415
Revised: August 21, 2009
Accepted: August 28, 2009
Published online: September 21, 2009
AIM: To assess the use of capecitabine-based therapy and associated complication rates in patients with gastroesophageal cancer (GEC) in a real-world treatment setting.
METHODS: Patients with claims between 2004 and 2005 were identified from the Thomson Reuters MarketScan® databases. Capecitabine regimens were compared with 5-fluorouracil (5-FU) and other chemotherapy regimens, and were stratified by treatment setting.
RESULTS: We identified 1013 patients with GEC: approximately half had treatment initiated with a 5-FU regimen, whereas 11% had therapy initiated with a capecitabine regimen. The mean capecitabine dose overall was 2382 ± 1118 mg/d, and capecitabine was used as monotherapy more often than in combination. Overall, 5-FU regimens were the most common treatment option in neoadjuvant and adjuvant settings, while other non-capecitabine regimens were used more widely in first- and second-line settings. The overall unadjusted complication rate for capecitabine regimens was about half of that seen with 5-FU regimens. In multivariate analyses, capecitabine recipients had a 51% (95% CI: 26%-81%) lower risk of developing any complication than 5-FU recipients did. The risk of developing bone marrow, constitutional, gastrointestinal tract, infectious, or skin complications was lower with capecitabine therapy than with 5-FU.
CONCLUSION: Capecitabine appeared to have a favorable side effect profile compared with 5-FU, which indicates that it may be a treatment option for GEC.
- Citation: Saif MW, Shi N, Zelt S. Capecitabine treatment patterns in patients with gastroesophageal cancer in the United States. World J Gastroenterol 2009; 15(35): 4415-4422
- URL: https://www.wjgnet.com/1007-9327/full/v15/i35/4415.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.4415
| Complication | ICD-9-CM | Procedure codes | Treatment |
| Anemia | 281.xx, 283.xx, 284.xx, 285.xx | ||
| Alopecia | 704.0x, A9282 | ||
| Asthenia | 780.7x | HCPCS codes G9029-G9032 | |
| Constipation | 546.0x | Therapeutic class 150-156 | |
| Cough | 786.2x, 786.3x, 786.4x | Therapeutic class 128, 131 | |
| Dehydration | 276.50, 276.51 | ||
| Dermatitis | 693.0x, 693.8x, 693.9x | ||
| Diarrhea | 007.xx, 009.x, 787.91 | Therapeutic class 148 | |
| Esophagitis | 530.1x | ||
| Fever | 780.6x | ||
| Gastritis | 535.xx | ||
| Headache | 784.0x | ||
| Infection | 001.xx-018.xx, 030.xx-041.xx, 045.xx-057.xx, | Therapeutic class 2-20 | |
| 070.xx-079.xx, | |||
| 110.xx-118.xx, 130.xx-136.xx, 480.xx-486.xx, 995.91, 995.92 | |||
| Insomnia | 780.51, 780.52 | Therapeutic class 74, Eszopiclone, Zaleplon, Zolpidem Tartrate | |
| Mucositis | 528.0x, 528.1x , 528.2x, 528.3x, 528.6, 529.0x, 054.2x | ||
| Nausea and vomiting | 787.0x, | CPT codes G9022, G9023, G9024, or | Therapeutic class 160 |
| HCPCS codes for antiemetics | |||
| Neutropenia | 288.0x | HCPCS codes for neutropenia treatment | |
| Night sweats | 780.8x | ||
| Weight loss | 783.2x, 783.0x | ||
| Complications of vascular access devices | 999.3x, 996.62, 996.74, 512.0x, 512.1x, 512.8x, 287.4x |
| 5-FU (n = 257) | CAP (n = 105) | 5-FU combination (n = 344) | CAP combination (n = 76) | Any 5-FU (n = 540) | Any CAP (n = 166) | Total (n = 1013)1 | |
| Demographics2 | |||||||
| Female | 180 (70.0) | 77 (73.3) | 264 (76.7) | 61 (80.3) | 395 (73.2) | 127 (76.5) | 746 (73.6) |
| Mean age (yr) | 63.2 (12.1) | 63.6 (12.4) | 60.3 (11.6) | 60.4 (12.0) | 61.7 (11.9) | 63.0 (12.3) | 62.6 (11.8) |
| Covered by Medicare | 127 (49.4) | 50 (47.6) | 126 (36.6) | 30 (39.5) | 231 (42.8) | 78 (47.0) | 465 (45.9) |
| Residing in urban area | 202 (78.6) | 83 (79.1) | 259 (75.3) | 64 (84.2) | 416 (77.0) | 136 (81.9) | 784 (77.4) |
| Clinical characteristics3 | |||||||
| CCI | 5.5 (3.2) | 5.9 (3.0) | 4.9 (3.0) | 5.1 (3.1) | 5.1 (3.1) | 5.5 (3.0) | 5.2 (3.1) |
| CDS | 4.2 (3.5) | 4.5 (3.7) | 4.6 (3.4) | 4.3 (3.5) | 4.4 (3.4) | 4.6 (3.6) | 4.6 (3.5) |
| Metastatic disease4 | 161 (62.7) | 55 (52.4) | 248 (72.1) | 47 (61.8) | 364 (67.4) | 97 (58.4) | 675 (66.6) |
| Previous treatment | |||||||
| Surgery | 125 (48.6) | 27 (25.7) | 46 (13.4) | 11 (14.5) | 154 (28.5) | 34 (20.5) | 239 (23.6) |
| Radiotherapy | 115 (44.8) | 25 (23.8) | 162 (47.1) | 15 (19.7) | 252 (46.7) | 40 (24.1) | 410 (40.5) |
| Baseline expenditure ($)5 | 5479 (4730) | 4965 (6277) | 4598 (5019) | 4178 (4005) | 5014 (5061) | 4607 (5573) | 4763 (5193) |
| Chemotherapy regimen | Neoadjuvant (n = 84) | Adjuvant (n = 213) | First-line (n = 743) | Second-line (n = 354) | Any setting (n = 1013) |
| All 5-FU-based regimens | 51 (60.7) | 140 (65.7) | 319 (42.9) | 139 (39.3) | 540 (53.5) |
| 5-FU monotherapy | 16 (19.0) | 104 (48.8) | 89 (12.0) | 68 (19.2) | 257 (25.4) |
| 5-FU + platinum | 24 (28.6) | 19 (8.9) | 104 (14.0) | 25 (7.1) | 163 (16.1) |
| 5-FU + platinum + non-platinum | 14 (16.7) | 18 (8.5) | 96 (12.9) | 31 (8.8) | 145 (14.3) |
| 5-FU + non-platinum | 2 (2.4) | 5 (2.3) | 30 (4.0) | 40 (11.3) | 73 (7.2) |
| Capecitabine-based regimens | 4 (4.8) | 24 (11.3) | 90 (12.1) | 72 (20.3) | 166 (16.4) |
| Capecitabine monotherapy | 2 (2.4) | 18 (8.5) | 50 (6.7) | 42 (11.9) | 105 (10.4) |
| Capecitabine + platinum | 2 (2.4) | 1 (0.5) | 11 (1.5) | 8 (2.3) | 19 (1.9) |
| Capecitabine + platinum + non-platinum | 0 (0.0) | 6 (2.8) | 16 (2.2) | 11 (3.1) | 31 (3.1) |
| Capecitabine + non-platinum | 0 (0.0) | 1 (0.5) | 13 (1.7) | 19 (5.4) | 32 (3.2) |
| Complication | 5-FU monotherapy | Capecitabine monotherapy | 5-FU combination therapy | Capecitabine combination therapy | Any 5-FU regimen | Any capecitabine regimen |
| Bone marrow | 233 | 110 | 349 | 205 | 307 | 151 |
| Constitutional symptom | 254 | 108 | 323 | 295 | 293 | 183 |
| Gastrointestinal tract symptoms | 465 | 183 | 577 | 284 | 529 | 224 |
| Infection | 199 | 114 | 268 | 150 | 238 | 131 |
| Skin complications | 0 | 0 | 2 | 0 | 1 | 0 |
| Other | 14 | 14 | 22 | 32 | 19 | 22 |
| Any complication | 764 | 336 | 835 | 460 | 806 | 387 |
Gastroesophageal cancer (GEC), which includes cancers that originate in the esophagus, gastroesophageal junction and stomach, is common in many countries. For example, gastric cancer is, by some estimates, the fourth most common cancer in the world, and esophageal cancer is eighth[1]. The incidence of GEC shows a marked geographic variation, with high-prevalence areas including Asia, Southern and Eastern Africa, and South America[2,3].
GEC is relatively uncommon in the United States compared with colorectal, lung, breast, and prostate cancers; however, the incidence of esophageal cancer (both squamous cell and adenocarcinoma) appears to be increasing[4-6]. It is estimated that in 2008, 37 970 cases of GEC will have been diagnosed and 25 160 deaths will have been attributed to these cancers[4].
A patient’s outcome depends on cancer stage at diagnosis; approximately 50% of patients with gastric or esophageal cancer have advanced disease (at least extending beyond the locoregional confines) at diagnosis[2,3]. The disease is highly metastatic; as a result, 70%-80% of resected specimens will have metastasized to the regional lymph nodes at the time of resection. The disease is associated with a poor prognosis: overall 5-year survival has been estimated at < 10%[7].
Patients with resectable disease are candidates for adjuvant chemotherapy. 5-fluorouracil (5-FU) plus leucovorin and radiotherapy significantly improve median overall survival compared with surgery alone in patients with resected adenoma of the stomach or gastroesophageal junction (36 mo vs 27 mo; P = 0.005)[8]. Regimens that may be considered for preoperative use or as definitive chemoradiation therapy for localized esophageal carcinoma include fluoropyrimidine-cisplatin, taxane-based, and oxaliplatin, and irinotecan-based regimens[2].
Chemotherapy should also be considered for patients with metastatic disease. A meta-analysis of randomized trials in a total of 390 patients with advanced gastric cancer showed a greater 1-year survival rate and improved quality of life with chemotherapy than with supportive care[9]. In this analysis, the 1-year survival rate achieved with supportive care was 8%, compared with 20% with chemotherapy (P = 0.05), and 30% of the patients in the chemotherapy group attained a 6-mo symptom-free period, vs 12% in the supportive care group (P < 0.01).
Chemotherapy options for advanced gastric or esophageal cancer include 5-FU/leucovorin, fluoropyrimidine-, cisplatin-, oxaliplatin-, and irinotecan-based combinations, and taxane-based regimens[2].
Another treatment option currently being investigated in patients with GEC is the fluoropyrimidine capecitabine. Capecitabine has been used widely to treat patients with colorectal and breast cancer, and has a well-established safety profile. Capecitabine is administered orally, therefore, there is no requirement for intravenous access, and the associated complications and morbidity seen with IV 5-FU are absent.
Two recent phase III trials have suggested that survival with capecitabine-based regimens compares favorably with that with 5-FU-based regimens as first-line therapy for GEC[10,11]. The REAL-2 study randomized 1002 patients with advanced GEC to receive first-line triple therapy with epirubicin and cisplatin plus either 5-FU (ECF) or capecitabine (ECX), or triple therapy with epirubicin and oxaliplatin plus either 5-FU (EOF) or capecitabine (EOX). The primary endpoint was non-inferiority in overall survival for the regimens that contained capecitabine as compared with 5-FU, and oxaliplatin as compared with cisplatin. Capecitabine-containing regimens demonstrated non-inferiority in overall survival compared with 5-FU-containing regimens. Median survival times in the ECF, ECX, EOF, and EOX groups were 9.9, 9.9, 9.3, and 11.2 mo, respectively.
In the other phase III trial, Kang and colleagues compared first-line doublet therapy with capecitabine/cisplatin vs 5-FU/cisplatin in 316 patients with advanced gastric cancer. The primary endpoint was non-inferiority in progression-free survival (PFS) between the two regimens. Non-inferiority was reached, with the capecitabine regimen achieving a median PFS of 5.6 mo vs 5.0 mo with the 5-FU regimen (unadjusted HR 0.81; 95% CI, 0.63-1.04; P < 0.001 vs non-inferiority margin of 1.25).
The current study was undertaken to assess the usage of capecitabine-based therapies and associated complication rates in patients with GEC in a real-world treatment setting. Capecitabine is currently not approved by the US FDA for this indication.
This retrospective analysis used claims data from the Thomson Reuters Healthcare MarketScan® Commercial Claims and Encounters and Medicare Supplemental and Coordination of Benefits databases to determine treatment patterns in patients with GEC. These databases contain data from 25 million individuals of all ages who are covered in the United States under employer- and government-funded (Medicare) health insurance plans, including a variety of fee-for-service and capitated provider reimbursement schemes. The MarketScan databases capture information on inpatient and outpatient health care claims, as well as outpatient pharmacy-dispensed drug claims. In compliance with the Health Insurance Portability and Accountability Act (HIPAA), patient data included in the analysis were statistically de-identified and therefore exempt from Institutional Review Board approval.
Patients included in the current analysis were required to have had at least three claims with a primary or secondary diagnosis of stomach or esophageal cancer [International Classification of Diseases, Ninth Revision (ICD-9) diagnosis codes 150 or 151] between January 1, 2000 and December 31, 2005. Because predominant treatments change over time and this study sought to compare current chemotherapy regimens, only patients with at least one claim for an identifiable chemotherapy agent between 2004 and 2005 were retained.
Patients were required to have been enrolled in the plan for at least 6 mo before the first chemotherapy administration (index date) in 2004 or 2005, and for at least 30 d after index. Patients with index dates between January 1, 2004 and February 14, 2004 were required to have had no claims for chemotherapy for at least 45 d before the index date.
Availability of data for both medical and pharmacy coverage was also required. Patients with only a chemotherapy diagnosis or revenue codes were excluded. Patients were followed from diagnosis until death, disenrollment, or study end (December 31, 2005).
Patient-level demographic variables measured as of the index date included age, geographic region, area of residence (urban vs rural), payer type (commercial vs Medicare), and length of follow-up (mo).
Clinical variables evaluated during the 6-mo pre-index period included: total direct health care costs; the Charlson Comorbidity Index (CCI); the Chronic Disease Score (CDS); select comorbidities (anemia, anxiety or depression, cerebrovascular disease, congestive heart failure, chronic obstructive pulmonary disease, diabetes, hypertension, or hypercalcemia); and pre-index treatments (radiotherapy, surgery). Other variables included the year of first cancer diagnosis (between 2000 and 2005), year of first chemotherapy (between 2000 and 2005), and the presence of metastases at any point during the pre-index period.
A treatment episode-level analysis file was created, which contained all chemotherapy treatments identified from the claims database. A treatment episode was defined as the time from treatment initiation until the addition of a new agent not seen in the first 30 d, or until a gap of ≥ 45 d in treatment. Neoadjuvant treatment regimens ended on the date of surgery. Patients could contribute more than one treatment episode to the study if they received multiple chemotherapy regimens during the 2004-2005 study period.
Each chemotherapy episode was categorized according to treatment regimen and setting. Treatment regimens of interest included capecitabine monotherapy; capecitabine in combination with a platinum agent; capecitabine in combination with a platinum and a non-platinum agent; capecitabine in combination with a non-platinum agent; 5-FU monotherapy; 5-FU in combination with a platinum agent; 5-FU in combination with a platinum and a non-platinum agent; 5-FU in combination with a non-platinum agent; and other chemotherapy. Treatment settings included neoadjuvant (90 d before surgery), adjuvant (within 90 d after surgery), first line (> 90 d after surgery), and second line (any subsequent treatment regimens).
Complications of chemotherapy were identified at the treatment episode level, using diagnosis codes, procedure codes, or pharmacological treatment specific to adverse events (Table 1). Adverse events were grouped into six categories: bone marrow complications (anemia, neutropenia, secondary thrombocytopenia); constitutional symptoms (asthenia, cough, fever, headache, insomnia, night sweats); gastrointestinal tract symptoms (constipation, diarrhea, esophagitis, gastritis, mucositis, nausea and vomiting, weight loss); infection (including central-line infection); skin complications (alopecia, dermatitis); and “other” (central-line thrombosis, pneumothorax). The complication rate was standardized per 1000 person-months on treatment. Person-month accumulation was based on time to event for treatment episodes with a complication event, and treatment episode length for those without an event.
Descriptive analyses of patient demographics, clinical characteristics, complication rates, and treatment characteristics were performed for each individual treatment regimen. Categorical variables were summarized in frequency tables. Continuous and other numeric variables were summarized by presenting the number of observations, mean, and standard deviation.
Multivariate analyses were performed to adjust for differences in patient demographic and clinical factors that confounded the complication rates from the descriptive analysis. Cox proportional hazard models were used to estimate time to first complication event for each of the six complication categories described above. Covariates employed in the models included patients’ age, sex, region, cancer type, presence or absence of metastases during each treatment episode, treatment setting, cancer diagnosis year, pre-index anemia status, pre-index CCI score, and pre-index surgery status.
Complications that occurred in patients treated with capecitabine were compared to those occurring in patients who received 5-FU regimens (i.e. capecitabine monotherapy was compared with 5-FU monotherapy; capecitabine combination regimens were compared with 5-FU combination regimens; and “any capecitabine” regimens were compared with “any 5-FU” regimens).
A total of 1013 patients with GEC were identified from the database and met the inclusion criteria for this analysis. These patients had 1349 treatment episodes during 2004 and 2005. Their characteristics are summarized in Table 2. There were more females than males in the selected population (73.6% of total patients), and the average age was 62.6 (± 11.8) years (most patients were in the 50-64-year age group). Patients were followed-up on average for 9.9 (± 6.9) mo after the initiation of the index chemotherapy.
Approximately two-thirds of patients were classified as having non-metastatic disease at the time of index treatment. Patients had high comorbidity scores at baseline (mean CCI score 5.2 ± 3.0; mean CDS 4.6 ± 3.5). Hypertension was the most common comorbidity and occurred in 30.6% of patients during the 6-mo pre-index period. Other common comorbidities included anemia, diabetes, and coronary artery disease.
Radiotherapy was used in 40.5% of patients in the 6-mo pre-index period, and 23.6% of patients underwent surgery.
Comparison of index treatment patterns: Approximately half of all patients included in the current analysis received a 5-FU regimen as their index treatment (540 of 1013; 53.3%) (Table 2). 5-FU combination therapy was initiated in 33.9% of patients, and 25.3% received 5-FU monotherapy. In comparison, 16.4% of patients had therapy initiated with a capecitabine regimen (166 of 1013): 10.4% received capecitabine monotherapy, and 7.5% received capecitabine combination therapy.
Patients who received capecitabine as index treatment were on average 1.5 ± 0.4 years older than those who were treated with 5-FU-based regimens (mean age: 63.0 ± 12.3 years vs 61.7 ± 11.9 years). Aggregate measures of comorbidity and chronic disease were similar between patients given 5-FU- and capecitabine-based regimens (Table 2). Patients who received capecitabine-based therapy were followed for about 1 mo less than those given 5-FU-based regimens (mean follow-up 9.1 ± 6.4 mo vs 10.3 ± 6.9 mo).
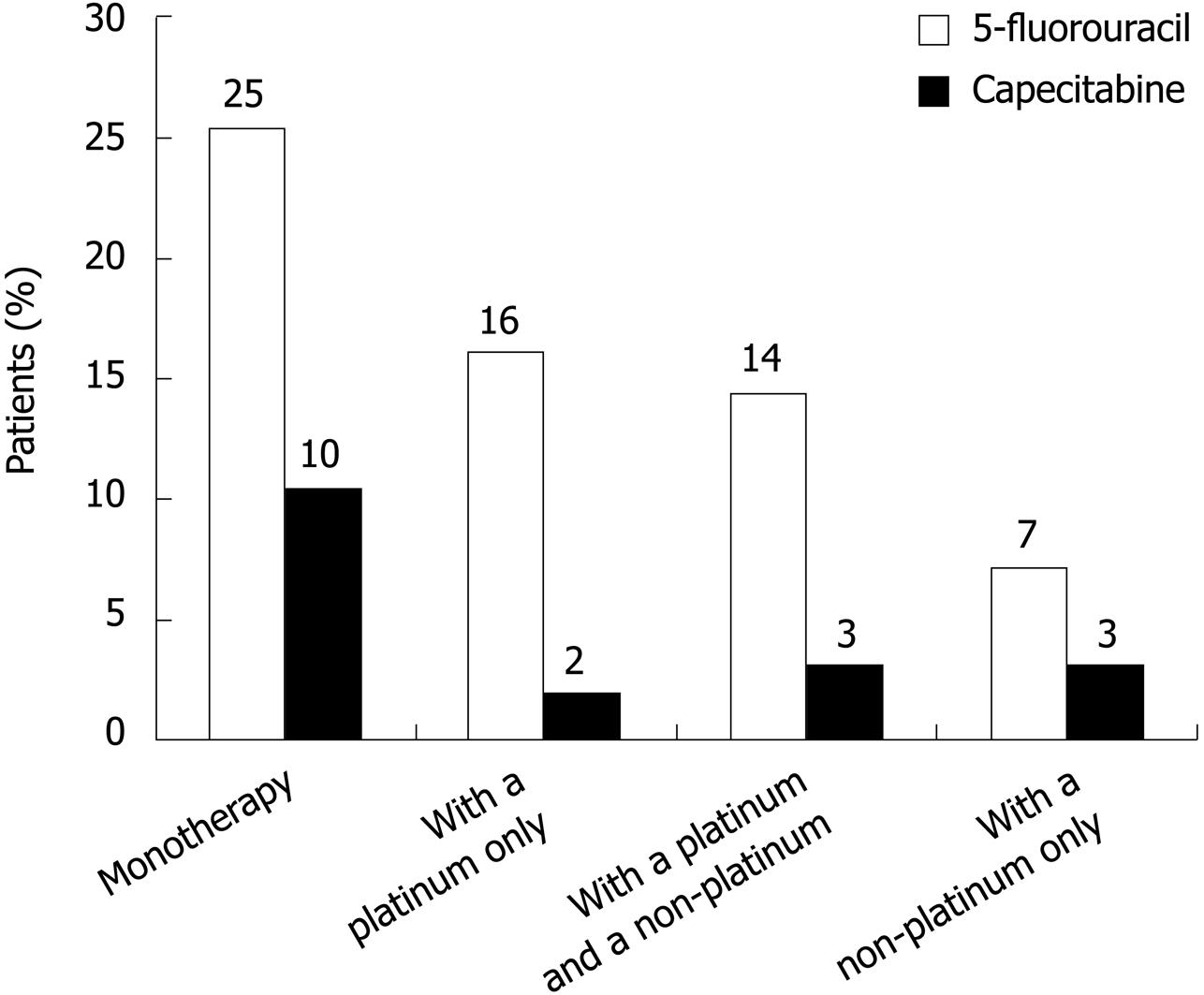
Comparison of treatment patterns by drug regimen and treatment setting: Combined data for all treatment episodes (index and subsequent treatments) showed that 5-FU regimens remained the most widely used treatments (53.3% of patients) and 5-FU monotherapy was the most popular 5-FU regimen (25.4% of patients) (Figure 1 and Table 3). Overall, 16.4% of patients received capecitabine across all treatment settings: capecitabine monotherapy was the preferred regimen, given to 10.4% of patients (1.9% received a capecitabine-platinum combination; 3.1% received a capecitabine-platinum-non-platinum combination; and 3.2% received a capecitabine-non-platinum combination). Overall, 51.7% of patients received other chemotherapy regimens.
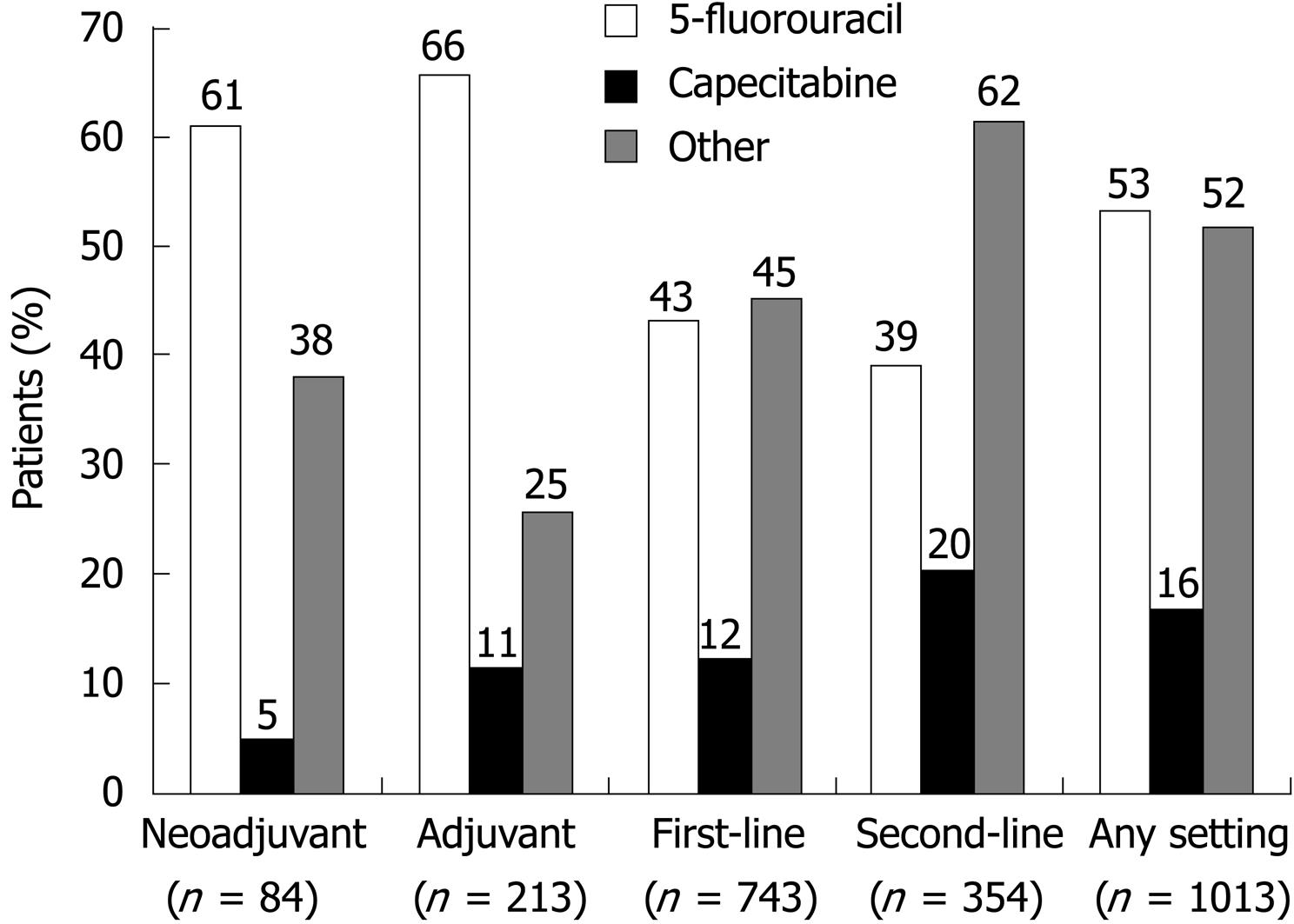
First-line treatment was the most common treatment setting in the selected population (73.3%), followed by second-line therapy (34.9%), adjuvant therapy (21.0%), and neoadjuvant therapy (8.3%) (Table 3). Patients could have more than one treatment episode; therefore, the sum of the percentage of patients receiving each treatment exceeded 100%.
5-FU was used widely in the neoadjuvant and adjuvant settings (in 60.7% and 65.7% of patients, respectively) (Figure 2 and Table 3). Capecitabine was used infrequently in the neoadjuvant setting (4.8% of patients), but was used more often in the adjuvant setting (11.3% of patients). Other chemotherapy regimens were used in 38.1% and 25.4% of patients in these respective treatment settings.
In the first-line setting, 42.9% and 12.1% of patients, respectively, were treated with 5-FU or capecitabine regimens. Although still a popular choice, 5-FU was used less often in the second-line setting than in other settings (in 39.3% of patients), and other chemotherapy regimens became the most popular choice (used in 61.6% of patients). The second-line treatment setting was the most popular choice for capecitabine-based therapies (given to 20.3% of patients). Capecitabine monotherapy was used in 11.9% of patients in this setting; cisplatin plus a non-platinum compound was used in 5.4% of patients (Table 3).
Capecitabine dosage regimens: The mean overall capecitabine dose was 2382 (± 1118) mg/d; a lower dose was used in combination regimens than in monotherapy [mean overall dose: 2349 (± 1052) vs 2410 (± 1175) mg/d, respectively).
Complication rates: Capecitabine compared favorably with 5-FU in terms of crude (unadjusted) complication rates (Table 4). The unadjusted complication rate (per 1000 person-months) for capecitabine-based regimens was half that for 5-FU-based regimens (387 vs 806 per 1000 person-months). Patients who received capecitabine monotherapy were 56% less likely to have a complication during treatment than patients given 5-FU monotherapy (336 vs 764 per 1000 person-months), and patients who received capecitabine combination therapy were 45% less likely to have a complication during treatment than those given 5-FU combination therapy (460 vs 835 per 1000 person-months).
Complication rates were lower with capecitabine regimens than with 5-FU across four individual categories: bone marrow (151 vs 307; 51% lower); constitutional symptoms (183 vs 293; 37% lower); gastrointestinal tract (224 vs 529; 58% lower); and infection (131 vs 238; 45% lower). Skin complications were rare in both comparison cohorts.
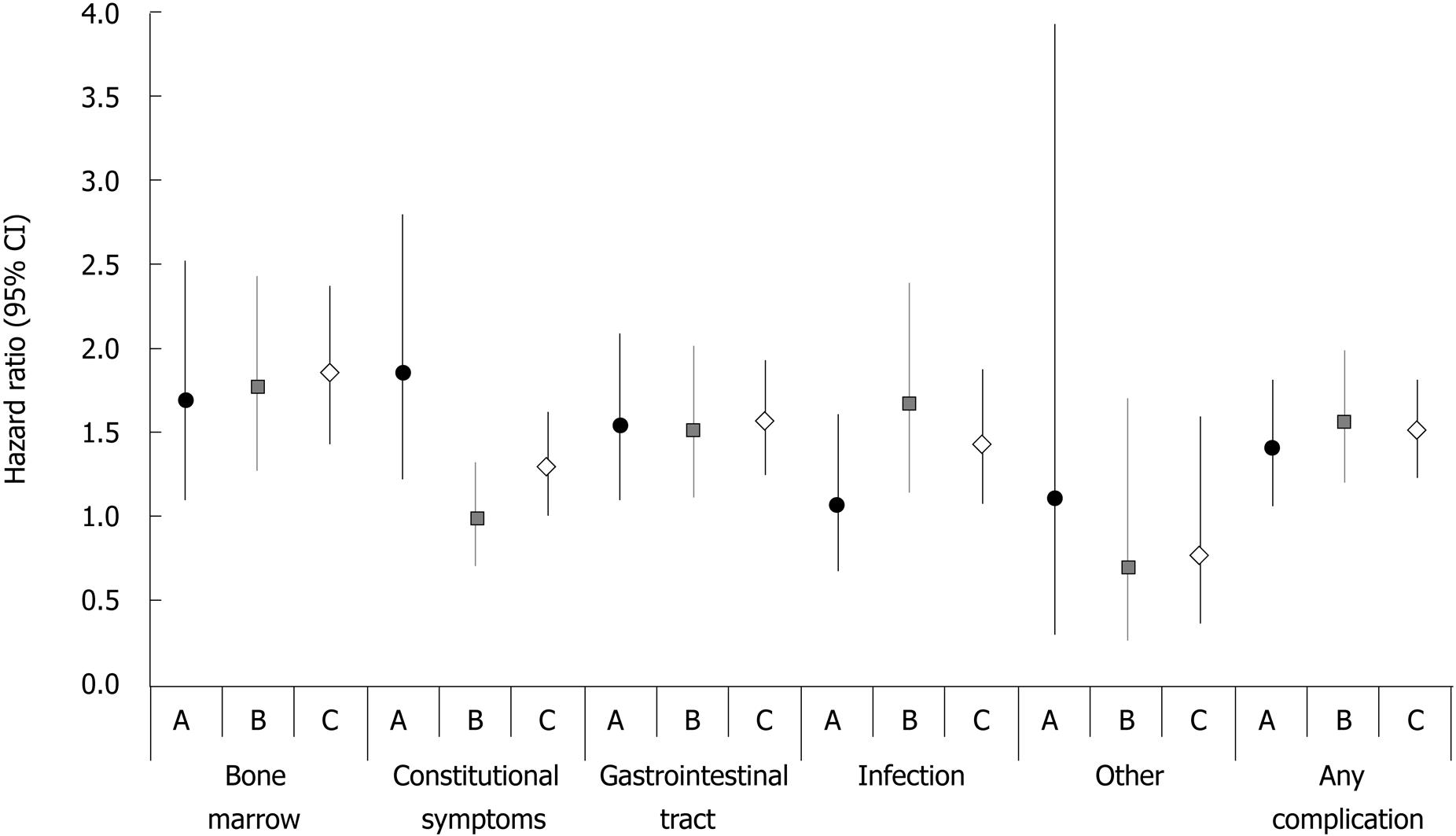
Relative risk of complications: The results of multivariate analyses supported the findings of the crude rate calculations (Figure 3). Cox proportional hazard models showed that, overall, 5-FU therapy was associated with a significantly higher risk for any complication than capecitabine (adjusted HR: 1.51, 95% CI: 1.26-1.81) (Figure 3). Specifically, the higher risk of developing a complication associated with 5-FU therapy was observed for bone marrow events (HR: 1.84, 95% CI: 1.42-2.37), constitutional symptoms (HR: 1.28, 95% CI, 1.01-1.63), and gastrointestinal tract events (HR: 1.56, 95% CI: 1.27-1.93). No difference was detected in the risk for infection or “other” complications, possibly because of the low number of reported events (skin: 1 vs 0; “other”: 19 vs 22, for capecitabine and 5-FU regimens, respectively).
The risk of complication with 5-FU monotherapy was markedly higher than with capecitabine monotherapy for bone marrow (HR: 1.68, 95% CI: 1.11-2.53), constitutional symptoms (HR: 1.85, 95% CI: 1.23-2.79), and gastrointestinal tract complications (HR: 1.52, 95% CI: 1.10-2.09). The overall risk for any complication also differed significantly between the two monotherapy treatment groups (HR: 1.39, 95% CI: 1.07-1.81) (Figure 3).
Similar to monotherapy, the difference in overall risk of any complication was significantly higher with 5-FU combination regimens than with capecitabine combination regimens (HR: 1.55, 95% CI: 1.21-1.99). The risk of bone marrow complications was 76% higher (HR: 1.76, 95% CI: 1.27-2.44), and the risk of developing a gastrointestinal tract event was higher (HR: 1.50, 95% CI: 1.13-2.01) with 5-FU than with capecitabine combination regimens (Figure 3).
Of the 1013 patients with GEC identified in this retrospective database analysis, approximately 50% had treatment initiated with a 5-FU regimen, whereas only 11% had therapy initiated with a capecitabine regimen. The mean capecitabine dose overall was 2382 (SD ± 1118) mg/d, and capecitabine was used as monotherapy more often than in combination. Overall, 5-FU regimens were the most common treatment option in the neoadjuvant and adjuvant settings, whereas other non-capecitabine regimens were used more widely in the first- and second-line settings.
The retrospective analysis of complication rates with capecitabine and 5-FU therapies indicates that capecitabine-based therapy may be a better tolerated treatment option than 5-FU in patients with GEC. The overall unadjusted complication rate for capecitabine regimens was about half of that seen with 5-FU regimens: in multivariate analyses, capecitabine recipients had a 51% lower risk of developing any complication than 5-FU recipients did. Lower complication rates were observed in bone marrow, constitutional, gastrointestinal tract, infection, and skin complications. Overall, patients who received capecitabine therapy (monotherapy or combination) were 38% less likely than their 5-FU counterparts to have a complication associated with therapy.
The two recent phase 3 trials of first-line capecitabine in GEC that were described earlier[10,11] have demonstrated non-inferiority in survival parameters for capecitabine- vs 5-FU-based regimens, as well as similar tolerability between the regimens. Although the incidence of grade 3/4 neutropenia was higher with the ECX regimen compared with ECF (51.1% vs 41.7%), in the study by Cunningham and colleagues, the incidence of these events was similar or slightly lower with the capecitabine regimen EOX vs EOF (27.6% vs 29.9%)[10], and capecitabine/cisplatin vs 5-FU/cisplatin (16% vs 19%) in the study by Kang and colleagues[11]. Other common treatment-related grade 3/4 adverse events in the latter study were vomiting (7% vs 8%) and stomatitis (2% vs 6%) with capecitabine/cisplatin vs 5-FU/cisplatin, respectively. Thus, the clinical efficacy of capecitabine demonstrated in the two phase three studies, combined with the lower complication rate observed in the current retrospective analysis, support capecitabine use for the treatment of patients with GEC. Capecitabine therapy also has the advantage of convenient oral administration, which eliminates the need for intravenous access and associated complications.
The treatment patterns observed in our analysis correspond with standard recommended treatment protocols for GEC: 5-FU-based regimens were used in about 50% of patients and other non-capecitabine chemotherapy regimens were also a common choice. Although not approved for this indication, there was considerable use of capecitabine for the treatment of GEC in the study period between 2004 and 2005. Overall, 16% of patients received a capecitabine-based regimen. Monotherapy was the preferred capecitabine regimen, and was given to 10% of patients overall across all treatment settings (1.9% received capecitabine plus a platinum agent; 3.1% received a capecitabine-platinum-non-platinum combination; 3.2% received a capecitabine-non-platinum combination). Capecitabine was most likely used in the second-line setting, and least likely used as neoadjuvant therapy.
In interpreting our findings, several limitations of the analysis should be considered. Although the current study was based on a large, diverse sample of patients with GEC, it was not a random population sample, and may not represent the United States population as a whole. Moreover, diagnostic information is recorded by physicians and hospitals to support their claims for reimbursement for particular services, but additional clinical information is limited. We relied exclusively on billing and coding by health care providers to identify cancer type, treatment setting, treatment regimen, treatment episode, and complication events. As such, particular conditions common among patients undergoing chemotherapy may not have been recorded on a claim unless deemed clinically relevant. In addition, clinical measures were not used to confirm the presence of complications. We also presumed that the use of known treatment for a complication was evidence of its existence. However, as prophylaxis is often used for specific events, the frequency of complications reported in the current study may have been overstated. Alternatively, the frequency of complications may have been understated because patients may have experienced complications that did not result in the generation of a health care claim for reimbursement. For example, hand-foot syndrome was recorded using the ICD-9 code 693, which includes dermatitis caused by substances taken internally. To ensure that hand-foot syndrome was not being miscoded, we conducted a sensitivity analysis using all ICD-9 codes indicative of an inflammatory dermatologic reaction (codes 690-698), but rates still remained low. Cases of hand-foot syndrome may not have been clinically significant to warrant coding. Finally, a key problem that often plagues observational studies is the lack of randomization in assigning individuals to either treatment or control groups. Given this concern, the estimation of the effects of treatment may have been biased by the existence of confounding factors. The current study used multivariate models to adjust for these pretreatment differences. Given the strength of the selection bias observed in the current study, it may be desirable for future studies to incorporate a propensity model approach in combination with multivariate adjustment.
In conclusion, in the United States, 5-FU-based regimens represented approximately 50% of the regimens used to treat patients with GEC between 2004 and 2005, which reflects current treatment recommendations for this indication. Capecitabine-containing regimens represented 16% of all regimens used for this indication during this time period, and were used most frequently in the second-line setting. Capecitabine appeared to have a favorable side-effect profile compared with 5-FU and, thus, could be a useful treatment option for patients with GEC.
Capecitabine is an oral cytotoxic agent with comparable efficacy to and a better safety profile than intravenous 5-fluorouracil (5-FU), as seen in clinical trials of gastroesophageal cancer (GEC). This study compared the occurrence of select adverse events (AEs) in patients treated with capecitabine and 5-FU in a real-world setting.
Although two recent phase III trials have suggested that survival with capecitabine-based regimens compares favorably with that with 5-FU-based regimens as first-line therapy for GEC (REAL-2 study and Kang et al study), data are lacking for the use of capecitabine in patients with GEC in a real-world treatment setting. This study was undertaken to assess the usage of capecitabine-based therapy and associated complication rates in patients with GEC in a real-world treatment setting.
The complication rate of a capecitabine regimen was nearly half that of 5-FU (387/1000 vs 806/1000 person-months). These findings held when comparing monotherapy (336/1000 vs 764/1000 person-months) and combination (460/1000 vs 835/1000 person-months) regimens. After adjusting for differences in demographic and clinical profile, patients on capecitabine monotherapy had significantly lower risk for any complication compared with patients on 5-FU alone (HR: 1.39, 95% CI: 1.07-1.81). Patients on a capecitabine combination regimen had significantly lower risk of complication compared with patients on a 5-FU combination regimen (HR: 1.55, 95% CI: 1.21-1.99).
Capecitabine is currently not approved by the US FDA for treatment of GEC. Consistent with trial data, in this real-world setting, capecitabine alone and in combination with other agents had lower rates of AEs than 5-FU did. These findings support that, in the treatment of GEC, capecitabine regimens produce a similarly favorable safety profile in the real-world setting as in controlled clinical trials.
Charlson Comorbidity Index (CCI): The CCI predicts the 1-year mortality for a patient who may have comorbid conditions, such as heart disease, diabetes, or cancer (a total of 17 conditions). Each condition is assigned a score of 1, 2, 3 or 6 depending on the associated risk of dying from this condition. Scores for individual comorbidities are added and the sum is used as a predictor for mortality. The CCI aids in directing how aggressively a condition should be treated (e.g. although a patient may have cancer, additional comorbidities may be severe enough that the costs and risks of treatment may outweigh the short-term benefit from treatment of the cancer). Chronic Disease Score (CDS): The CDS is a risk-adjustment measure based on age, sex, and history of dispensed drugs.
It is useful to publish the practice of the utilization of these drugs. This was a retrospective study and although there are biases related to the data collection and lack of control related to the selection of patients for different treatments, these limitations are recognized and the study makes an important contribution to our knowledge of the potential of capecitabine in the treatment of GEC.
| 1. | Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137-2150. |
| 4. | American Cancer Society. Cancer facts and figures 2006. 2006; Available from: http://www.cancer.org/downloads/STT/CAFF2006PWSecured.pdf. |
| 5. | Blot WJ, Devesa SS, Kneller RW, Fraumeni JF Jr. Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA. 1991;265:1287-1289. |
| 6. | Younes M, Henson DE, Ertan A, Miller CC. Incidence and survival trends of esophageal carcinoma in the United States: racial and gender differences by histological type. Scand J Gastroenterol. 2002;37:1359-1365. |
| 7. | Corporaal S, Smit WM, Russel MG, van der Palen J, Boot H, Legdeur MC. Capecitabine, epirubicin and cisplatin in the treatment of oesophagogastric adenocarcinoma. Neth J Med. 2006;64:141-146. |
| 8. | Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, Haller DG, Ajani JA, Gunderson LL, Jessup JM. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725-730. |
| 9. | Casaretto L, Sousa PL, Mari JJ. Chemotherapy versus support cancer treatment in advanced gastric cancer: a meta-analysis. Braz J Med Biol Res. 2006;39:431-440. |
| 10. | Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, Middleton G, Daniel F, Oates J, Norman AR. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36-46. |
Peer reviewer: Ross C Smith, Professor, Department of Surgery, University of Sydney, Royal North Shore Hospital, St Leonards, New South Wales 2065, Australia
S- Editor Tian L L- Editor Kerr C E- Editor Ma WH