MATERIALS AND METHODS
During January 2000 to January 2007, a total of 632 patients had emergent endoscopy for bleeding in the upper GI tract in our hospital, and 155 patients were given endoscopic therapy. Among them, 68 cases with nonvariceal bleeding were given endoscopic hemoclip application. Written informed consent was obtained from all the patients or their relatives before the treatment. The 68 cases had ages ranging from 9 to 70 years (average 54.4, male:female = 42:26). The presenting manifestations were hematemesis in 26 cases (38.2%), melena in nine cases (13.3%), and both in 33 cases (48.5%). Some of the patients had basal disease, including cardiovascular disease (myocardial infarction, congestive heart failure, or significant cardiac arrhythmia) in eight cases (11.8%), liver cirrhosis in two cases (2.94%) and respiratory disease (chronic obstructive pulmonary disease) in six cases (8.82%). Twenty-eight cases were in a state of shock, and 44 cases were given blood transfusions of more than 400 mL; the systolic blood pressures of 12 cases were still less than 90 mmHg when they were given the endoscopic treatment. The electrocardiogram, blood pressure, and oxygen saturation were monitored for those who were in a severe condition.
The type of hemoclip applied was MD 850 (Olympus Corp.) with a rotatable clip application device (HX-5L, Olympus Corp.). After finding the bleeding point, we exposed the clip from the sheath, rotated it to a desired axis, and opened the clip to the maximum width. The clip was then pressed against the lesion and deployed. If needed, the procedure was repeated. The mean number of hemoclips applied was four. All of the patients were given physical care after endoscopic therapy, such as monitoring vital signs, fasting, intravenous fluid, intravenous administration of Histamine-2 receptor antagonists or proton pump inhibitors, hemostatic agents, and some were given blood transfusions.
RESULTS
The causes of the nonvariceal bleeding in the upper GI tract can be listed as followings: gastric ulcer in 29 cases, duodenal ulcer in 11 cases, Dieulafoy’s lesion in 11 cases, Mallory-Weiss syndrome in six cases, post-operative in three cases, post-polypectomy bleeding in five cases, and post-sphincterotomy bleeding in three cases.
Hemostasis was defined as endoscopic cessation of bleeding for at least one minute after hemoclip application. Clinically, hemostasis was defined as no decrease in hemoglobin concentration, and correction of shock by blood transfusion and intravenous fluid. Hemostasis was obtained by hemoclip placement in 59 cases. Six patients underwent emergent surgery, in which three cases had peptic ulcers (two located in the posterior wall of the gastric body and one duodenal ulcer located in the posterior wall near the lesser curvature), one case had Dieulafoy’s lesion, and two cases were caused by sphincterotomy. Three patients died due to cardiovascular failure and liver cirrhosis (two had Dieulafoy’s lesion and one was caused by sphincterotomy). To evaluate the long-term outcomes of the treatment, the patients were followed-up for 30 d. All 59 cases achieved permanent hemostasis, and one of them had recurrent bleeding because of Dieulafoy’s lesion 10 mo later, but in a different location (initially in the proximal one third of the stomach and later in the duodena). The patient underwent endoscopic hemoclip application again, and also achieved a satisfactory result.
DISCUSSION
Despite the development of pharmacology and endoscopic therapy, nonvariceal bleeding in the upper GI tract remains a serious problem, especially for those who have active bleeding. It is associated with an approximately 20% rebleeding rate and its mortality ranges from 10% to 36%[7-9]. The etiology of acute nonvariceal bleeding in the upper GI tract has changed little in the past 20 years, peptic ulcers (including gastric ulcer and duodenal ulcer) are still the most common causes of acute hemorrage in the upper GI tract[10]. In our group, it accounted for 58.8% of bleeding episodes. After endoscopic therapy, acid suppression is essential for those who have bleeding caused by peptic ulcer disease. In a low pH environment, platelets can lose their function, and blood clots might be dissolved by pepsin, resulting in further bleeding. Among the 40 bleeding peptic ulcers, 92.5% achieved permanent hemostasis, only three cases underwent emergent surgery.
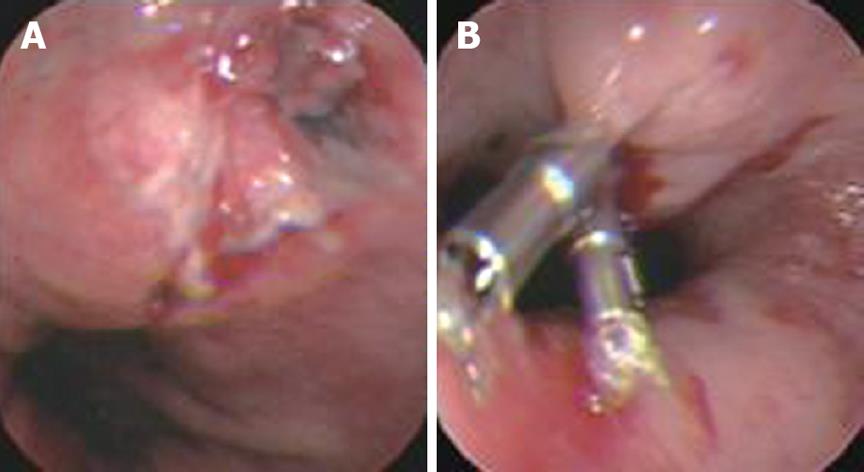
Tears at the gastroesophageal junction (Mallory-Weiss syndrome) account for 5% to 15% of all cases of nonvariceal bleeding in the upper GI tract[11]. These lesions are usually associated with repeated nausea and vomiting. For nonbleeding cases, conservative treatments are usually sufficient. In our group, six cases with active bleeding were given hemoclip application and all had excellent outcomes. Compared with other endoscopic treatments, such as sclerotherapy, epinephrine injection, and heater probe, the hemoclip is a safer choice, without adverse effects[12].
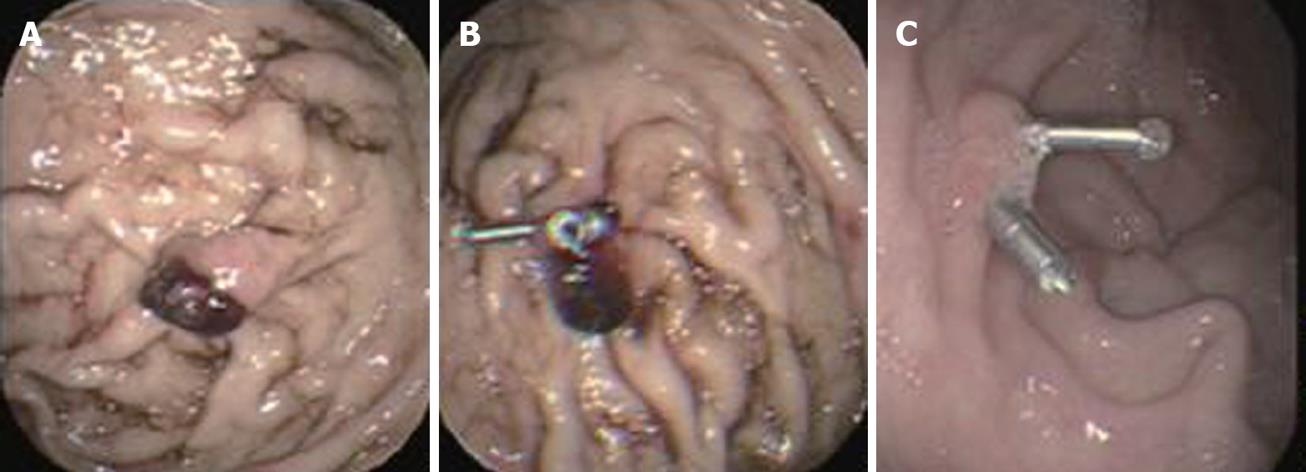
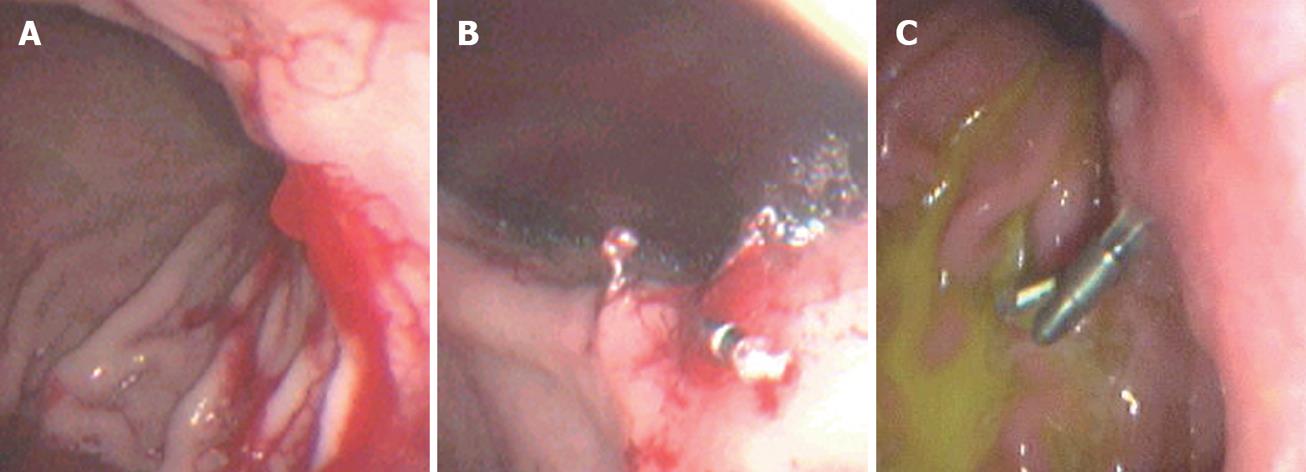
Dieulafoy’s lesion, an important cause of potentially life-threatening GI bleeding, was first described in 1896 and is a submucosal artery protruding from a minute defective mucosa surrounded by normal tissue[13,14]. Its histopathologic description is “a caliber-persistent artery” in the submucosal tissue[14]. It was regarded as a rare disease in the past because the caliber-persistent artery often retracts after bleeding[9], but with the development of technology and familiarity with this disease, it is now estimated to represent about 5% the etiology of acute upper-GI bleeding[9]. Endoscopic therapy is now considered the first-line method of achieving hemostasis, and hemoclip application has achieved satisfactory results with no reported ulcerative complications[10]. It can cause occlusion of the bleeding vessel, which results of immediate local hemostasis and prevent delayed recanalization and recurrent bleeding[5]. In most cases, the hemoclip can replace surgery as the first choice therapy for patients with Dieulafoy’s lesion. However, because the lesions are often located in the proximal stomach, usually along the lesser curvature, it might be technically difficulty to apply a hemoclip. In our group, one case underwent emergent surgery and two cases were dead due to cardiovascular failure.
Upper GI bleeding caused by endoscopic treatment, such as resection of polyps, mucosal resection, and sphincterotomy, is becoming more and more frequent in clinics due to the increased endoscopic treatments. In our group, five cases were caused by resection of polyps by endoscopy and three cases were caused by sphincterotomy by endoscopy. Among them, two cases underwent emergent surgery and one case died from GI bleeding. These three cases were all caused by sphincterotomy. In most cases, hemoclipping can achieve satisfactory results; however, it is difficult to accomplish hemostasis through endoscopy in upper GI bleeding caused by sphincterotomy, even for experienced endoscopists, so if necessary, emergent surgery might be a better choice.
Patients who have active bleeding or have a high risk of recurrence of bleeding require effective hemostasis[15]. At present, endoscopic therapy has been recommended as the first choice for the treatment of acute nonvariceal upper GI bleeding[2]. Some endoscopic therapies, such as heat probe coagulation, injection of epinephrine, or sclerotic agency, have been proved to be effective for achieving hemostasis, but they might cause tissue injury at the same time, causing necrosis or even perforation[16,17].
As a mechanical method of hemostasis, the hemoclip application was first introduced in 1975[18,19]. Due to its simplicity, low cost, easy availability, repetition, minimal damage to the localized field, and reduced risk of adverse effects, hemoclips have been widely used for the treatment of nonvariceal bleeding in the upper GI tract, such as bleeding peptic ulcer[20,21], Dieulafoy’s lesion bleeding[22,23] (Figures 1 and 2), Mallory-Weiss syndrome[24,25] (Figure 3), post-polypectomy bleeding[26], and post-sphincterotomy bleeding[27]. It is suggested that hemoclips are particularly helpful when active bleeding is encountered and/or there is a specific point of bleeding[28]. In these cases, hemoclip application is fast and very effective in controlling bleeding. Theoretically[6], clips can provide immediate hemostasis comparable with surgery by ligation of the bleeding vessel, with minimal injury to the adjacent tissue. DiMaio et al[10] reported that Hemoclip application has excellent results for initial (97.6%) and permanent hemostasis (95.1%). An experimental study[29] showed that only this mechanical method was effective for control of bleeding from vessels greater than 2 mm in diameter. Cipolletta et al[30] also thought that clipping was superior to a standard therapy such as injection epinephrine, with significantly less further bleeding, fewer units of blood transfused, a shorter hospital stay, and limited damage to surrounding tissue. However, Gevers et al[31] and Lin et al[32] produced different results.
These inconsistent results in randomized controlled trials suggest that some other factors, such as age, the reasons and the locations of the lesions, shock, presence of multiple comorbidities, could all be associated with the failure of endoscopic hemoclips for bleeding[33]. For example, some lesions are located in difficult-to-reach sites, which make it hard to apply the clips to the bleeding spot with a perpendicular angle[34]. The tissue of an ulcer is very brittle, the clip can easily fall off if located on it, so the clip must be located on the normal tissue across the ulcer. If the ulcer is very large and beyond the width of the clip, we can not achieve hemostasis using a hemoclip. However, the experience of endoscopists appears to play a major role in successful clip application. In some cases, it is difficult to deploy hemoclips to the lesion with active bleeding, and only experienced endoscopists can accomplish this task. Thus, hemoclipping is more operator-dependent than other therapies, with some endoscopists achieving excellent results and others having less success. This could explain why the results have great variation in the hemoclip group. The devices themselves might also affect the final results. At present, a number of design improvements have been made to achieve better results. For example, the clip device can be rotated to a desired axis, which makes it easier to adjust the clip position before deployment. The installation of the clips is easier than before, which can save time, because in many ceses, more than one clip is needed to achieve hemostasis. Of course, the results will be better if the device could deploy multiple clips at the same time and larger, stronger clips are designed to control the bleeding from large vessels. In summary, endoscopic hemoclip application is an effective and safe method for control nonvariceal bleeding in the upper GI tract with satisfactory outcomes, but the clip and the application device require further improvements.