Published online Sep 14, 2009. doi: 10.3748/wjg.15.4240
Revised: July 15, 2009
Accepted: July 22, 2009
Published online: September 14, 2009
Several advances in diagnosis, treatment and palliation of cholangiocarcinoma (CC) have occurred in the last decades. A multidisciplinary approach to this disease is therefore recommended. CC is a relatively rare tumor and the main risk factors are: chronic inflammation, genetic predisposition and congenital abnormalities of the biliary tree. While the incidence of intra-hepatic CC is increasing, the incidence of extra-hepatic CC is trending down. The only curative treatment for CC is surgical resection with negative margins. Liver transplantation has been proposed only for selected patients with hilar CC that cannot be resected who have no metastatic disease after a period of neoadjuvant chemo-radiation therapy. Magnetic resonance imaging/magnetic resonance cholangiopancreatography, positron emission tomography scan, endoscopic ultrasound and computed tomography scans are the most frequently used modalities for diagnosis and tumor staging. Adjuvant therapy, palliative chemotherapy and radiotherapy have been relatively ineffective for inoperable CC. For most of these patients biliary stenting provides effective palliation. Photodynamic therapy is an emerging palliative treatment that seems to provide pain relief, improve biliary patency and increase survival. The clinical utility of other emerging therapies such as transarterial chemoembolization, hepatic arterial chemoinfusion and high intensity intraductal ultrasound needs further study.
- Citation: Aljiffry M, Walsh MJ, Molinari M. Advances in diagnosis, treatment and palliation of cholangiocarcinoma: 1990-2009. World J Gastroenterol 2009; 15(34): 4240-4262
- URL: https://www.wjgnet.com/1007-9327/full/v15/i34/4240.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.4240
Cholangiocarcinomas (CC) are malignant tumors originating from epithelial cells lining the biliary tree and gallbladder[1]. Intrahepatic CCs (ICC) arise within the liver and extra-hepatic CCs (ECC) originate in the bile duct along the hepato-duodenal ligament. ICCs usually present as masses in the liver while jaundice is the most common presentation of ECCs. CCs are relatively rare tumors although their incidence is rising worldwide[2,3]. Several advances in the diagnosis, therapy and palliation of patients affected by CC have occurred during the last decades. The aim of this article is to review the most recent high quality literature on this topic.
We sought studies that reported at least one of the following aspects of CC: epidemiology, diagnosis, therapy (e.g. surgery, radiotherapy, chemotherapy, phototherapy), and palliation. Preference was given to randomized controlled trials (RCT) and prospective observational studies. For each of these topics we searched MEDLINE, Ovid MEDLINE In-Process, Cochrane Database of Systematic Reviews, Database of Systematic Reviews, Database of Abstracts of Reviews of Effects, EMBASE, PubMed, National Library of Medicine Gateway by established systematic review methods (Jadad Scale for RCT controlled studies, Downs and Black checklist for observational studies[4-6]. We further reviewed reference lists and articles from the authors’ libraries. We limited our search to English-language articles published from January 1990 to June 2009. We then developed a comprehensive and current database to catalog the medical literature on CC. The evidence database for the catalog was assembled only for CC arising in the intra- and extra-hepatic bile ducts. Our review did not include the management of gallbladder cancer, as several other comprehensive articles had already covered this topic[7-10]. To identify all potential papers, we searched medical subject headings reported in Table 1. Two authors (Aljiffry M and Molinari M) independently performed the selection of the articles based on the content of titles and abstracts. When in doubt, each article was reviewed entirely. The decision to include articles in this review was reached by consensus. For conciseness, a full list of search strategies, search results, and quality assessment for each included study are available on request from the corresponding author.
| Primary MeSH terms | Secondary MeSH terms (Epidemiology, diagnosis) | Secondary MeSH terms (treatment, palliation) |
| Cholangiocarcinoma(s) | Epidemiology | Hepatectomy |
| Adenocarcinoma(s) | Classification | Resection |
| Carcinoma(s) | Diagnosis | Therapeutic(s) |
| Bile duct neoplasm(s) | Differential diagnosis | Treatment outcome(s) |
| Biliary tract neoplasm(s) | Early diagnosis | Surgery |
| Common bile duct neoplasm(s) | Risk factor(s) | Transplantation |
| Liver neoplasm(s) | Diagnostic imaging | Biliary tract |
| Bile duct(s) | Magnetic resonance imaging | Surgical procedures |
| Common bile duct | Endosonography | Liver transplantation |
| Intrahepatic bile duct(s) | Ultrasonography | Organ transplantation |
| Extrahepatic bile duct(s) | Emission computed tomography | Clinical trial |
| Biliary tract disease(s) | Radionuclide imaging | Controlled clinical trial(s) |
| Bile duct disease(s) | Positron emission tomography | Randomized controlled trial(s) |
| X-ray | Clinical trial (phase I) | |
| Computed tomography | Clinical trial (phase II) | |
| Biopsy (needle) | Clinical trial (phase III) | |
| Biopsy (fine needle) | Clinical trial (phase IV) | |
| Cytology | Drug therapy | |
| Cytodiagnosis | Chemotherapy | |
| Tumor markers (biological) antigen(s) | Adjuvant | |
| Carcinoembryonic antigen | Antineoplastic agent(s) | |
| Ca 19-9 antigen | Combined modality therapy | |
| Ca 125 antigen | Antineoplastic | |
| Endoscopic retrograde cholangiopancreatography | Combined chemotherapy protocols | |
| Cholangiography | Neoadjuvant therapy | |
| In situ hybridization | Radiotherapy | |
| Fluorescence in situ hybridization | Adjuvant embolization | |
| Nucleic acid hybridization | Portal vein embolization | |
| Computed assisted image processing | Drainage | |
| Cholestasis | ||
| Obstructive jaundice |
The incidence of CC is rising in most countries and it is the second most common primary malignancy of the liver after hepatocellular carcinoma[1]. In the USA, approximately 5000 new cases are diagnosed every year[11] accounting for almost 3% of all tumors of the gastrointestinal tract[12]. While the incidence of ICC is rising, the occurrence of ECC is trending down[13,14] suggesting that different risk factors may be involved[15]. Caution should be used when interpreting these results as misclassification bias may have influenced these observations[2,16]. In fact, analysis of the Surveillance Epidemiology and End Results database from 1975 until 1999 has shown that most hilar tumors (more than 90%) were classified as ICC[2,16] while ECC were often combined with gallbladder cancers[2,13]. Nevertheless, evidence that ICC and ECC may be dissimilar tumors is supported by the recent discovery that, in vitro, they express diverse cellular proteins and have different cellular shape, doubling time, chromosome karyotype and chemosensitivity[17]. Similarly, researchers from France showed that hilar CC (HCC) express higher levels of MUC5AC (60% vs 22%), Akt2 (64% vs 36%), CK8 (98% vs 82%), annexin (56% vs 44%) and less vascular epithelial growth factor (22% vs 78%) in comparison to ICC[18]. These findings support the hypothesis that the heterogeneous protein and receptor expression of ECC and ICC may be due to different carcinogenic pathways[17,18].
The estimated age-adjusted incidence rates of ICC in the USA has increased by 165% over the last thirty years (from 0.32 per 100 000 in 1975-1979 to 0.85 per 100 000 in 1995-1999[2,19] accounting for 10% to 15% of all primary hepatic cancers[20]. The average age at presentation is the seventh decade of life[2] with a male to female ratio of 1.5[20]. The mortality rate and incidence of ICC have parallel trends[13] as age-adjusted mortality rate increased from 0.07 per 100 000 in 1973 to 0.69 per 100 000 in 1997[21].
In the USA, the age-adjusted incidence of ECC has decreased by 14% compared to data from two decades earlier. Currently it is 1.2 per 100 000 in men and 0.8 per 100 000 in women[2,22]. Similarly to ICC, 65% of ECC present in the seventh decade of life[22]. The mortality rate of ECC parallels its incidence and in the USA, the age-adjusted mortality rates for ECC declined from 0.6 per 100 000 in 1979 to 0.3 per 100 000 in 1998[14,21].
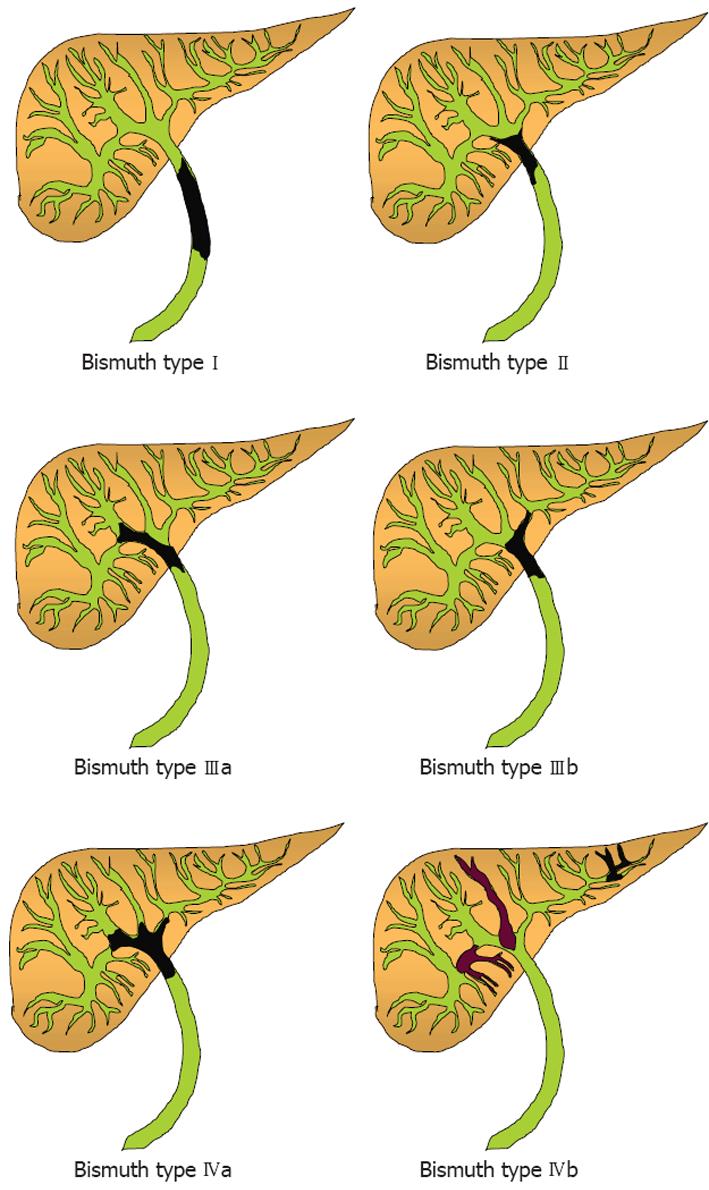
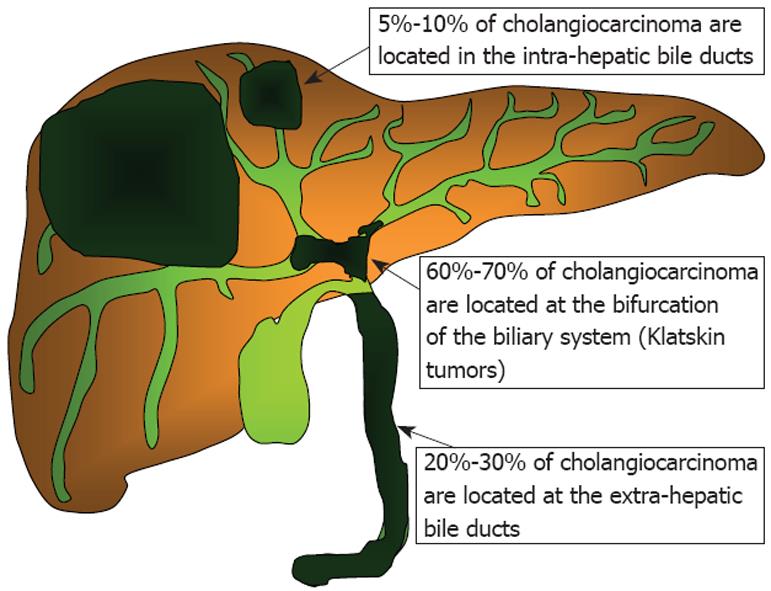
ICCs arise within the liver parenchyma while ECCs involve the biliary tree within the hepatoduodenal ligament and gallbladder. ECCs are further divided into hilar or distal tumors. HCC, also called Klatskin tumors, are located within 2 cm from the bifurcation of the common duct and were described for the first time by Klatskin in 1965[22]. Ten years later, Bismuth and Corlette proposed a clinical classification that stratifies these tumors by anatomical location (Figure 1)[23]. Approximately 60% to 70% of CC are located in the hylum, 20% to 30% are ECC, and 5% to 10% are ICC (Figure 2)[24,25].
More than 90% of CC are well- to moderately-differentiated adenocarcinomas[26,27] with tendency to develop desmoplastic reaction and early perineural invasion. Macroscopically, ICC may develop in solid masses, infiltrate periductal tissues, grow intraductally or have mixed characteristics. On the other hand, ECC develop nodular lesions, sclerosing strictures, or papillary growth patterns. Sclerosing CC are the most common[28] while papillary adenocarcinomas are rare and associated with more favorable prognosis[22].
Only a minority of patients presenting with CC have known risk factors such as chronic biliary inflammation, cholestasis or congenital abnormalities (Table 2)[29].
| General risk factors |
| Old age (older than 65 years) |
| Smoking |
| Obesity |
| Diabetes |
| Post surgical |
| Biliary-enteric anastomosis |
| Chronic inflammatory diseases |
| Primary sclerosing cholangitis (PSC) |
| Hepatolithiasis (Oriental Cholangiohepatitis) |
| Hepatitis C |
| Hepatitis B |
| Human Immunodeficiency Virus (HIV) |
| Liver cirrhosis |
| Parasitic infections |
| Opisthorchis viverrini |
| Clonorchis sinensis |
| Congenital |
| Choledochal cysts |
| Caroli’s disease |
| Congenital hepatic fibrosis |
| Chemical agents |
| Thorotrast |
| Dioxin |
| Nitrosamines |
| Asbestos |
| Medications |
| Oral Contraceptive Pills |
| Isoniazid |
In Western countries, PSC is the most important predisposing factor for CC[30,31]. The cumulative annual risk of CC in patients with PSC is 1.5% per year after the development of jaundice[32] and the prevalence of CC in patients with PSC ranges between 8% and 40%[30,33,34]. A recent epidemiological study from the Netherlands has shown that the risk of CC for patients with PSC is 9% after 10 years from the time of the diagnosis[35]. In patients with concomitant inflammatory bowel disease, the 10-year and 20-year risks for CC are 14% and 31% respectively, which are significantly higher than patients without inflammatory bowel disease (2% and 2% respectively; P = 0.008)[35]. Individuals with PSC frequently develop CC at younger age (30-50 years) compared to the general population (60-70 years)[30,32]. The diagnosis of CC in this group is challenging because clinical presentation and radiological findings of CC and PSC are similar. As a result, most cases of CC complicating PSC are detected at advanced stages and have poor prognosis[36]. Predictive factors of CC in PSC patients are: sudden progressive jaundice, unintentional weight loss, marked dilation of bile ducts proximal to biliary strictures, serum level of Ca 19-9 tumor marker above 100 U/mL, and presence of cellular dysplasia on cytological specimens obtained by brushing of the biliary ducts[37].
Infestation with liver flukes (Opisthorchis viverrini and Clonorchis sinensis) has been strongly associated with an increased risk of CC in South-East Asia[3,38,39]. In areas where Opisthorchis viverrini is endemic, the adjusted prevalence for CC by age and gender is as high as 14%[40]. The pathophysiology causing CC in these patients is not completely understood, however it is hypothesized that parasites colonize the biliary system causing chronic inflammation and predisposing to malignant transformation.
Oriental cholangiohepatitis (also known as recurrent pyogenic cholangiohepatitis) has a prevalence of 20% in South-East Asia[41] and almost 10% of affected patients develop ICC[42-45]. Recurrent episodes of cholangitis aggravate the chronic inflammatory process that persists between flare ups. Risk factors associated with CC in these patients are: age over 40 years, long history of hepatolithiasis, unintentional weight loss, increasing serum alkaline phosphatase, decreasing serum albumin, and serum CEA tumor marker above 4.2 ng/mL[46].
Patients with choledochal cysts have low risk for CC if the cyst is excised early in their life[46]. On the other hand, the incidence of malignant degeneration is between 10% and 20% if the cyst is not excised by the age of 20 years[28,47]. The mechanism of malignant transformation is not totally understood although biliary stasis and reflux of pancreatic secretions are suspected of causing neoplasia through chronic inflammation[48]. CC can occur years after resection of the cyst suggesting that there might be a genetic defect predisposing to tumors of the biliary system[49].
The risk of developing CC in cirrhotic patients is ten-fold higher than the general population (0.7% vs 10.7%)[2,50]. Among patients with CC in the USA, the prevalence of hepatitis C viral infection (HCV) was found to be four times higher than the general population (0.8% vs 0.2%)[20]. These results have been confirmed in Italy[51], in Taiwan[52] and in Japan where HCV and viral hepatitis B (HBV) infection were detected in 23% and 11.5% of CC compared to 6% and 5.5% of controls, respectively, with cumulative rates 1000-times greater than the general population[53]. Similar results were recently confirmed in a case-control study performed in China, where researchers found that at multivariate analysis, significant risk factors for the development of ICC were hepatolithiasis (adjusted OR: 5.7; 95% CI: 1.9-16.8) and HBV infection (adjusted OR: 8.8; 95% CI: 5.9-13.1)[15]. A large epidemiological study from the United States validated that HCV infection is a risk factor for ICC (hazard ratio 2.55; 95% CI: 1.3-4.9) but not for ECC (hazard ratio 1.5; 95% CI: 0.6-1.85)[54]. Although human immunodeficiency virus (HIV) does not cause cirrhosis per se, 0.5% of patients infected with HIV have been found to have CC in comparison to 0.1% among controls, confirming previous observations which suggested that chronic viral infections might predispose to neoplastic transformation of some cell lines[20].
Several compounds have been suspected of causing CC. Thorotrast (thorium dioxide) needs a special mention because it was used as a radiological contrast in the period between 1920-1950 and was found to increase the risk of CC up to 300-times in comparison to the general population[16,55]. Because of its long biological half-life (400 years), the latency period of thorotrast-induced CC ranges between 16 to 45 years[56], with the highest incidence between 20 to 30 years after exposure[57]. A few studies have shown an association between CC and other chemical agents such as asbestos[58], vinyl chloride[59] and nitrosamines[60]. Medications such as isoniazide[61] and first generation of oral contraceptives[62] are also suspected of increasing the risk of CC.
Tobacco smoking is weakly associated with CC in the general population[20] while it appears to be a strong risk factor for PSC patients[63]. Other predisposing factors for CC are diabetes, obesity, presence of bile duct adenomas and biliary papillomatosis[64,65]. Although there is no evidence that endoscopic sphincterotomy increases the risk of CC, biliary-enteric bypasses may do so[66].
CCs are usually clinically silent or associated with nonspecific symptoms in early stage (Table 3)[67,68]. ICCs are often diagnosed by imaging tests, and rarely during physical exams, as asymptomatic hepatic masses[26]. On the other hand, ECC usually present with painless jaundice[69] and symptoms related to biliary obstruction such as itching, clay-colored stool and hyperpigmented urine[69]. Only 10% of cases present with ascending cholangitis[70]. Jaundice is usually persistent and progressive while intermittent biliary obstruction may be observed in patients with papillary lesions that cause a ball-valve effect[71]. Physical examination of patients with CC may reveal hepatomegaly, palpable gallbladder (Courvoisier sign), or signs of portal hypertension due to portal vein thrombosis secondary to tumor invasion or compression[33,69].
| Symptoms | Percentage (%) |
| Jaundice | 84 |
| Weight loss | 35 |
| Abdominal pain | 30 |
| Nausea and vomiting | 20 |
| Fever | 10 |
Serum biochemical tests usually support the clinical suspicion of CC but they are rarely diagnostic. Jaundice occurs only if there is obstruction of the two main intra-hepatic biliary ducts or common bile duct. In these circumstances, elevation of the serum levels of bilirubin and markers of biliary epithelial injury, such as alkaline phosphatase (ALP) and gamma glutamyltransferase (GTT)[33], are common[72,73]. On the other hand, in the presence of unilateral intrahepatic biliary obstruction, elevation of ALP or GTT may be present without increase in the serum bilirubin level[33]. Other abnormal laboratory findings include hypoalbuminemia and prolonged prothrombin time, which reflect the combination of diminished hepatic synthetic function, cachexia and malabsorption of vitamin K[33].
Several tumor markers may support the diagnosis of CC, although none of them is sensitive enough to be used for screening purposes. The most commonly used markers are carbohydrate antigen (Ca 19-9) and carcinoembryonic antigen (CEA)[73]. These tumor markers are not very specific as they can be elevated in the presence of other malignancies (e.g. pancreas and stomach) and with benign conditions such as cholangitis and hepatolithiasis[73-75]. In patients without PSC, serum Ca 19-9 values above 100 U/mL have a sensitivity of 53% and specificity of 75%-90% for the diagnosis of CC[74]. In patients with PSC, serum Ca 19-9 levels above 100 U/mL have sensitivity of 75%-89% and specificity of 80%-86% for the diagnosis of CC[75-77]. In a recent study from the Mayo Clinic, the optimal cutoff value for serum Ca 19-9 in patients with PSC was 20 U/mL which provided a sensitivity of 78%, specificity of 67%, positive predictive values of 23% and negative predictive value of 96%[78]. Serum Ca 19-9 combined with either ultrasonography, computed tomography, or magnetic resonance imaging provided a sensitivity of 91%, 100% and 96% respectively for CC diagnosis[78]. The levels of Ca 19-9 seem to correlate with the stage of the disease. Patel et al[72] reported that the sensitivity of Ca 19-9 above 100 U/mL for the diagnosis of CC in patients with resectable tumors was 33% compared to 72% in patients with unresectable tumors. Using more than one tumor marker for patients with PSC may improve the detection rate of CC. In one study, using Ca 19-9 levels above 180 U/mL in combination with CEA levels above 5.2 ng/mL had a sensitivity of 100% and a specificity of 78.4%[79]. Several new markers are currently being investigated. The human mucin 5, subtypes A and C (MUC5AC) are the most promising for future clinical use with sensitivity and specificity of 71% and 90%, respectively[80].
Imaging modalities are essential for the diagnosis and treatment planning of patients with CC[73].
US is usually the initial imaging test performed to evaluate patients with biliary obstruction[81]. The sensitivity and accuracy of US for ECC diagnosis are 89%[82] and 80%-95%, respectively[83,84]. On the other hand, ICC are difficult to distinguish from other solid intra-hepatic masses as they lack specific US features[83,84]. The use of duplex US with color Doppler technology is helpful in assessing portal venous invasion and hepatic parenchymal involvement. In a small series of patients with HCC, duplex US detected portal vein invasion correctly in 86% of patients[85]. In a larger study, duplex US was 93% sensitive and 99% specific for detecting portal vein involvement[86]. As the sensitivity and specificity of US are operator-dependent, most patients with suspected CC undergo further imaging modalities to confirm and stage suspected tumors[87]. The sensitivity of US improves significantly in the presence of elevated serum tumor marker Ca 19-9[80]. Serum level of Ca 19-9 above 20 U/mL in patients with PSC has been shown to increase the diagnostic sensitivity of abdominal US up to 91%, with specificity of 62%, positive predictive value of 23%, and negative predictive value of 98%[80].
Triple-phase CT scan is widely used to diagnose and stage CC[88] as it provides valuable information regarding local spread, vascular invasion, lymph node involvement and presence of distant metastases[89,90]. On CT scans, ICC usually present as hypodense lesions with irregular margins on initial images and a variable degree of delayed venous phase enhancement[86]. These characteristics have been shown to correlate with prognosis as hyperattenuating CC have a more aggressive behavior[91]. Other CT findings of ICC include dilatation and thickening of the peripheral intra-hepatic bile ducts and liver capsular retraction[92]. ECC may be seen as a focal thickening of the ductal wall with various enhancement patterns[93]. However, in many cases of ECC, visualization of the neoplasms is not definitive because they are too small to be detected. More recent studies[92,94] have shown that modern contrast-enhanced multidetector row computed tomography was 78.6%-92.3% accurate for the diagnosis of ECC, although there was a strong tendency to underestimate the longitudinal extension of the tumor (77.8%) in comparison with the pathological results of the excised specimens[95,96]. Four-channel multidetector-row CT has been shown to correctly diagnose hepatic artery invasion with 100% sensitivity and 90% specificity and portal vein invasion with 92.3% sensitivity and 90.2% specificity[96]. Regular enhanced CT can be extremely useful by showing indirect signs of ECC such as biliary ductal dilatation and hepatic lobar atrophy. Atrophy of one hepatic lobe could be associated with hypertrophy of the opposite lobe, a condition known as the atrophy-hypertrophy complex. This phenomenon is seen when CC obstruct the biliary outflow of a single lobe and invade the ipsilateral portal vein causing compensatory hypertrophy of the opposite hepatic lobe[97]. The sensitivity of triple-phase helical CT in the detection of HCC is in the range of 90% to 100%[92,98] and it is even more sensitive in detecting ICC greater than 1 cm in size[90]. These results show a marked improvement in the diagnostic yield of CT compared to previous reports in which the tumor detection rate was only 60%[99]. CT is also useful for assessing the vascular and lymph node status of patients affected by CC. In a series of 55 patients with HCC, CT accurately predicted portal vein invasion, arterial invasion, and lymph node involvement in 86%, 93%, and 84% of patients, respectively[100]. The overall accuracy of CT for determining resectability of CC is in the range of 60% to 85%[90,100,101]. Recently, CT cholangiography (CTC) has been shown to be a promising modality for delineating the biliary tree. In a large study, CTC was superior to conventional CT or US and equal to endoscopic retrograde cholangiopancreaticography (ERCP) for the diagnosis of HCC[102]. In another smaller study, the sensitivity and specificity of CTC for malignant biliary obstruction were both 94%[103]. One of the limitations of CTC is that optimal imaging quality depends on the secretory function of the liver[104]. For patients affected by PSC, the combination of tumor serology (serum level of Ca 19-9 above 20 U/mL) and contrast-enhanced abdominal CT scan has been shown to improve the diagnostic sensitivity (100%), specificity (38%), positive predictive value (22%) and negative predictive value (100%) of the test[80].
MRI with concurrent MRCP can provide three-dimensional reconstruction of the biliary tree by using magnetic resonance technology[105]. Multiple studies have demonstrated the utility of MRCP in evaluating patients with CC[106,107]. MRCP has diagnostic accuracy comparable to invasive cholangiographic techniques such as ERCP or percutaneous transhepatic cholangiography (PTC)[108-111]. A further advantage of MRCP over invasive cholangiographies is that it does not require biliary instrumentation[112]. Therefore, MRI along with MRCP is considered the radiological modality of choice for evaluating patients with suspected CC[113]. MRCP/MRI allows definition of the anatomy and extent of CC within the hepatobiliary system[108,110,114] vascular invasion, local lymphadenopathy and distant metastases[108,113,115]. Ideally, MRCP should be performed before decompressing the biliary tree[86]. ICC appear as a hypointense lesion on T1- and hyperintense on T2-weighted images with pooling of contrast within the tumor on delayed pictures as seen with CT[116,117]. On MRCP, ECC may appear as extrahepatic lesions with similar signal intensity of ICC on both T1- and T2-weighted images, in addition to proximal biliary dilatation[106,116]. A meta-analysis of 67 studies (4711 patients) evaluating MRCP performance in patients with suspected biliary diseases showed an overall sensitivity of 88% and specificity of 95%[107]. In a series of 99 patients with HCC, MRCP accurately determined the longitudinal extension of the tumor in 88% of patients[118]. In another smaller study, MRCP predicted the extent of biliary ductal involvement in 96% of cases with malignant hilar obstructions[115]. Regarding surrounding structures, MRI has been shown to have 66% accuracy for detection of lymph node metastases[119], 78% sensitivity and 91% specificity for portal vein invasion, 58%-73% sensitivity[120] and 93% specificity for arterial invasion[121]. In a comparative study the relationship of ICC to the vessels and surrounding organs was more easily evaluated on CT compared to MRI[89]. For patients affected by PSC and CC, the diagnostic capacity of MRI is enhanced by the presence of serum tumor marker Ca 19-9 above 20 U/mL; as a recent study has shown that the sensitivity of the test in this case was 96%, specificity 37%, positive predictive value 24%, and negative predictive value 98%[80].
ERCP and PTC provide dynamic images but require invasive access to the biliary system. Both techniques can detect biliary abnormalities and determine the location and extent of ECC within the biliary tree. The choice between PTC and ERCP is generally dictated by the availability of local expertise and the anatomical characteristics of the tumor[69]. In patients with complete biliary obstruction, ERCP often cannot assess the proximal biliary tree while PTC cannot assess the distal extent of the tumor[33,122].
The sensitivity and specificity of cholangiography range between 75%-85%, and 70%-75%, respectively[109,110] with accuracy of 95%[123]. Recent data have shown that in the presence of PSC, the association of an elevated level of serum Ca 19-9 increases the diagnostic utility of ERCP as its sensitivity was 91%, specificity 69%, positive predictive value 42%, and negative predictive value was 96%[80]. A drawback of these invasive procedures is the risk of complications such as post-ERCP pancreatitis (4%-10%)[123], bacteriobilia (30%-100%)[73], bleeding, sepsis, vascular injury and death[124]. On the other hand, ERCP and PTC have the advantage of providing brush cytology and bioptic specimens that can confirm the diagnosis of CC. The sensitivity of biopsy and brush cytology for diagnosing CC has been low due to the desmoplastic reaction associated with the tumor which is characterized by the presence of few malignant cholangiocytes within an extensive fibrous stroma[11]. In a large prospective study, the sensitivity of routine cytology varied from 9%-24% and the specificity varied from 61%-100% with a high rate of inter-pathologist variation; the best diagnostic yield was obtained when the pathologists were aware of the patient’s clinical condition[125]. This was recently confirmed by a study from the Mayo Clinic which showed that in patients affected by PSC, the simultaneous presence of an elevated serum tumor marker level (Ca 19-9 above 20 U/mL) increased the sensitivity (50%), specificity (97%), positive predictive value (86%) and negative predictive value (88%) of cytological specimens[80]. In another study, repeated brushing appeared to be a valuable strategy to improve the sensitivity of cytological analysis up to 44%[126]. Endoscopic transpapillary forceps biopsies had a diagnostic sensitivity of 52% and a specificity of 100%[127]. Advanced cytologic techniques, including digitized image analysis (DIA) and fluorescence in situ hybridization (FISH), have been recently used to increase the sensitivity of cytology[128] especially in patients with PSC[80]. The DIA technique quantitates nuclear DNA via special stains to assess the presence of aneuploidy, whereas FISH analysis detects chromosomal polysomy by using fluorescent probes. In a prospective study, DIA increased the sensitivity from 18% to 39% but decreased the specificity from 98% to 77%[129]. In another comparative study, FISH increased the sensitivity from 15% to 34% compared to routine cytology, with similar specificities (91% for FISH and 98% for routine cytology)[130]. For patients with PSC, the presence of elevated serum level of Ca 19-9 (above 20 U/mL) increases the diagnostic capacity of DIA and FISH; as a recent study measuring these parameters has shown that their sensitivity was 57% and 86%, specificity 94% and 83%, positive predictive value 89% and 80%, and negative predictive value was 74% and 88%, respectively[80]. The use of peroral cholangioscopy (POCS) or choledochoscopy has been shown to improve the diagnostic capacity of ERCP by directing tissue sampling. Fukuda et al[131] reported a sensitivity of 100% and a specificity of 87% for diagnosing the etiology of bile duct strictures by adding POCS to ERCP. At this moment, the availability of POCS is limited to a few centers due to lack of expertise and the high costs of instrumentation. The introduction of new technologies such as SpyGlass®, a single operator peroral cholangiopancreatoscopy, has eliminated the need for two ERCP operators and has the potential of becoming an important tool to improve the diagnostic capacities of endoscopic techniques, and it is currently under investigation[132-134].
EUS is performed by using high frequency ultrasound probes placed on the endoscope. EUS has the advantage of interrogating tissues and organs in direct proximity to the stomach and duodenum, increasing the ability to detect abnormalities that would not be easily identified by percutaneous approach. In a prospective study of patients with suspected CC, EUS had a diagnostic sensitivity of 79% and specificity of 62%[111]. This was confirmed in a recent meta-analysis where EUS had sensitivity and specificity of 78% and 84%, respectively[135]. Two of the most attractive features of EUS are the ability to perform direct-guided fine needle aspirations (FNA) of the tumors in patients with negative cytology or the ability to sample enlarged lymph nodes for preoperative staging[136-138]. However, caution should be applied in patients with potentially curative CC as this approach has some risk of peritoneal seeding[65,130]. A recent prospective study evaluated the diagnostic yield of EUS-guided FNA of suspected HCC in potentially operable patients with negative brush cytology. The study showed sensitivity and specificity of 89% and 100%, respectively, and changed the preplanned surgical approach in 61% of patients[136]. In another prospective study, EUS-guided FNA of suspected CC reported a diagnostic sensitivity of 86%, with a specificity of 100%. In the same study, EUS-guided FNA had a positive impact on the treatment management of 84% of patients[139].
IDUS is performed by using high frequency US probes placed into the common bile duct under ERCP guidance[140]. Malignant biliary strictures often appear on IDUS as a hypoechoic infiltration of the ductal wall with irregular margins[141,142]. In a prospective study of 62 patients with biliary strictures, IDUS had a diagnostic sensitivity of 90% and specificity of 93%[143]. In another study by Stavropoulos et al[144], IDUS increased the diagnostic accuracy of ERCP from 58% to 90% in a series of patients with biliary strictures and no mass detected on CT.
PET is a non-invasive imaging modality that provides functional images by detecting radiotracer 18F-fluorodeoxyglucose (FDG) uptake in neoplastic cells[145]. Currently it is considered a standard modality for the staging of many malignancies[146]. In the last decade, integrated PET and CT imaging systems (PET/CT) have combined the ability to obtain anatomical and functional images[146,147]. PET and PET/CT are proven to be useful in the diagnosis and staging of CC. In a recent study, PET showed sensitivity and specificity of 90% and 78% respectively[148]. In another study by Anderson et al[149], PET had sensitivity of 85% for CC measuring at least 1 cm in size although its sensitivity was only 18% for infiltrating CC. These values were confirmed by Kluge et al[150] who reported sensitivity of 92% and specificity of 93% for the detection of any type of CC by PET scan. A more recent study has shown that the sensitivity of PET/CT is correlated with the stage of CC as the sensitivity of the study was 25% for T2 tumors, 70% for T3 tumors and 66.7% for T4 tumors[151]. The rate of detecting distant metastases by PET and PET/CT in patients with CC is in the range of 70% to 100%, while the detection of regional lymph node metastases is only about 12%[152]. The sensitivity and specificity of PET/CT for detecting lymph node metastasis and distant metastasis were 41.7% and 80%, and 55.6% and 87.5%, respectively[146]. Another study from the Memorial Sloan Kettering Cancer Center has shown that PET/CT had an overall sensitivity for identifying the primary tumor of 80% (78% for CC and 86% for gallbladder cancer) and changed management in nearly a quarter of all patients[153]. PET has been shown to be useful in monitoring tumor response to treatment. In a small series by Chikamoto et al[154], PET had a sensitivity of 80% for detecting local recurrence after resection in patients with HCC. One of the limitations of PET is that patients with biliary inflammatory conditions such as PSC or cholangitis may have false positive results[152,155] while patients with mucinous CC may have falsely negative scans due to poor uptake of FDG[155].
OCT is a new technique that produces cross-sectional images using infrared light. Preliminary studies have demonstrated the ability of OCT to generate high resolution images of the biliary tree that correlate with histological findings[156,157]. OCT has the potential to identify early CC[104] but it is not widely available except in a few centers. Therefore the role of OCT in the diagnostic workup of CC is not yet established.
Non-diagnostic cytology or biopsy results should not rule out CC in the presence of appropriate clinical and radiological findings[73]. In the absence of other explainable causes of biliary strictures, patients should be considered to have CC and treated as such, accepting that 10% to 15% will have benign lesions on final pathology[158,159]. For high risk patients, no surveillance or screening programs have been validated. Some authors advocate annual follow up with non-invasive modalities (tumor markers and radiological tests), reserving invasive methods only when cytology and bioptic specimens or stenting are indicated[160].
Understanding the patterns of spread of CC is essential for staging and treatment planning. CC can spread along biliary ducts, invade perineural and vascular tissues, spread directly into adjacent structures, invade lymph nodes or develop distant metastasis. Longitudinal extension of CC consists of mucosal (superficial) or submucosal (invasive) infiltration depending on the tumor growth pattern. Mucosal extension is predominantly seen with papillary (intraductal) and nodular (mass-forming) tumors, while submucosal extension is mainly seen with sclerosing (infiltrating) tumors[161]. The length of longitudinal extension is determined by the type of invasion, with a mean length of 6-10 mm for the submucosal spread and 10-20 mm for the mucosal spread[162]. Therefore, a gross surgical margin of more than 1 cm in the infiltrating type and more than 2 cm in the papillary and nodular types is recommended to achieve negative microscopic resection margins. One of the special characteristics of CC is the presence of perineural invasion that is seen in about 75% of cases[163,164]. Perineural invasion is a prognostic factor for poor survival[164,165]. In a retrospective review by He et al[164], the 5-year survival rate was 47% for patients without perineural invasion compared to 13% for those with perineural invasion. HCC can spread directly into the hepatic parenchyma and the hepatoduodenal ligament where the proper hepatic artery and the portal vein are in close proximity to the bile duct, while distal ECC may directly infiltrate into the pancreas or the duodenum[166]. Up to 80% of HCC have extension into the liver parenchyma[166,167] by direct infiltration or by longitudinal extension along the biliary ducts[168]. The latter mechanism explains the caudate lobe involvement by HCC and tumors involving the left hepatic duct[169]. Hence, the practice of partial hepatectomy with caudate lobectomy for the surgical treatment of patients with hilar tumors is associated with improved survival[168]. Tumors at the biliary confluence involve the portal vein in 30% of cases and often result in hepatic lobar atrophy[166,167]. The significance of portal vein involvement in patients’ survival is controversial. Some studies have shown that tumor invasion of the portal vein has a negative impact[170,171] while other investigators reported opposite findings[166]. This is most likely due to the fact that patients with portal vein invasion may tolerate more extensive surgical resections as the contralateral lobe becomes hypertrophied, therefore decreasing the risk of perioperative mortality and enhancing the chances of negative resection margins. Lymph node metastases usually involve the regional hilar nodes and to a lesser extent the para-aortic lymphatic nodes[172]. The prevalence of lymph node involvement is approximately 45% for all CC with distal ECC having the highest incidence of nodal metastases[68,172]. Several studies have confirmed that lymphatic tumor involvement is an important prognostic factor. Survival rates for patients undergoing surgical resection with positive lymphatic invasion at 5 years are 10% to 15% in comparison to 30% to 40% for patients without lymph node metastasis[164,172]. Presence of distant metastases (e.g. lung, bone, peritoneal, distant lymph nodes) is observed in 30% of patients at the time of diagnosis and is associated with survival of only a few months[166].
ICC and ECC are staged differently.
ICC are classified as primary liver malignancies in the new American Joint Committee on Cancer (AJCC) staging system, also known as the TNM staging (Table 4)[173]. The AJCC staging system for primary liver tumors was based on data provided by patients affected by hepatocellular carcinomas and therefore is not sufficiently accurate for ICC[174]. A new staging system for ICC was proposed by Nathan et al[174] based on the number of tumors, vascular invasion, lymph node status and presence of metastatic disease. The presence of multiple tumors may be indicative of satellite neoplastic deposits or intrahepatic metastatic disease from hematogenous or lymphatic spread; similarly to vascular and lymph node invasion, it is associated with poor survival.
| Stage | Tumor | Node | Metastasis |
| I | T1 | N0 | M0 |
| II | T2 | N0 | M0 |
| IIIA | T3 | N0 | M0 |
| IIIB | T4 | N0 | M0 |
| IIIC | Any T | N1 | M0 |
| IV | Any T | Any N | M1 |
Giving the proximity of ECCs to the portal vein and hepatic artery, the goal of staging is to determine the local extent of the disease as it predicts resectability and the extent of the resection. The AJCC staging system for ECC (Table 5)[173] is based on pathological data useful in identifying the patients’ prognosis but with little applicability for assessing the feasibility of surgical treatment[174]. Bismuth-Corlette classification for HCC is useful for describing the tumor location and its spread within the biliary tree but it is not predictive of resectability. The Memorial Sloan-Kettering Cancer Center (MSKCC) has proposed a staging system known as T-stage criteria[174]. The MSKCC staging system is based on the location and extent of ductal involvement, presence or absence of portal vein invasion, and presence or absence of hepatic lobar atrophy irrespective of metastases or lymph node status (Table 6). The MSKCC staging system for HCC correlates with resectability and survival, as 59% of T1 lesions are resectable with median survival of 20 mo compared to 0% resectability for T3 lesions with a median survival of only 8 mo[174].
| Stage | Tumor | Node | Metastasis |
| 0 | Tis | N0 | M0 |
| IA | T1 | N0 | M0 |
| IB | T2 | N0 | M0 |
| IIA | T3 | N0 | M0 |
| IIB | T1-T3 | N1 | M0 |
| III | T4 | Any N | M0 |
| IV | Any T | Any N | M1 |
| Stage | Criteria |
| T1 | Tumor involving biliary confluence with or without unilateral extension to second-order biliary radicles |
| T2 | Tumor involving biliary confluence with or without unilateral extension to second-order biliary radicles and ipsilateral portal vein involvement with or without ipsilateral hepatic lobar atrophy |
| T3 | Tumor involving biliary confluence with bilateral extension to second-order biliary radicles; or unilateral extension to second-order biliary radicles with contralateral portal vein involvement; or unilateral extension to second-order biliary radicles with contralateral hepatic lobar atrophy; or main or bilateral portal vein involvement |
Tumor resection is the only potential cure for CC and the median survival of patients with unresectable disease is 6 to 12 mo[26]. All patients with resectable ICC and HCC, and the majority of patients with ECC, require partial hepatectomy to increase the chances of negative resection margins. Preoperative patients’ evaluation includes an extensive assessment of their fitness for major surgery, the absence of any metastatic disease and the possibility of resection margins free from cancer[175]. If any of these conditions are not satisfied, surgical therapy is not indicated and palliative modalities should be recommended.
Many patients are not considered surgical candidates because of the presence of comorbidities or advanced age. A patient’s performance status, nutritional conditions, and comorbidities need to be carefully evaluated before considering surgery[176]. A retrospective review of patients with resected HCC showed that the presence of preoperative serum albumin less than 3 g/dL was a significant predictive factor for high postoperative mortality[177]. In the same study, a preoperative serum total bilirubin above 10 mg/dL was associated with lower survival rates[177]. The role of preoperative biliary drainage (PBD) in jaundiced patients remains controversial. A recent meta-analysis failed to demonstrate any benefit[178]. Furthermore, PBD seems to increase the risk of perioperative infections and a longer postoperative hospital stay[124,178].
Nevertheless, prolonged preoperative biliary obstruction is associated with increased postoperative morbidity and mortality after hepatic resection due to the presence of severe cholestatic liver dysfunction[177,179]. Currently, preoperative PBD is not routinely recommended, but it has been shown to be beneficial in the presence of cholangitis, severe malnutrition, coagulation abnormalities[180,181] and when patients require major hepatic resection[175,182]. When preoperative drainage is performed, definitive surgery should be deferred for a few weeks to allow sufficient restoration of hepatic function[168]. The use of liver volumetric and/or hepatic functional studies is warranted when anticipating an extended hepatic resection, to estimate the future liver remnant and minimize the risk of liver failure caused by insufficient function or small residual liver parenchyma.
Meticulous interpretation of all the available clinical and radiological data is recommended to determine resectability and avoid unnecessary interventions. Despite the improvement of diagnostic modalities, about 16%-25% of patients are found to have more extensive disease preventing resection at the time of laparotomy[68,183]. The major determinants of resectability are the extent of tumor within the biliary tree, the amount of hepatic parenchyma involved, vascular invasion, hepatic lobar atrophy, and metastatic disease[166,179]. A review of 294 cases of CC demonstrated that resectability rates are higher for more distal tumors[69]. The determination of resectability is most challenging in patients with HCC. It is reported that about half of patients with HCC deemed to be resectable preoperatively have unresectable disease when explored[174]. The radiological criteria defining unresectability in patients with HCC are listed in Table 7[174,175]. With regard to distal ECC and ICC, AJCC stages III and IV are generally considered unresectable (Table 8).
| Local tumor invasion |
| Bilateral hepatic duct involvement up to secondary biliary radicles |
| Encasement or occlusion of the main portal vein |
| Unilateral tumor extension to secondary biliary radicles with contralateral portal vein or hepatic artery encasement or occlusion |
| Hepatic lobar atrophy with contralateral portal vein or hepatic artery encasement or occlusion |
| Hepatic lobar atrophy with contralateral tumor extension to secondary biliary radicles |
| Metastatic disease |
| Lymph node metastases beyond the hepatoduodenal ligament (N2 lymph nodes)1 |
| Distant metastasis (e.g. lung, liver, peritoneal) |
| Author (yr) | Resections (n) | Overall 5-year survival (%) | R0 5-year survival (%) |
| DeOliveira et al[67], 2007 | 34 | 40 | 63 |
| Miwa et al[195], 2006 | 41 | 29 | 36 |
| Jan et al[196], 2005 | 81 | 15 | NR |
| Ohtsuka et al[197], 2003 | 50 | 23 | NR |
| Uenishi et al[198], 2001 | 28 | 27 | 67 |
| Inoue et al[199], 2000 | 52 | 36 | 55 |
| Yamamoto et al[200], 1999 | 83 | 23 | 53 |
| Madariaga et al[201], 1998 | 34 | 35 | 41 |
Generally, invasion of the main portal vein or invasion of the vasculature supplying the planned hepatic remnant preclude resection. Nevertheless, recent reports have shown that en-bloc resection with vascular reconstruction may achieve negative margins and potential cure with only 10% perioperative mortality in very selected patients[184,185]. The application of staging laparoscopy has been recently advocated as it can reduce the number of unnecessary laparotomies by identifying metastatic lesions in the liver and in the peritoneal cavity[186]. The yield of laparoscopy for detecting unresectability in patients with potentially resectable CC on preoperative imaging modalities is about 25% with an overall accuracy of 50%[187,188]. Moreover, laparoscopy offers the addition of intraoperative hepatic US, which can increase the diagnostic yield up to 42%[189]. One of the limitations of laparoscopy is the inability to detect vascular or nodal involvement[188,189]. Peritoneal washings to obtain cytology specimens have not been shown to predict occult metastasis in patients with CC[190]. Ultimately, true resectability is determined after a complete abdominal exploration.
The goal of surgery is to obtain complete excision of the tumor with negative histological margins (R0 resection), as this is associated with marked survival advantages compared to margin positive resections (R1 or R2 resection)[26,68,176,191]. To confirm histologically-negative margins, many authors advocate the use of intraoperative frozen section examinations of the bile ducts[174]. A very important study from the MSKCC has recently evaluated the clinical significance of intraoperative frozen section for patients affected by HCC[192]. The primary aim of this study was to assess the importance of obtaining frozen sections of the bile duct margins for the planning of the extent of the surgical dissection. Frozen sections were obtained in 101 patients: among them 20 (19.8%) had positive and 81 (80.1%) had negative results. Among the patients who had negative frozen sections, 8 (9.8%) individuals were found to have positive margins at subsequent histopathology. In this study, intraoperative frozen section was shown to be 71.4% sensitive, 100% specific, and with a positive predictive value of 100% and negative predictive value of 80.2%[192].
Surgical therapy for ICC is based on the same principles used for hepatic resections performed for hepatocellular carcinomas or secondary tumors. The operative approach should be aimed at ensuring R0 resection margins whenever it is possible. Lymph node dissection during resection of ICC is not recommended as it does not improve patients’ survival[193,194]. Current outcomes after surgical resection have improved in comparison to historical data with 5-year survival rates ranging from 20% to 40% (Table 9)[195-201]. Predictors of poor outcomes include: positive resection margins, lymphatic and vascular invasion and periductal infiltrating disease[202,203]. The most common site of recurrence after surgical resection is within the liver[196].
| Author (yr) | Resections (n) | Liver resection (%) | R0 resection (%) | Overall 5-year survival (%) | R0 5-year survival (%) |
| Hasegawa et al[213], 2007 | 49 | 92 | 78 | 40 | 50 |
| DeOliveira et al[67], 2007 | 173 | 20 | 19 | 10 | 30 |
| Dinant et al[214], 2006 | 99 | 38 | 31 | 27 | 33 |
| Hemming et al[205], 2005 | 53 | 98 | 80 | 35 | 45 |
| Rea et al[208], 2004 | 46 | 100 | 80 | 26 | 30 |
| Kawasaki et al[182], 20031 | 79 | 96 | 68 | NR | 40 |
| Kawarada[215], 2002 | 87 | 75 | 64 | 26 | NR |
| Jarnagin et al[166], 2001 | 80 | 78 | 78 | 37 | NR |
| Tabata et al[216], 2000 | 75 | 71 | 60 | 23 | 40 |
| Kosuge et al[217], 1999 | 65 | 88 | 52 | 35 | 52 |
| Miyazaki et al[218], 1998 | 76 | 86 | 71 | 26 | 40 |
Curative surgery of HCC usually requires the excision of the extrahepatic bile duct, regional lymphadenectomy, cholecystectomy and in most cases some sort of partial hepatectomy including the caudate lobe, especially for tumors mainly extending in the left hepatic duct[174,207]. The rationale behind performing partial hepatectomies in HCC is to ensure histologically negative margins. Several studies have shown that this strategy increases R0 resections in up to 80% of patients[174,182,205]. Extended lymphadenectomy is not recommended as there is no evidence showing survival advantage[168,172].
Radical resection of HCC has 5%-10% perioperative mortality rate, especially when extended hepatectomy (5 or more segments) is required[174,206-208]. This partly relates to the increased rate of postoperative liver failure with major hepatic resections. Portal vein embolization (PVE) is a valuable preoperative measure when anticipating extensive liver resections with subsequent small hepatic residual volume[209]. A compensatory hypertrophy of the remnant hepatic parenchyma is induced by selectively occluding the main portal vein branch to the lobe that will be resected. This can increase the volume of the anticipated liver remnant by 12%-20%, thereby reducing the rate of postoperative liver dysfunction[210,211]. PVE is useful when the anticipated liver remnant volume is less than 20%-25% of the total liver volume in patients with normal liver function, and when the anticipated liver remnant volume is 40% or less in patients with compromised liver function[212].
The average 5-year survival rates post-resection for HCC are 25%-40% (Table 10)[68,174,182,209,213-218]. Factors associated with favorable outcome include; R0 resection, no lymph node metastasis, absence of perineural invasion, and well-differentiated histological grade[174,185].
The same principle of achieving a negative resection margin applies to ECC. The resectability rate has been reported as being up to 90% with distal extrahepatic tumors[68,219]. Complete removal of distal ECC usually requires a pancreaticoduodenectomy (Whipple procedure)[73,220,221]. Even in these circumstances, extended lymphadenectomy is not justified as it does not provide survival advantages and it is associated with increased perioperative morbidity[222]. Segmental bile duct excision is rarely an option, except for CC located in the middle of the common duct in the absence of periductal invasion or spread to the surrounding structures. Only 10% of patients undergoing bile duct excision alone obtain curative resection margins on final pathology[222,223]. Most commonly, when approaching patients with CC arising midway along the extrahepatic duct, surgeons should assess whether a pancreaticoduodenectomy or a partial hepatectomy is more appropriate with regard to the tumor extension. In these patients, curative resections are associated with a 25%-50% 5-year survival rate (Table 10)[68,220,223-225]. The main determinants of poor outcomes are positive surgical margins and lymph node involvement[220,223]. Other factors associated with unfavorable prognosis include pancreatic invasion, duodenal invasion, perineural invasion, and a poorly-differentiated histology[68,220].
Transplantation is an emerging therapy for unresectable CC without evidence of metastatic disease. Candidates are individuals who would require a total hepatectomy to achieve clear margins and those with underlying liver failure precluding hepatic resection. The early experience of transplantation for CC reported early recurrence rates of more than 50% and a 5-year survival of 10%-20%[226-228]. More recently, in highly selected patients undergoing neoadjuvant protocols, promising results have been reported. In 2002, Sudan et al[229] reported a series of 11 patients transplanted for CC after neoadjuvant chemoradiation with 45% tumor free survival and median follow up of 7.5 years. Similar reports have been reported by Becker et al[230] who observed a 45% 5-year survival for patients who were diagnosed as being affected by CC prior to undergoing transplantation, and a 33% 5-year survival was observed by Sotiropoulos et al[231] in Germany. At the Mayo Clinic, Rosen et al[232,233] have developed a liver transplantation protocol for HCC that provides a disease-free 5-year survival of 82%. This protocol is aimed at treating unresectable HCC or CC in PSC patients. To be eligible for this protocol, the diagnosis of CC is confirmed histologically, considered unresectable and with no evidence of metastatic disease. Eligible patients receive neoadjuvant chemoradiation therapy followed by staging laparotomy to rule out metastatic disease followed by living-related or cadaveric liver transplantation. Currently, the use of liver transplantation for the treatment of CC is reserved only for highly selected patients in specialized centers.
The use of postoperative chemotherapy, radiotherapy or chemoradiation therapy have been evaluated as means of improving disease-free survival in patients with resected tumors since CC have high rates of local and distant recurrence.
Postoperative chemotherapy has failed to show significant survival benefits[234,235]. A recent multicenter RCT evaluated the effect of postoperative chemotherapy with mitomycin C and 5-fluorouracil (5FU) versus surgery alone for individuals affected by cancers of the pancreas and biliary system[236]. Among 508 patients post-R0 resection, 139 individuals were affected by CC and for these individuals no survival benefit was seen after chemotherapy treatment[237].
The use of postoperative external beam radiation with or without intraoperative radiotherapy and intraluminal radiotherapy (brachytherapy) has been explored in the adjuvant setting without significant benefits after R0 resections[125,237,238]. On the other hand, several studies showed that adjuvant radiotherapy may benefit patients with positive resection margins[239-241]. Todoroki et al[241] showed that the 5-year survival in patients with R1 resections was 34% when adjuvant radiotherapy (intraoperative and external beam) was used compared to 14% with surgery alone.
The radiosensitizing effect of chemotherapeutic agents has been evaluated in the adjuvant setting with positive results only for distal ECC. In a retrospective cohort study of 94 individuals who underwent resection for CC, 34% received postoperative chemoradiation[242]. Longer survival was seen in patients who received adjuvant therapy (median survival 41 mo vs 24 mo)[243]. Other retrospective studies demonstrated similar results and showed that patients with distal ECC had a superior survival advantage in comparison to more proximal CC following adjuvant therapy[243,244]. Recently, Hughes et al[245] have confirmed a slight 5-year survival advantage with postoperative chemoradiation therapy in patients with distal ECC compared with surgical resection alone (35% vs 27%). In line with these findings, Figueras et al[246] did not demonstrate a significant survival benefit with adjuvant chemoradiation therapy for HCC. These results need to be confirmed further with larger prospective trials. For ICC, evidence to support the use of adjuvant chemoradiation therapy is very limited. In a recent retrospective study of 3839 patients, Shinohara et al[247] have shown that the overall survival rate was significantly different between groups receiving surgery alone and surgery plus adjuvant radiation therapy (P = 0.014) and between radiation therapy alone and no treatment (P < 0.0001). The combination of surgery and adjuvant radiation therapy conferred the greatest benefit on overall survival (HR: 0.40; 95% CI: 0.3-0.47), followed by surgery alone (HR: 0.49; 95% CI: 0.44-0.54) and radiation therapy alone (HR: 0.68; 95% CI: 0.59-0.77) compared with no treatment.
There is a lack of RCT evaluating the utility of adjuvant therapy following R0 resections of CC. Moreover, most of the current studies are small and retrospective in nature and incorporated CC with cancers of the gallbladder and pancreas. Therefore, no standard adjuvant modalities are universally embraced for the treatment of CC.
The role of preoperative chemoradiation therapy has been evaluated in a small series of patients with ECC[248]. Among nine patients who underwent neoadjuvant therapy, McMasters et al[248] observed pathological complete response in 3 individuals and negative resection margins were obtained in all subjects. More recently, neoadjuvant therapy has been used in the setting of liver transplantation for CC with promising results. Further trials are required to better assess its efficacy.
Nearly half of the patients with CC are considered candidates only for palliative treatments due to the advanced stage of their disease at the time of diagnosis or the presence of significant comorbidities that prevent surgical therapy[68,174]. Therefore, palliation plays an important role in the management of these individuals. The primary aim of palliative interventions is to improve quality of life by relieving symptoms and prolonging survival by preventing cholestatic liver failure. In the presence of incurable CC, tissue diagnosis should be obtained whenever possible to direct palliative therapy planning.
Biliary obstruction is the major cause of morbidity and mortality in patients with CC. The goals of biliary decompression are to relieve jaundice, pain, pruritus, and to prevent cholangitis and cholestatic liver failure[249]. Different modalities are currently available to drain the biliary system and these include: endoscopic, percutaneous and surgical bypass. The ideal palliative biliary decompression should be effective, provide durable results, and have low risks of morbidity and mortality.
Biliary stenting can be achieved endoscopically or percutaneously. Endoscopic biliary stenting is the most widely used method and the percutaneous approach is usually performed when endoscopic drainage fails or cannot be performed. Percutaneous stents can be either internal, external or both. External stents have the disadvantage of draining bile without the ability of enteric recycling and are associated with more discomfort and reduced quality of life. Little is known as to whether percutaneous or endoscopic biliary drainage have different overall efficacy in palliating patients with unresectable disease. Generally, only patients with advanced tumors that are totally obstructed are candidates for percutaneous external biliary drainage. A recent multicenter retrospective study from South Korea has shown that the placement of percutaneous self-expanding metallic stents across HCCs is associated with a higher success rate and lower risk of procedure-induced cholangitis[250].
Endoscopic stents can be either self-expanding metallic or plastic (polyethylene). Metal stents are more expensive than plastic stents but have larger diameters and provide better patency rates[251]. Metal stents can be either uncovered or covered by sealing the metallic mesh with a membrane which prevents tumor growth through the stent, increasing patency rates. Plastic stents often need to be changed at 2 to 3-mo intervals, while metal stents can remain patent up to 9 mo[252]. Several RCT have compared metal to plastic stents for the treatment of patients with inoperable malignant biliary obstruction[253,254]. These studies concluded that metal stents are more cost-effective for patients who are expected to survive more than 5 mo as they need less interventions and shorter hospitalizations[254,255]. Patency rates are generally higher for ECC[255] and metal stents provide superior palliation for HCC as compared to plastic stents[256,257]. Draining about 25% of the hepatic parenchyma is usually sufficient for adequate palliation in the absence of infection[250]. A RCT comparing unilateral versus bilateral drainage in patients with malignant hilar obstruction found that drainage of one functional hepatic lobe is sufficient to relieve obstruction with no difference in complication and survival rates[258]. It is important to note that stents placed for hilar lesions will require re-intervention in about 30% of patients due to stent occlusion[259,260]. A RCT comparing covered to uncovered stents in patients with unresectable distal biliary malignancies showed that the patency of covered stents was significantly higher than that of uncovered stents[261]. However, multiple studies report an increased risk of cholecystitis (5%) with the covered stents due to cystic duct occlusion[262].
Biliary-enteric anastomosis can be performed by open or laparoscopic approach. Studies comparing surgical to non-surgical biliary drainage showed similar overall palliative effects but with higher perioperative morbidity and mortality[263,264]. Surgical drainage has the advantage of superior patency rates and prevents the need for stent exchanges required when using endoscopic or percutaneous stents due to clogging[265]. Currently, the main candidates for surgical drainage are patients found to have unresectable CC at the time of exploration, individuals who are not able to undergo repeat endoscopic or percutaneous stent exchanges, and those who have long expected survival and who are fit for surgery[183,266].
Palliative radiotherapy may benefit patients with locally advanced unresectable CC or those who have undergone palliative bypass in the absence of distant metastases. The use of palliative radiotherapy has beneficial effects on pain relief, biliary patency and overall patient survival[267,268]. The two most commonly used radiotherapy modalities are external beam radiation with 30 to 50 Gy, intraluminal brachytherapy with 10 to 20 Gy or the combination of both. Intraluminal brachytherapy is delivered by using iridium-192 seeds mounted on a catheter that is deployed across the tumor by endoscopic or percutaneous approach[269]. It appears that brachytherapy is able to deliver more effective doses of radiation without damaging the surrounding organs. Generally, the majority of studies that demonstrated benefit of radiotherapy used combinations of both modalities with median patient survival ranging between 9 and 14 mo[270-273]. Palliative radiotherapy is associated with increased incidence of complications such as cholangitis, gastroduodenitis and longer hospital stay in comparison to best supportive care and therefore it is not routinely used in many centers[274]. Moreover, higher doses of radiation (more than 55 Gy) may be required to obtain an improved survival, with increased toxicity rates[275]. Controlled studies are required to better evaluate the effectiveness and safety of these palliative treatments. For ICC, brachytherapy can be delivered by radioembolization with yttrium-90 microspheres[276]. This approach has been shown to provide partial response in 27% of patients and stable disease in 68% with limited side effects; therefore it is not surprising that it has become the leading modality for palliation of CC in centers where this technique is available[277].
There is no standard chemotherapy option for patients with CC. Patients with widespread disease considered for palliative chemotherapy undergo treatment in an attempt to control the disease and improve their overall survival. Owing to the lack of RCT and the retrospective nature of observational studies with heterogenous patient populations currently available, the interpretation of the survival benefit of palliative chemotherapy is difficult. Various chemotherapeutic agents with different dosing regimens have been tested with overall poor survival improvement. Historically, 5FU was the first chemotherapeutic agent used for palliation of CC patients with only 10% response rates when used alone. Several subsequent studies have evaluated 5FU in combination with other agents such as leucovorin, interferon-alpha, cisplatin, and oxaliplatin, with an overall response rate of 25% to 55% and a median survival ranging between 6 and 12 mo[278-281]. Multiple phase II trials have evaluated the use of oral 5FU prodrugs (uracil-tegafur and capecitabine) in patients with advanced CC[282,283]. For example, the combination of capecitabine and cisplatin had mild toxicity and produced a response in 41% of patients with a median survival of 12 mo[283]. Gemcitabine proved to have good efficacy as a single agent in biliary malignancies with response rates of 30%[284]. Several gemcitabine-based combinations, including cisplatin, capecitabine, and oxaliplatin have reported response rates up to 36% and median survival of 10 to 15 mo[285-287]. Finally, a recent analysis of all the published chemotherapy trials in individuals affected by advanced CC from 1985 to 2006 concluded that gemcitabine combined with platinum compounds (cisplatin or oxaliplatin) had the best patient response rates[288,289]. Recently, the roles of transcatheter arterial chemoembolization (TACE) and transcatheter arterial chemoinfusion (TACI) have been assessed for patients affected by unresectable ICC[290,291]. Although TACE and TACI with gemcitabine, cisplatin and doxorubicin in different combinations[292] are well tolerated, survival benefits have not been proven in large studies and will require further evidence before becoming widely accepted in the scientific community.
PDT is an emerging palliative strategy based on the intravenous administration of photosensitizing agents that preferentially accumulate in malignant cells. After the delivery of these photosensitizing agents, specific wavelengths of light are administered causing activation of the photosensitizer and thus tumor cell necrosis[293]. The depth of tumor necrosis obtained by this technique is between 4 mm and 6 mm[294]. This modality is currently used as a palliative measure in conjunction with biliary stenting for nonresectable CCs. Improvements in quality of life, biliary drainage, and survival in patients with advanced CCs post-PDT have been reported in several case series[294]. Furthermore, a RCT compared PDT with endoscopic stenting to stenting alone in patients with unresectable CC[295]. The study was terminated prematurely because PDT proved to be markedly superior to simple stenting. The PDT group in that trial had higher median survival (493 d vs 98 d), improved biliary drainage and better quality of life than the stenting alone group[296]. Recently, PDT was investigated as a neoadjuvant modality before surgical resection of advanced HCC in 7 patients[296]. Tumor-free resection margins were achieved in all patients with a 1-year recurrence-free survival rate of 83%[297]. A recent study confirmed that the use of PDT as a neoadjuvant therapy is safe and can downstage tumors from unresectable to resectable[297]. The main side effects of PDT include photosensitivity caused by the administration of photosensitizer agents and cholangitis related to biliary instrumentation[294,298].
Other palliative measures: Several other palliative modalities have shown some benefits in selected groups of patients. Radiofrequency ablation has been used for patients unfit for surgery who have small intrahepatic CC[299]. In a single-centre cohort of patients with unresectable intrahepatic CC, TACE has shown some survival advantage in comparison to best supportive care (median survival: 23 mo)[300]. Hepatic arterial chemoinfusion offers tumor-directed chemotherapy and it has been proven to be safe[301] as has localized ablation of tumor cells by high intensity intraductal ultrasound[302]. Another promising area is the use of molecular targeting agents for chemoprevention and adjuvant therapy of CC such as cyclooxygenase-2 and nitric oxide inhibitors[303]. The clinical utility of these emerging therapies needs further investigation before gaining wide acceptance.
Over the last decades several advances have occurred in the fields of epidemiology, diagnostic modalities, medical and surgical treatment of CC as well as in palliation. The diagnosis, staging and further management of patients affected by this disease may be a complex issue and requires expertise in many fields. To optimize the outcome of patients with suspected or proven CC, a multidisciplinary approach is recommended.
| 2. | Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24:115-125. |
| 4. | Cook DJ, Mulrow CD, Haynes RB. Systematic reviews: synthesis of best evidence for clinical decisions. Ann Intern Med. 1997;126:376-380. |
| 5. | Mulrow CD, Oxman AD. Cochrane Collaboration Handbook. Oxford: Update Software 1997; 1. |
| 6. | Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377-384. |
| 7. | Sikora SS, Singh RK. Surgical strategies in patients with gallbladder cancer: nihilism to optimism. J Surg Oncol. 2006;93:670-681. |
| 8. | Thomas MB. Biological characteristics of cancers in the gallbladder and biliary tract and targeted therapy. Crit Rev Oncol Hematol. 2007;61:44-51. |
| 9. | Tang B, Cuschieri A. Conversions during laparoscopic cholecystectomy: risk factors and effects on patient outcome. J Gastrointest Surg. 2006;10:1081-1091. |
| 10. | Oikarinen H. Diagnostic imaging of carcinomas of the gallbladder and the bile ducts. Acta Radiol. 2006;47:345-358. |
| 11. | Lazaridis KN, Gores GJ. Cholangiocarcinoma. Gastroenterology. 2005;128:1655-1667. |
| 12. | Vauthey JN, Blumgart LH. Recent advances in the management of cholangiocarcinomas. Semin Liver Dis. 1994;14:109-114. |
| 13. | Khan SA, Taylor-Robinson SD, Toledano MB, Beck A, Elliott P, Thomas HC. Changing international trends in mortality rates for liver, biliary and pancreatic tumours. J Hepatol. 2002;37:806-813. |
| 14. | Patel T. Worldwide trends in mortality from biliary tract malignancies. BMC Cancer. 2002;2:10. |
| 15. | Zhou YM, Yin ZF, Yang JM, Li B, Shao WY, Xu F, Wang YL, Li DQ. Risk factors for intrahepatic cholangiocarcinoma: a case-control study in China. World J Gastroenterol. 2008;14:632-635. |
| 16. | Khan SA, Thomas HC, Davidson BR, Taylor-Robinson SD. Cholangiocarcinoma. Lancet. 2005;366:1303-1314. |
| 17. | He XR, Wu XP. Difference in biological characteristics and sensitivity to chemotherapy and radiotherapy between intrahepatic and extrahepatic cholangiocarcinoma cells in vitro. Chin Med Sci J. 2008;23:54-59. |
| 18. | Guedj N, Martine P, Degos F, Zhan Q, Valla D, Belghiti J, Farges O, Bedossa P, Paradis V. Are hilar and intrahepatic cholangiocarcinomas different entities? J Hepatology. 2007;46:242A. |
| 19. | Shaib YH, Davila JA, McGlynn K, El-Serag HB. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol. 2004;40:472-477. |
| 20. | Shaib YH, El-Serag HB, Davila JA, Morgan R, McGlynn KA. Risk factors of intrahepatic cholangiocarcinoma in the United States: a case-control study. Gastroenterology. 2005;128:620-626. |
| 21. | Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33:1353-1357. |
| 22. | Klatskin G. Adenocarcinoma of the hepatic duct at its bifurcation within the porta hepatis. An unusual tumor with distinctive clinical and pathological features. Am J Med. 1965;38:241-256. |
| 23. | Bismuth H, Corlette MB. Intrahepatic cholangioenteric anastomosis in carcinoma of the hilus of the liver. Surg Gynecol Obstet. 1975;140:170-178. |
| 24. | Patel T. Cholangiocarcinoma. Nat Clin Pract Gastroenterol Hepatol. 2006;3:33-42. |
| 25. | Ishak KG, Anthony PP, Sobin LH. World Health Organization International Histologic Classification of Tumors: Histological Typing of Tumors to the Liver. 2nd ed. Berlin: Springer Verlag 1994; . |
| 26. | Carriaga MT, Henson DE. Liver, gallbladder, extrahepatic bile ducts, and pancreas. Cancer. 1995;75:171-190. |
| 27. | Lim JH, Park CK. Pathology of cholangiocarcinoma. Abdom Imaging. 2004;29:540-547. |
| 29. | Broomé U, Olsson R, Lööf L, Bodemar G, Hultcrantz R, Danielsson A, Prytz H, Sandberg-Gertzén H, Wallerstedt S, Lindberg G. Natural history and prognostic factors in 305 Swedish patients with primary sclerosing cholangitis. Gut. 1996;38:610-615. |
| 30. | Bergquist A, Broomé U. Hepatobiliary and extra-hepatic malignancies in primary sclerosing cholangitis. Best Pract Res Clin Gastroenterol. 2001;15:643-656. |
| 31. | Bergquist A, Ekbom A, Olsson R, Kornfeldt D, Lööf L, Danielsson A, Hultcrantz R, Lindgren S, Prytz H, Sandberg-Gertzén H. Hepatic and extrahepatic malignancies in primary sclerosing cholangitis. J Hepatol. 2002;36:321-327. |
| 32. | Farrant JM, Hayllar KM, Wilkinson ML, Karani J, Portmann BC, Westaby D, Williams R. Natural history and prognostic variables in primary sclerosing cholangitis. Gastroenterology. 1991;100:1710-1717. |
| 33. | Pitt HA, Dooley WC, Yeo CJ, Cameron JL. Malignancies of the biliary tree. Curr Probl Surg. 1995;32:1-90. |
| 34. | Rosen CB, Nagorney DM, Wiesner RH, Coffey RJ Jr, LaRusso NF. Cholangiocarcinoma complicating primary sclerosing cholangitis. Ann Surg. 1991;213:21-25. |
| 35. | Claessen MM, Vleggaar FP, Tytgat KM, Siersema PD, van Buuren HR. High lifetime risk of cancer in primary sclerosing cholangitis. J Hepatol. 2009;50:158-164. |
| 36. | Kaya M, de Groen PC, Angulo P, Nagorney DM, Gunderson LL, Gores GJ, Haddock MG, Lindor KD. Treatment of cholangiocarcinoma complicating primary sclerosing cholangitis: the Mayo Clinic experience. Am J Gastroenterol. 2001;96:1164-1169. |
| 38. | Haswell-Elkins MR, Mairiang E, Mairiang P, Chaiyakum J, Chamadol N, Loapaiboon V, Sithithaworn P, Elkins DB. Cross-sectional study of Opisthorchis viverrini infection and cholangiocarcinoma in communities within a high-risk area in northeast Thailand. Int J Cancer. 1994;59:505-509. |
| 39. | Jang KT, Hong SM, Lee KT, Lee JG, Choi SH, Heo JS, Choi DW, Choi D, Lim JH. Intraductal papillary neoplasm of the bile duct associated with Clonorchis sinensis infection. Virchows Arch. 2008;453:589-598. |
| 40. | Su CH, Shyr YM, Lui WY, P'Eng FK. Hepatolithiasis associated with cholangiocarcinoma. Br J Surg. 1997;84:969-973. |
| 41. | Chen MF, Jan YY, Wang CS, Hwang TL, Jeng LB, Chen SC, Chen TJ. A reappraisal of cholangiocarcinoma in patient with hepatolithiasis. Cancer. 1993;71:2461-2465. |
| 42. | Kubo S, Kinoshita H, Hirohashi K, Hamba H. Hepatolithiasis associated with cholangiocarcinoma. World J Surg. 1995;19:637-641. |
| 43. | Lesurtel M, Regimbeau JM, Farges O, Colombat M, Sauvanet A, Belghiti J. Intrahepatic cholangiocarcinoma and hepatolithiasis: an unusual association in Western countries. Eur J Gastroenterol Hepatol. 2002;14:1025-1027. |
| 44. | Chu KM, Lo CM, Liu CL, Fan ST. Malignancy associated with hepatolithiasis. Hepatogastroenterology. 1997;44:352-357. |
| 45. | Kim YT, Byun JS, Kim J, Jang YH, Lee WJ, Ryu JK, Kim SW, Yoon YB, Kim CY. Factors predicting concurrent cholangiocarcinomas associated with hepatolithiasis. Hepatogastroenterology. 2003;50:8-12. |
| 46. | Hewitt PM, Krige JE, Bornman PC, Terblanche J. Choledochal cysts in adults. Br J Surg. 1995;82:382-385. |
| 47. | Lipsett PA, Pitt HA, Colombani PM, Boitnott JK, Cameron JL. Choledochal cyst disease. A changing pattern of presentation. Ann Surg. 1994;220:644-652. |
| 48. | Ohtsuka T, Inoue K, Ohuchida J, Nabae T, Takahata S, Niiyama H, Yokohata K, Ogawa Y, Yamaguchi K, Chijiiwa K. Carcinoma arising in choledochocele. Endoscopy. 2001;33:614-619. |
| 49. | Goto N, Yasuda I, Uematsu T, Kanemura N, Takao S, Ando K, Kato T, Osada S, Takao H, Saji S. Intrahepatic cholangiocarcinoma arising 10 years after the excision of congenital extrahepatic biliary dilation. J Gastroenterol. 2001;36:856-862. |
| 50. | Sorensen HT, Friis S, Olsen JH, Thulstrup AM, Mellemkjaer L, Linet M, Trichopoulos D, Vilstrup H, Olsen J. Risk of liver and other types of cancer in patients with cirrhosis: a nationwide cohort study in Denmark. Hepatology. 1998;28:921-925. |
| 51. | Donato F, Gelatti U, Tagger A, Favret M, Ribero ML, Callea F, Martelli C, Savio A, Trevisi P, Nardi G. Intrahepatic cholangiocarcinoma and hepatitis C and B virus infection, alcohol intake, and hepatolithiasis: a case-control study in Italy. Cancer Causes Control. 2001;12:959-964. |
| 52. | Lee CH, Chang CJ, Lin YJ, Yeh CN, Chen MF, Hsieh SY. Viral hepatitis-associated intrahepatic cholangiocarcinoma shares common disease processes with hepatocellular carcinoma. Br J Cancer. 2009;100:1765-1770. |
| 53. | Kobayashi M, Ikeda K, Saitoh S, Suzuki F, Tsubota A, Suzuki Y, Arase Y, Murashima N, Chayama K, Kumada H. Incidence of primary cholangiocellular carcinoma of the liver in japanese patients with hepatitis C virus-related cirrhosis. Cancer. 2000;88:2471-2477. |
| 54. | El-Serag HB, Engels EA, Landgren O, Chiao E, Henderson L, Amaratunge HC, Giordano TP. Risk of hepatobiliary and pancreatic cancers after hepatitis C virus infection: A population-based study of U.S. veterans. Hepatology. 2009;49:116-123. |
| 55. | Sahani D, Prasad SR, Tannabe KK, Hahn PF, Mueller PR, Saini S. Thorotrast-induced cholangiocarcinoma: case report. Abdom Imaging. 2003;28:72-74. |
| 56. | Lipshutz GS, Brennan TV, Warren RS. Thorotrast-induced liver neoplasia: a collective review. J Am Coll Surg. 2002;195:713-718. |
| 57. | Rubel LR, Ishak KG. Thorotrast-associated cholangiocarcinoma: an epidemiologic and clinicopathologic study. Cancer. 1982;50:1408-1415. |
| 58. | Szendröi M, Németh L, Vajta G. Asbestos bodies in a bile duct cancer after occupational exposure. Environ Res. 1983;30:270-280. |
| 59. | Wong O, Whorton MD, Foliart DE, Ragland D. An industry-wide epidemiologic study of vinyl chloride workers, 1942-1982. Am J Ind Med. 1991;20:317-334. |
| 60. | Mitacek EJ, Brunnemann KD, Hoffmann D, Limsila T, Suttajit M, Martin N, Caplan LS. Volatile nitrosamines and tobacco-specific nitrosamines in the smoke of Thai cigarettes: a risk factor for lung cancer and a suspected risk factor for liver cancer in Thailand. Carcinogenesis. 1999;20:133-137. |
| 62. | Yen S, Hsieh CC, MacMahon B. Extrahepatic bile duct cancer and smoking, beverage consumption, past medical history, and oral-contraceptive use. Cancer. 1987;59:2112-2116. |
| 63. | Bergquist A, Glaumann H, Persson B, Broomé U. Risk factors and clinical presentation of hepatobiliary carcinoma in patients with primary sclerosing cholangitis: a case-control study. Hepatology. 1998;27:311-316. |
| 64. | Oh SW, Yoon YS, Shin SA. Effects of excess weight on cancer incidences depending on cancer sites and histologic findings among men: Korea National Health Insurance Corporation Study. J Clin Oncol. 2005;23:4742-4754. |
| 65. | Malhi H, Gores GJ. Review article: the modern diagnosis and therapy of cholangiocarcinoma. Aliment Pharmacol Ther. 2006;23:1287-1296. |
| 66. | Hakamada K, Sasaki M, Endoh M, Itoh T, Morita T, Konn M. Late development of bile duct cancer after sphincteroplasty: a ten- to twenty-two-year follow-up study. Surgery. 1997;121:488-492. |
| 67. | DeOliveira ML, Cunningham SC, Cameron JL, Kamangar F, Winter JM, Lillemoe KD, Choti MA, Yeo CJ, Schulick RD. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg. 2007;245:755-762. |
| 68. | Nakeeb A, Pitt HA, Sohn TA, Coleman J, Abrams RA, Piantadosi S, Hruban RH, Lillemoe KD, Yeo CJ, Cameron JL. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996;224:463-473; discussion 473-475. |
| 69. | Khan SA, Davidson BR, Goldin R, Pereira SP, Rosenberg WM, Taylor-Robinson SD, Thillainayagam AV, Thomas HC, Thursz MR, Wasan H. Guidelines for the diagnosis and treatment of cholangiocarcinoma: consensus document. Gut. 2002;51 Suppl 6:VI1-VI9. |
| 70. | Washburn WK, Lewis WD, Jenkins RL. Aggressive surgical resection for cholangiocarcinoma. Arch Surg. 1995;130:270-276. |
| 71. | Jarnagin WR. Cholangiocarcinoma of the extrahepatic bile ducts. Semin Surg Oncol. 2000;19:156-176. |
| 72. | Patel AH, Harnois DM, Klee GG, LaRusso NF, Gores GJ. The utility of CA 19-9 in the diagnoses of cholangiocarcinoma in patients without primary sclerosing cholangitis. Am J Gastroenterol. 2000;95:204-207. |
| 73. | Chen CY, Shiesh SC, Tsao HC, Lin XZ. The assessment of biliary CA 125, CA 19-9 and CEA in diagnosing cholangiocarcinoma--the influence of sampling time and hepatolithiasis. Hepatogastroenterology. 2002;49:616-620. |
| 75. | Maestranzi S, Przemioslo R, Mitchell H, Sherwood RA. The effect of benign and malignant liver disease on the tumour markers CA19-9 and CEA. Ann Clin Biochem. 1998;35:99-103. |
| 76. | Chalasani N, Baluyut A, Ismail A, Zaman A, Sood G, Ghalib R, McCashland TM, Reddy KR, Zervos X, Anbari MA. Cholangiocarcinoma in patients with primary sclerosing cholangitis: a multicenter case-control study. Hepatology. 2000;31:7-11. |
| 77. | Nichols JC, Gores GJ, LaRusso NF, Wiesner RH, Nagorney DM, Ritts RE Jr. Diagnostic role of serum CA 19-9 for cholangiocarcinoma in patients with primary sclerosing cholangitis. Mayo Clin Proc. 1993;68:874-879. |
| 78. | Charatcharoenwitthaya P, Enders FB, Halling KC, Lindor KD. Utility of serum tumor markers, imaging, and biliary cytology for detecting cholangiocarcinoma in primary sclerosing cholangitis. Hepatology. 2008;48:1106-1117. |
| 79. | Siqueira E, Schoen RE, Silverman W, Martin J, Rabinovitz M, Weissfeld JL, Abu-Elmaagd K, Madariaga JR, Slivka A. Detecting cholangiocarcinoma in patients with primary sclerosing cholangitis. Gastrointest Endosc. 2002;56:40-47. |
| 80. | Bamrungphon W, Prempracha N, Bunchu N, Rangdaeng S, Sandhu T, Srisukho S, Boonla C, Wongkham S. A new mucin antibody/enzyme-linked lectin-sandwich assay of serum MUC5AC mucin for the diagnosis of cholangiocarcinoma. Cancer Lett. 2007;247:301-308. |
| 81. | Saini S. Imaging of the hepatobiliary tract. N Engl J Med. 1997;336:1889-1894. |
| 82. | Sharma MP, Ahuja V. Aetiological spectrum of obstructive jaundice and diagnostic ability of ultrasonography: a clinician's perspective. Trop Gastroenterol. 1999;20:167-169. |
| 83. | Bloom CM, Langer B, Wilson SR. Role of US in the detection, characterization, and staging of cholangiocarcinoma. Radiographics. 1999;19:1199-1218. |
| 84. | Slattery JM, Sahani DV. What is the current state-of-the-art imaging for detection and staging of cholangiocarcinoma? Oncologist. 2006;11:913-922. |
| 85. | Hann LE, Greatrex KV, Bach AM, Fong Y, Blumgart LH. Cholangiocarcinoma at the hepatic hilus: sonographic findings. AJR Am J Roentgenol. 1997;168:985-989. |
| 86. | Bach AM, Hann LE, Brown KT, Getrajdman GI, Herman SK, Fong Y, Blumgart LH. Portal vein evaluation with US: comparison to angiography combined with CT arterial portography. Radiology. 1996;201:149-154. |
| 87. | Robledo R, Muro A, Prieto ML. Extrahepatic bile duct carcinoma: US characteristics and accuracy in demonstration of tumors. Radiology. 1996;198:869-873. |
| 88. | Valls C, Gumà A, Puig I, Sanchez A, Andía E, Serrano T, Figueras J. Intrahepatic peripheral cholangiocarcinoma: CT evaluation. Abdom Imaging. 2000;25:490-496. |
| 89. | Zhang Y, Uchida M, Abe T, Nishimura H, Hayabuchi N, Nakashima Y. Intrahepatic peripheral cholangiocarcinoma: comparison of dynamic CT and dynamic MRI. J Comput Assist Tomogr. 1999;23:670-677. |
| 90. | Tillich M, Mischinger HJ, Preisegger KH, Rabl H, Szolar DH. Multiphasic helical CT in diagnosis and staging of hilar cholangiocarcinoma. AJR Am J Roentgenol. 1998;171:651-658. |
| 91. | Asayama Y, Yoshimitsu K, Irie H, Tajima T, Nishie A, Hirakawa M, Nakayama T, Kakihara D, Taketomi A, Aishima S. Delayed-phase dynamic CT enhancement as a prognostic factor for mass-forming intrahepatic cholangiocarcinoma. Radiology. 2006;238:150-155. |
| 92. | Kim TK, Choi BI, Han JK, Jang HJ, Cho SG, Han MC. Peripheral cholangiocarcinoma of the liver: two-phase spiral CT findings. Radiology. 1997;204:539-543. |
| 93. | Han JK, Choi BI, Kim AY, An SK, Lee JW, Kim TK, Kim SW. Cholangiocarcinoma: pictorial essay of CT and cholangiographic findings. Radiographics. 2002;22:173-187. |
| 94. | Watadani T, Akahane M, Yoshikawa T, Ohtomo K. Preoperative assessment of hilar cholangiocarcinoma using multidetector-row CT: correlation with histopathological findings. Radiat Med. 2008;26:402-407. |
| 95. | Seo H, Lee JM, Kim IH, Han JK, Kim SH, Jang JY, Kim SW, Choi BI. Evaluation of the gross type and longitudinal extent of extrahepatic cholangiocarcinomas on contrast-enhanced multidetector row computed tomography. J Comput Assist Tomogr. 2009;33:376-382. |
| 96. | Okumoto T, Sato A, Yamada T, Takase K, Matsuhashi T, Tsuda M, Seiji K, Ishibashi T, Higano S, Katayose Y. Correct diagnosis of vascular encasement and longitudinal extension of hilar cholangiocarcinoma by four-channel multidetector-row computed tomography. Tohoku J Exp Med. 2009;217:1-8. |
| 97. | Hann LE, Getrajdman GI, Brown KT, Bach AM, Teitcher JB, Fong Y, Blumgart LH. Hepatic lobar atrophy: association with ipsilateral portal vein obstruction. AJR Am J Roentgenol. 1996;167:1017-1021. |
| 98. | Feydy A, Vilgrain V, Denys A, Sibert A, Belghiti J, Vullierme MP, Menu Y. Helical CT assessment in hilar cholangiocarcinoma: correlation with surgical and pathologic findings. AJR Am J Roentgenol. 1999;172:73-77. |
| 99. | Yamashita Y, Takahashi M, Kanazawa S, Charnsangavej C, Wallace S. Parenchymal changes of the liver in cholangiocarcinoma: CT evaluation. Gastrointest Radiol. 1992;17:161-166. |
| 100. | Lee HY, Kim SH, Lee JM, Kim SW, Jang JY, Han JK, Choi BI. Preoperative assessment of resectability of hepatic hilar cholangiocarcinoma: combined CT and cholangiography with revised criteria. Radiology. 2006;239:113-121. |
| 101. | Aloia TA, Charnsangavej C, Faria S, Ribero D, Abdalla EK, Vauthey JN, Curley SA. High-resolution computed tomography accurately predicts resectability in hilar cholangiocarcinoma. Am J Surg. 2007;193:702-706. |
| 102. | Xu AM, Cheng HY, Jiang WB, Chen D, Jia YC, Wu MC. Multi-slice three-dimensional spiral CT cholangiography: a new technique for diagnosis of biliary diseases. Hepatobiliary Pancreat Dis Int. 2002;1:595-603. |
| 103. | Ahmetoğlu A, Koşucu P, Kul S, Dinç H, Sari A, Arslan M, Alhan E, Gümele HR. MDCT cholangiography with volume rendering for the assessment of patients with biliary obstruction. AJR Am J Roentgenol. 2004;183:1327-1332. |
| 104. | Singh P, Patel T. Advances in the diagnosis, evaluation and management of cholangiocarcinoma. Curr Opin Gastroenterol. 2006;22:294-299. |
| 105. | Romagnuolo J, Bardou M, Rahme E, Joseph L, Reinhold C, Barkun AN. Magnetic resonance cholangiopancreatography: a meta-analysis of test performance in suspected biliary disease. Ann Intern Med. 2003;139:547-557. |
| 106. | Guthrie JA, Ward J, Robinson PJ. Hilar cholangiocarcinomas: T2-weighted spin-echo and gadolinium-enhanced FLASH MR imaging. Radiology. 1996;201:347-351. |
| 107. | Schwartz LH, Coakley FV, Sun Y, Blumgart LH, Fong Y, Panicek DM. Neoplastic pancreaticobiliary duct obstruction: evaluation with breath-hold MR cholangiopancreatography. AJR Am J Roentgenol. 1998;170:1491-1495. |
| 108. | Manfredi R, Brizi MG, Masselli G, Vecchioli A, Marano P. [Malignant biliary hilar stenosis: MR cholangiography compared with direct cholangiography]. Radiol Med. 2001;102:48-54. |
| 109. | Rösch T, Meining A, Frühmorgen S, Zillinger C, Schusdziarra V, Hellerhoff K, Classen M, Helmberger H. A prospective comparison of the diagnostic accuracy of ERCP, MRCP, CT, and EUS in biliary strictures. Gastrointest Endosc. 2002;55:870-876. |
| 110. | Park MS, Kim TK, Kim KW, Park SW, Lee JK, Kim JS, Lee JH, Kim KA, Kim AY, Kim PN. Differentiation of extrahepatic bile duct cholangiocarcinoma from benign stricture: findings at MRCP versus ERCP. Radiology. 2004;233:234-240. |
| 111. | Varghese JC, Farrell MA, Courtney G, Osborne H, Murray FE, Lee MJ. A prospective comparison of magnetic resonance cholangiopancreatography with endoscopic retrograde cholangiopancreatography in the evaluation of patients with suspected biliary tract disease. Clin Radiol. 1999;54:513-520. |
| 112. | Loperfido S, Angelini G, Benedetti G, Chilovi F, Costan F, De Berardinis F, De Bernardin M, Ederle A, Fina P, Fratton A. Major early complications from diagnostic and therapeutic ERCP: a prospective multicenter study. Gastrointest Endosc. 1998;48:1-10. |
| 113. | Manfredi R, Barbaro B, Masselli G, Vecchioli A, Marano P. Magnetic resonance imaging of cholangiocarcinoma. Semin Liver Dis. 2004;24:155-164. |
| 114. | Lopera JE, Soto JA, Múnera F. Malignant hilar and perihilar biliary obstruction: use of MR cholangiography to define the extent of biliary ductal involvement and plan percutaneous interventions. Radiology. 2001;220:90-96. |
| 115. | Fulcher AS, Turner MA. HASTE MR cholangiography in the evaluation of hilar cholangiocarcinoma. AJR Am J Roentgenol. 1997;169:1501-1505. |
| 116. | Choi BI, Lee JM, Han JK. Imaging of intrahepatic and hilar cholangiocarcinoma. Abdom Imaging. 2004;29:548-557. |
| 117. | Lee JW, Han JK, Kim TK, Kim YH, Choi BI, Han MC, Suh KS, Kim SW. CT features of intraductal intrahepatic cholangiocarcinoma. AJR Am J Roentgenol. 2000;175:721-725. |
| 118. | Lee SS, Kim MH, Lee SK, Kim TK, Seo DW, Park JS, Hwang CY, Chang HS, Min YI. MR cholangiography versus cholangioscopy for evaluation of longitudinal extension of hilar cholangiocarcinoma. Gastrointest Endosc. 2002;56:25-32. |
| 119. | Hänninen EL, Pech M, Jonas S, Ricke J, Thelen A, Langrehr J, Hintze R, Röttgen R, Denecke T, Winter L. Magnetic resonance imaging including magnetic resonance cholangiopancreatography for tumor localization and therapy planning in malignant hilar obstructions. Acta Radiol. 2005;46:462-470. |
| 120. | Masselli G, Manfredi R, Vecchioli A, Gualdi G. MR imaging and MR cholangiopancreatography in the preoperative evaluation of hilar cholangiocarcinoma: correlation with surgical and pathologic findings. Eur Radiol. 2008;18:2213-2221. |
| 121. | Lee MG, Park KB, Shin YM, Yoon HK, Sung KB, Kim MH, Lee SG, Kang EM. Preoperative evaluation of hilar cholangiocarcinoma with contrast-enhanced three-dimensional fast imaging with steady-state precession magnetic resonance angiography: comparison with intraarterial digital subtraction angiography. World J Surg. 2003;27:278-283. |
| 122. | Pitt HA, Nakeeb A, Abrams RA, Coleman J, Piantadosi S, Yeo CJ, Lillemore KD, Cameron JL. Perihilar cholangiocarcinoma. Postoperative radiotherapy does not improve survival. Ann Surg. 1995;221:788-797; discussion 797-798. |
| 123. | Kumar M, Prashad R, Kumar A, Sharma R, Acharya SK, Chattopadhyay TK. Relative merits of ultrasonography, computed tomography and cholangiography in patients of surgical obstructive jaundice. Hepatogastroenterology. 1998;45:2027-2032. |
| 124. | Hochwald SN, Burke EC, Jarnagin WR, Fong Y, Blumgart LH. Association of preoperative biliary stenting with increased postoperative infectious complications in proximal cholangiocarcinoma. Arch Surg. 1999;134:261-266. |
| 125. | Harewood GC, Baron TH, Stadheim LM, Kipp BR, Sebo TJ, Salomao DR. Prospective, blinded assessment of factors influencing the accuracy of biliary cytology interpretation. Am J Gastroenterol. 2004;99:1464-1469. |
| 126. | de Bellis M, Fogel EL, Sherman S, Watkins JL, Chappo J, Younger C, Cramer H, Lehman GA. Influence of stricture dilation and repeat brushing on the cancer detection rate of brush cytology in the evaluation of malignant biliary obstruction. Gastrointest Endosc. 2003;58:176-182. |
| 127. | Domagk D, Poremba C, Dietl KH, Senninger N, Heinecke A, Domschke W, Menzel J. Endoscopic transpapillary biopsies and intraductal ultrasonography in the diagnostics of bile duct strictures: a prospective study. Gut. 2002;51:240-244. |
| 128. | Baskin-Bey ES, Moreno Luna LE, Gores GJ. Diagnosis of cholangiocarcinoma in patients with PSC: a sight on cytology. J Hepatol. 2006;45:476-479. |
| 129. | Baron TH, Harewood GC, Rumalla A, Pochron NL, Stadheim LM, Gores GJ, Therneau TM, De Groen PC, Sebo TJ, Salomao DR. A prospective comparison of digital image analysis and routine cytology for the identification of malignancy in biliary tract strictures. Clin Gastroenterol Hepatol. 2004;2:214-219. |
| 130. | Kipp BR, Stadheim LM, Halling SA, Pochron NL, Harmsen S, Nagorney DM, Sebo TJ, Therneau TM, Gores GJ, de Groen PC. A comparison of routine cytology and fluorescence in situ hybridization for the detection of malignant bile duct strictures. Am J Gastroenterol. 2004;99:1675-1681. |
| 131. | Fukuda Y, Tsuyuguchi T, Sakai Y, Tsuchiya S, Saisyo H. Diagnostic utility of peroral cholangioscopy for various bile-duct lesions. Gastrointest Endosc. 2005;62:374-382. |
| 132. | Chen YK, Pleskow DK. SpyGlass single-operator peroral cholangiopancreatoscopy system for the diagnosis and therapy of bile-duct disorders: a clinical feasibility study (with video). Gastrointest Endosc. 2007;65:832-841. |
| 133. | Larghi A, Lecca PG, Ardito F, Rossi ED, Fadda G, Nuzzo G, Costamagna G. Evaluation of hilar biliary strictures by using a newly developed forward-viewing therapeutic echoendoscope: preliminary results of an ongoing experience. Gastrointest Endosc. 2009;69:356-360. |
| 134. | Fishman DS, Tarnasky PR, Patel SN, Raijman I. Management of pancreaticobiliary disease using a new intra-ductal endoscope: the Texas experience. World J Gastroenterol. 2009;15:1353-1358. |
| 135. | Garrow D, Miller S, Sinha D, Conway J, Hoffman BJ, Hawes RH, Romagnuolo J. Endoscopic ultrasound: a meta-analysis of test performance in suspected biliary obstruction. Clin Gastroenterol Hepatol. 2007;5:616-623. |
| 136. | Brugge WR. Advances in the endoscopic management of patients with pancreatic and biliary malignancies. South Med J. 2006;99:1358-1366. |
| 137. | Lee JH, Salem R, Aslanian H, Chacho M, Topazian M. Endoscopic ultrasound and fine-needle aspiration of unexplained bile duct strictures. Am J Gastroenterol. 2004;99:1069-1073. |
| 138. | Fritscher-Ravens A, Broering DC, Sriram PV, Topalidis T, Jaeckle S, Thonke F, Soehendra N. EUS-guided fine-needle aspiration cytodiagnosis of hilar cholangiocarcinoma: a case series. Gastrointest Endosc. 2000;52:534-540. |
| 139. | Eloubeidi MA, Chen VK, Jhala NC, Eltoum IE, Jhala D, Chhieng DC, Syed SA, Vickers SM, Mel Wilcox C. Endoscopic ultrasound-guided fine needle aspiration biopsy of suspected cholangiocarcinoma. Clin Gastroenterol Hepatol. 2004;2:209-213. |
| 140. | Chak A, Catanzaro A. Innovative methods of biliary tract diagnosis: intraductal ultrasound and tissue acquisition. Gastrointest Endosc Clin N Am. 2003;13:609-622. |
| 141. | Brugge WR. Endoscopic techniques to diagnose and manage biliary tumors. J Clin Oncol. 2005;23:4561-4565. |
| 142. | Vazquez-Sequeiros E, Baron TH, Clain JE, Gostout CJ, Norton ID, Petersen BT, Levy MJ, Jondal ML, Wiersema MJ. Evaluation of indeterminate bile duct strictures by intraductal US. Gastrointest Endosc. 2002;56:372-379. |
| 143. | Farrell RJ, Agarwal B, Brandwein SL, Underhill J, Chuttani R, Pleskow DK. Intraductal US is a useful adjunct to ERCP for distinguishing malignant from benign biliary strictures. Gastrointest Endosc. 2002;56:681-687. |
| 144. | Stavropoulos S, Larghi A, Verna E, Battezzati P, Stevens P. Intraductal ultrasound for the evaluation of patients with biliary strictures and no abdominal mass on computed tomography. Endoscopy. 2005;37:715-721. |
| 145. | Sun L, Wu H, Guan YS. Positron emission tomography/computer tomography: challenge to conventional imaging modalities in evaluating primary and metastatic liver malignancies. World J Gastroenterol. 2007;13:2775-2783. |
| 146. | Iglehart JK. The new era of medical imaging--progress and pitfalls. N Engl J Med. 2006;354:2822-2828. |
| 147. | Kapoor V, McCook BM, Torok FS. An introduction to PET-CT imaging. Radiographics. 2004;24:523-543. |
| 148. | Wakabayashi H, Akamoto S, Yachida S, Okano K, Izuishi K, Nishiyama Y, Maeta H. Significance of fluorodeoxyglucose PET imaging in the diagnosis of malignancies in patients with biliary stricture. Eur J Surg Oncol. 2005;31:1175-1179. |
| 149. | Anderson CD, Rice MH, Pinson CW, Chapman WC, Chari RS, Delbeke D. Fluorodeoxyglucose PET imaging in the evaluation of gallbladder carcinoma and cholangiocarcinoma. J Gastrointest Surg. 2004;8:90-97. |
| 150. | Kluge R, Schmidt F, Caca K, Barthel H, Hesse S, Georgi P, Seese A, Huster D, Berr F. Positron emission tomography with[(18)F]fluoro-2-deoxy-D-glucose for diagnosis and staging of bile duct cancer. Hepatology. 2001;33:1029-1035. |
| 151. | Li J, Kuehl H, Grabellus F, Müller SP, Radunz S, Antoch G, Nadalin S, Broelsch CE, Gerken G, Paul A. Preoperative assessment of hilar cholangiocarcinoma by dual-modality PET/CT. J Surg Oncol. 2008;98:438-443. |
| 152. | Petrowsky H, Wildbrett P, Husarik DB, Hany TF, Tam S, Jochum W, Clavien PA. Impact of integrated positron emission tomography and computed tomography on staging and management of gallbladder cancer and cholangiocarcinoma. J Hepatol. 2006;45:43-50. |
| 153. | Corvera CU, Blumgart LH, Akhurst T, DeMatteo RP, D'Angelica M, Fong Y, Jarnagin WR. 18F-fluorodeoxyglucose positron emission tomography influences management decisions in patients with biliary cancer. J Am Coll Surg. 2008;206:57-65. |
| 154. | Chikamoto A, Tsuji T, Takamori H, Kanemitsu K, Uozumi H, Yamashita Y, Baba H. The diagnostic efficacy of FDG-PET in the local recurrence of hilar bile duct cancer. J Hepatobiliary Pancreat Surg. 2006;13:403-408. |
| 155. | Fritscher-Ravens A, Bohuslavizki KH, Broering DC, Jenicke L, Schäfer H, Buchert R, Rogiers X, Clausen M. FDG PET in the diagnosis of hilar cholangiocarcinoma. Nucl Med Commun. 2001;22:1277-1285. |
| 156. | Poneros JM, Tearney GJ, Shiskov M, Kelsey PB, Lauwers GY, Nishioka NS, Bouma BE. Optical coherence tomography of the biliary tree during ERCP. Gastrointest Endosc. 2002;55:84-88. |
| 157. | Singh P, Chak A, Willis JE, Rollins A, Sivak MV Jr. In vivo optical coherence tomography imaging of the pancreatic and biliary ductal system. Gastrointest Endosc. 2005;62:970-974. |
| 158. | Gerhards MF, Vos P, van Gulik TM, Rauws EA, Bosma A, Gouma DJ. Incidence of benign lesions in patients resected for suspicious hilar obstruction. Br J Surg. 2001;88:48-51. |
| 159. | Nakayama A, Imamura H, Shimada R, Miyagawa S, Makuuchi M, Kawasaki S. Proximal bile duct stricture disguised as malignant neoplasm. Surgery. 1999;125:514-521. |
| 160. | Malhi H, Gores GJ. Cholangiocarcinoma: modern advances in understanding a deadly old disease. J Hepatol. 2006;45:856-867. |
| 161. | Sakamoto E, Nimura Y, Hayakawa N, Kamiya J, Kondo S, Nagino M, Kanai M, Miyachi M, Uesaka K. The pattern of infiltration at the proximal border of hilar bile duct carcinoma: a histologic analysis of 62 resected cases. Ann Surg. 1998;227:405-411. |
| 162. | Ebata T, Watanabe H, Ajioka Y, Oda K, Nimura Y. Pathological appraisal of lines of resection for bile duct carcinoma. Br J Surg. 2002;89:1260-1267. |
| 163. | Yamaguchi K, Chijiiwa K, Saiki S, Shimizu S, Takashima M, Tanaka M. Carcinoma of the extrahepatic bile duct: mode of spread and its prognostic implications. Hepatogastroenterology. 1997;44:1256-1261. |
| 164. | He P, Shi JS, Chen WK, Wang ZR, Ren H, Li H. Multivariate statistical analysis of clinicopathologic factors influencing survival of patients with bile duct carcinoma. World J Gastroenterol. 2002;8:943-946. |
| 165. | Bhuiya MR, Nimura Y, Kamiya J, Kondo S, Fukata S, Hayakawa N, Shionoya S. Clinicopathologic studies on perineural invasion of bile duct carcinoma. Ann Surg. 1992;215:344-349. |
| 166. | Jarnagin WR, Fong Y, DeMatteo RP, Gonen M, Burke EC, Bodniewicz BS J, Youssef BA M, Klimstra D, Blumgart LH. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234:507-517; discussion 517-519. |
| 167. | Nagorney DM, Kendrick ML. Hepatic resection in the treatment of hilar cholangiocarcinoma. Adv Surg. 2006;40:159-171. |
| 168. | Seyama Y, Makuuchi M. Current surgical treatment for bile duct cancer. World J Gastroenterol. 2007;13:1505-1515. |
| 169. | D'Angelica MI, Jarnagin WR, Blumgart LH. Resectable hilar cholangiocarcinoma: surgical treatment and long-term outcome. Surg Today. 2004;34:885-890. |
| 170. | Ebata T, Nagino M, Kamiya J, Uesaka K, Nagasaka T, Nimura Y. Hepatectomy with portal vein resection for hilar cholangiocarcinoma: audit of 52 consecutive cases. Ann Surg. 2003;238:720-727. |
| 171. | Miyazaki M, Kato A, Ito H, Kimura F, Shimizu H, Ohtsuka M, Yoshidome H, Yoshitomi H, Furukawa K, Nozawa S. Combined vascular resection in operative resection for hilar cholangiocarcinoma: does it work or not? Surgery. 2007;141:581-588. |
| 172. | Kitagawa Y, Nagino M, Kamiya J, Uesaka K, Sano T, Yamamoto H, Hayakawa N, Nimura Y. Lymph node metastasis from hilar cholangiocarcinoma: audit of 110 patients who underwent regional and paraaortic node dissection. Ann Surg. 2001;233:385-392. |
| 173. | Greene FL, Page DL, Fleming ID. AJCC (American Joint Committee on Cancer) Cancer Staging Manual. 6th ed. New York: Springer-Verlag 2002; . |
| 174. | Nathan H, Aloia TA, Vauthey JN, Abdalla EK, Zhu AX, Schulick RD, Choti MA, Pawlik TM. A proposed staging system for intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2009;16:14-22. |
| 175. | Jarnagin WR, Shoup M. Surgical management of cholangiocarcinoma. Semin Liver Dis. 2004;24:189-199. |
| 176. | Shimoda M, Kubota K. Multi-disciplinary treatment for cholangiocellular carcinoma. World J Gastroenterol. 2007;13:1500-1504. |
| 177. | Su CH, Tsay SH, Wu CC, Shyr YM, King KL, Lee CH, Lui WY, Liu TJ, P'eng FK. Factors influencing postoperative morbidity, mortality, and survival after resection for hilar cholangiocarcinoma. Ann Surg. 1996;223:384-394. |
| 178. | Sewnath ME, Karsten TM, Prins MH, Rauws EJ, Obertop H, Gouma DJ. A meta-analysis on the efficacy of preoperative biliary drainage for tumors causing obstructive jaundice. Ann Surg. 2002;236:17-27. |
| 179. | Anderson CD, Pinson CW, Berlin J, Chari RS. Diagnosis and treatment of cholangiocarcinoma. Oncologist. 2004;9:43-57. |
| 180. | Aly EA, Johnson CD. Preoperative biliary drainage before resection in obstructive jaundice. Dig Surg. 2001;18:84-89. |
| 181. | Nakeeb A, Pitt HA. The role of preoperative biliary decompression in obstructive jaundice. Hepatogastroenterology. 1995;42:332-337. |
| 182. | Kawasaki S, Imamura H, Kobayashi A, Noike T, Miwa S, Miyagawa S. Results of surgical resection for patients with hilar bile duct cancer: application of extended hepatectomy after biliary drainage and hemihepatic portal vein embolization. Ann Surg. 2003;238:84-92. |
| 183. | Forsmo HM, Horn A, Viste A, Hoem D, Ovrebo K. Survival and an overview of decision-making in patients with cholangiocarcinoma. Hepatobiliary Pancreat Dis Int. 2008;7:412-417. |
| 184. | Hemming AW, Kim RD, Mekeel KL, Fujita S, Reed AI, Foley DP, Howard RJ. Portal vein resection for hilar cholangiocarcinoma. Am Surg. 2006;72:599-604; discussion 604-605. |
| 185. | Shimada H, Endo I, Sugita M, Masunari H, Fujii Y, Tanaka K, Misuta K, Sekido H, Togo S. Hepatic resection combined with portal vein or hepatic artery reconstruction for advanced carcinoma of the hilar bile duct and gallbladder. World J Surg. 2003;27:1137-1142. |
| 186. | Corvera CU, Weber SM, Jarnagin WR. Role of laparoscopy in the evaluation of biliary tract cancer. Surg Oncol Clin N Am. 2002;11:877-891. |
| 187. | Goere D, Wagholikar GD, Pessaux P, Carrère N, Sibert A, Vilgrain V, Sauvanet A, Belghiti J. Utility of staging laparoscopy in subsets of biliary cancers : laparoscopy is a powerful diagnostic tool in patients with intrahepatic and gallbladder carcinoma. Surg Endosc. 2006;20:721-725. |
| 188. | Weber SM, DeMatteo RP, Fong Y, Blumgart LH, Jarnagin WR. Staging laparoscopy in patients with extrahepatic biliary carcinoma. Analysis of 100 patients. Ann Surg. 2002;235:392-399. |
| 189. | Connor S, Barron E, Wigmore SJ, Madhavan KK, Parks RW, Garden OJ. The utility of laparoscopic assessment in the preoperative staging of suspected hilar cholangiocarcinoma. J Gastrointest Surg. 2005;9:476-480. |
| 190. | Martin RC 2nd, Fong Y, DeMatteo RP, Brown K, Blumgart LH, Jarnagin WR. Peritoneal washings are not predictive of occult peritoneal disease in patients with hilar cholangiocarcinoma. J Am Coll Surg. 2001;193:620-625. |
| 191. | Uenishi T, Kubo S, Yamazaki O, Yamada T, Sasaki Y, Nagano H, Monden M. Indications for surgical treatment of intrahepatic cholangiocarcinoma with lymph node metastases. J Hepatobiliary Pancreat Surg. 2008;15:417-422. |
| 192. | Endo I, House MG, Klimstra DS, Gönen M, D'Angelica M, Dematteo RP, Fong Y, Blumgart LH, Jarnagin WR. Clinical significance of intraoperative bile duct margin assessment for hilar cholangiocarcinoma. Ann Surg Oncol. 2008;15:2104-2112. |
| 193. | Shimada M, Yamashita Y, Aishima S, Shirabe K, Takenaka K, Sugimachi K. Value of lymph node dissection during resection of intrahepatic cholangiocarcinoma. Br J Surg. 2001;88:1463-1466. |
| 194. | Shimada K, Sano T, Nara S, Esaki M, Sakamoto Y, Kosuge T, Ojima H. Therapeutic value of lymph node dissection during hepatectomy in patients with intrahepatic cholangiocellular carcinoma with negative lymph node involvement. Surgery. 2009;145:411-416. |
| 195. | Miwa S, Miyagawa S, Kobayashi A, Akahane Y, Nakata T, Mihara M, Kusama K, Soeda J, Ogawa S. Predictive factors for intrahepatic cholangiocarcinoma recurrence in the liver following surgery. J Gastroenterol. 2006;41:893-900. |
| 196. | Jan YY, Yeh CN, Yeh TS, Hwang TL, Chen MF. Clinicopathological factors predicting long-term overall survival after hepatectomy for peripheral cholangiocarcinoma. World J Surg. 2005;29:894-898. |
| 197. | Ohtsuka M, Ito H, Kimura F, Shimizu H, Togawa A, Yoshidome H, Shimamura F, Shimizu Y, Miyazaki M. Extended hepatic resection and outcomes in intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 2003;10:259-264. |
| 198. | Uenishi T, Hirohashi K, Kubo S, Yamamoto T, Hamba H, Tanaka H, Kinoshita H. Histologic factors affecting prognosis following hepatectomy for intrahepatic cholangiocarcinoma. World J Surg. 2001;25:865-869. |
| 199. | Inoue K, Makuuchi M, Takayama T, Torzilli G, Yamamoto J, Shimada K, Kosuge T, Yamasaki S, Konishi M, Kinoshita T. Long-term survival and prognostic factors in the surgical treatment of mass-forming type cholangiocarcinoma. Surgery. 2000;127:498-505. |
| 200. | Yamamoto M, Takasaki K, Yoshikawa T. Extended resection for intrahepatic cholangiocarcinoma in Japan. J Hepatobiliary Pancreat Surg. 1999;6:117-121. |
| 201. | Madariaga JR, Iwatsuki S, Todo S, Lee RG, Irish W, Starzl TE. Liver resection for hilar and peripheral cholangiocarcinomas: a study of 62 cases. Ann Surg. 1998;227:70-79. |
| 202. | Hanazaki K, Kajikawa S, Shimozawa N, Shimada K, Hiraguri M, Koide N, Adachi W, Amano J. Prognostic factors of intrahepatic cholangiocarcinoma after hepatic resection: univariate and multivariate analysis. Hepatogastroenterology. 2002;49:311-316. |
| 203. | Hirohashi K, Uenishi T, Kubo S, Yamamoto T, Tanaka H, Shuto T, Kinoshita H. Macroscopic types of intrahepatic cholangiocarcinoma: clinicopathologic features and surgical outcomes. Hepatogastroenterology. 2002;49:326-329. |
| 204. | Nimura Y, Hayakawa N, Kamiya J, Kondo S, Shionoya S. Hepatic segmentectomy with caudate lobe resection for bile duct carcinoma of the hepatic hilus. World J Surg. 1990;14:535-543; discussion 544. |
| 205. | Hemming AW, Reed AI, Fujita S, Foley DP, Howard RJ. Surgical management of hilar cholangiocarcinoma. Ann Surg. 2005;241:693-699; discussion 699-702. |
| 206. | Miyazaki M, Ito H, Nakagawa K, Ambiru S, Shimizu H, Okaya T, Shinmura K, Nakajima N. Parenchyma-preserving hepatectomy in the surgical treatment of hilar cholangiocarcinoma. J Am Coll Surg. 1999;189:575-583. |
| 207. | Todoroki T, Kawamoto T, Koike N, Takahashi H, Yoshida S, Kashiwagi H, Takada Y, Otsuka M, Fukao K. Radical resection of hilar bile duct carcinoma and predictors of survival. Br J Surg. 2000;87:306-313. |
| 208. | Rea DJ, Munoz-Juarez M, Farnell MB, Donohue JH, Que FG, Crownhart B, Larson D, Nagorney DM. Major hepatic resection for hilar cholangiocarcinoma: analysis of 46 patients. Arch Surg. 2004;139:514-523; discussion 523-525. |
| 209. | Nagino M, Kamiya J, Nishio H, Ebata T, Arai T, Nimura Y. Two hundred forty consecutive portal vein embolizations before extended hepatectomy for biliary cancer: surgical outcome and long-term follow-up. Ann Surg. 2006;243:364-372. |
| 210. | Abdalla EK, Barnett CC, Doherty D, Curley SA, Vauthey JN. Extended hepatectomy in patients with hepatobiliary malignancies with and without preoperative portal vein embolization. Arch Surg. 2002;137:675-680; discussion 680-681. |
| 211. | Abdalla EK, Hicks ME, Vauthey JN. Portal vein embolization: rationale, technique and future prospects. Br J Surg. 2001;88:165-175. |
| 212. | Hemming AW, Reed AI, Howard RJ, Fujita S, Hochwald SN, Caridi JG, Hawkins IF, Vauthey JN. Preoperative portal vein embolization for extended hepatectomy. Ann Surg. 2003;237:686-691; discussion 691-693. |
| 213. | Hasegawa S, Ikai I, Fujii H, Hatano E, Shimahara Y. Surgical resection of hilar cholangiocarcinoma: analysis of survival and postoperative complications. World J Surg. 2007;31:1256-1263. |
| 214. | Dinant S, Gerhards MF, Rauws EA, Busch OR, Gouma DJ, van Gulik TM. Improved outcome of resection of hilar cholangiocarcinoma (Klatskin tumor). Ann Surg Oncol. 2006;13:872-880. |
| 215. | Kawarada Y, Das BC, Naganuma T, Tabata M, Taoka H. Surgical treatment of hilar bile duct carcinoma: experience with 25 consecutive hepatectomies. J Gastrointest Surg. 2002;6:617-624. |
| 216. | Tabata M, Kawarada Y, Yokoi H, Higashiguchi T, Isaji S. Surgical treatment for hilar cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 2000;7:148-154. |
| 217. | Kosuge T, Yamamoto J, Shimada K, Yamasaki S, Makuuchi M. Improved surgical results for hilar cholangiocarcinoma with procedures including major hepatic resection. Ann Surg. 1999;230:663-671. |
| 218. | Miyazaki M, Ito H, Nakagawa K, Ambiru S, Shimizu H, Shimizu Y, Kato A, Nakamura S, Omoto H, Nakajima N. Aggressive surgical approaches to hilar cholangiocarcinoma: hepatic or local resection? Surgery. 1998;123:131-136. |
| 219. | Cheng Q, Luo X, Zhang B, Jiang X, Yi B, Wu M. Distal bile duct carcinoma: prognostic factors after curative surgery. A series of 112 cases. Ann Surg Oncol. 2007;14:1212-1219. |
| 220. | Seiler CA, Wagner M, Sadowski C, Kulli C, Büchler MW. Randomized prospective trial of pylorus-preserving vs Classic duodenopancreatectomy (Whipple procedure): initial clinical results. J Gastrointest Surg. 2000;4:443-452. |
| 221. | Riall TS, Cameron JL, Lillemoe KD, Campbell KA, Sauter PK, Coleman J, Abrams RA, Laheru D, Hruban RH, Yeo CJ. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma--part 3: update on 5-year survival. J Gastrointest Surg. 2005;9:1191-1204; discussion 1204-1206. |
| 222. | Fong Y, Blumgart LH, Lin E, Fortner JG, Brennan MF. Outcome of treatment for distal bile duct cancer. Br J Surg. 1996;83:1712-1715. |
| 223. | Wade TP, Prasad CN, Virgo KS, Johnson FE. Experience with distal bile duct cancers in U.S. Veterans Affairs hospitals: 1987-1991. J Surg Oncol. 1997;64:242-245. |
| 224. | Murakami Y, Uemura K, Hayashidani Y, Sudo T, Ohge H, Sueda T. Pancreatoduodenectomy for distal cholangiocarcinoma: prognostic impact of lymph node metastasis. World J Surg. 2007;31:337-342; discussion 343-344. |
| 225. | Yoshida T, Matsumoto T, Sasaki A, Morii Y, Aramaki M, Kitano S. Prognostic factors after pancreatoduodenectomy with extended lymphadenectomy for distal bile duct cancer. Arch Surg. 2002;137:69-73. |
| 226. | Meyer CG, Penn I, James L. Liver transplantation for cholangiocarcinoma: results in 207 patients. Transplantation. 2000;69:1633-1637. |
| 227. | Jeyarajah DR, Klintmalm GB. Is liver transplantation indicated for cholangiocarcinoma? J Hepatobiliary Pancreat Surg. 1998;5:48-51. |
| 228. | Pichlmayr R, Weimann A, Klempnauer J, Oldhafer KJ, Maschek H, Tusch G, Ringe B. Surgical treatment in proximal bile duct cancer. A single-center experience. Ann Surg. 1996;224:628-638. |
| 229. | Sudan D, DeRoover A, Chinnakotla S, Fox I, Shaw B Jr, McCashland T, Sorrell M, Tempero M, Langnas A. Radiochemotherapy and transplantation allow long-term survival for nonresectable hilar cholangiocarcinoma. Am J Transplant. 2002;2:774-779. |
| 230. | Becker NS, Rodriguez JA, Barshes NR, O'Mahony CA, Goss JA, Aloia TA. Outcomes analysis for 280 patients with cholangiocarcinoma treated with liver transplantation over an 18-year period. J Gastrointest Surg. 2008;12:117-122. |
| 231. | Sotiropoulos GC, Kaiser GM, Lang H, Molmenti EP, Beckebaum S, Fouzas I, Sgourakis G, Radtke A, Bockhorn M, Nadalin S. Liver transplantation as a primary indication for intrahepatic cholangiocarcinoma: a single-center experience. Transplant Proc. 2008;40:3194-3195. |
| 232. | Heimbach JK, Gores GJ, Haddock MG, Alberts SR, Nyberg SL, Ishitani MB, Rosen CB. Liver transplantation for unresectable perihilar cholangiocarcinoma. Semin Liver Dis. 2004;24:201-207. |
| 233. | Rea DJ, Heimbach JK, Rosen CB, Haddock MG, Alberts SR, Kremers WK, Gores GJ, Nagorney DM. Liver transplantation with neoadjuvant chemoradiation is more effective than resection for hilar cholangiocarcinoma. Ann Surg. 2005;242:451-458; discussion 458-461. |
| 234. | Todoroki T. Chemotherapy for bile duct carcinoma in the light of adjuvant chemotherapy to surgery. Hepatogastroenterology. 2000;47:644-649. |
| 235. | Thongprasert S. The role of chemotherapy in cholangiocarcinoma. Ann Oncol. 2005;16 Suppl 2:ii93-ii96. |
| 236. | Takada T, Amano H, Yasuda H, Nimura Y, Matsushiro T, Kato H, Nagakawa T, Nakayama T. Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma? A phase III multicenter prospective randomized controlled trial in patients with resected pancreaticobiliary carcinoma. Cancer. 2002;95:1685-1695. |
| 237. | Gerhards MF, van Gulik TM, González González D, Rauws EA, Gouma DJ. Results of postoperative radiotherapy for resectable hilar cholangiocarcinoma. World J Surg. 2003;27:173-179. |
| 238. | Sagawa N, Kondo S, Morikawa T, Okushiba S, Katoh H. Effectiveness of radiation therapy after surgery for hilar cholangiocarcinoma. Surg Today. 2005;35:548-552. |
| 239. | Stein DE, Heron DE, Rosato EL, Anné PR, Topham AK. Positive microscopic margins alter outcome in lymph node-negative cholangiocarcinoma when resection is combined with adjuvant radiotherapy. Am J Clin Oncol. 2005;28:21-23. |
| 240. | Itoh H, Nishijima K, Kurosaka Y, Takegawa S, Kiriyama M, Dohba S, Kojima Y, Saitoh Y. Magnitude of combination therapy of radical resection and external beam radiotherapy for patients with carcinomas of the extrahepatic bile duct and gallbladder. Dig Dis Sci. 2005;50:2231-2242. |
| 241. | Todoroki T, Ohara K, Kawamoto T, Koike N, Yoshida S, Kashiwagi H, Otsuka M, Fukao K. Benefits of adjuvant radiotherapy after radical resection of locally advanced main hepatic duct carcinoma. Int J Radiat Oncol Biol Phys. 2000;46:581-587. |
| 242. | Kelley ST, Bloomston M, Serafini F, Carey LC, Karl RC, Zervos E, Goldin S, Rosemurgy P, Rosemurgy AS. Cholangiocarcinoma: advocate an aggressive operative approach with adjuvant chemotherapy. Am Surg. 2004;70:743-748; discussion 748-749. |
| 243. | Serafini FM, Sachs D, Bloomston M, Carey LC, Karl RC, Murr MM, Rosemurgy AS. Location, not staging, of cholangiocarcinoma determines the role for adjuvant chemoradiation therapy. Am Surg. 2001;67:839-843; discussion 843-844. |
| 244. | Nelson JW, Ghafoori AP, Willett CG, Tyler DS, Pappas TN, Clary BM, Hurwitz HI, Bendell JC, Morse MA, Clough RW. Concurrent chemoradiotherapy in resected extrahepatic cholangiocarcinoma. Int J Radiat Oncol Biol Phys. 2009;73:148-153. |
| 245. | Hughes MA, Frassica DA, Yeo CJ, Riall TS, Lillemoe KD, Cameron JL, Donehower RC, Laheru DA, Hruban RH, Abrams RA. Adjuvant concurrent chemoradiation for adenocarcinoma of the distal common bile duct. Int J Radiat Oncol Biol Phys. 2007;68:178-182. |
| 246. | Figueras J, Llado L, Valls C, Serrano T, Ramos E, Fabregat J, Rafecas A, Torras J, Jaurrieta E. Changing strategies in diagnosis and management of hilar cholangiocarcinoma. Liver Transpl. 2000;6:786-794. |
| 247. | Shinohara ET, Mitra N, Guo M, Metz JM. Radiation therapy is associated with improved survival in the adjuvant and definitive treatment of intrahepatic cholangiocarcinoma. Int J Radiat Oncol Biol Phys. 2008;72:1495-1501. |
| 248. | McMasters KM, Tuttle TM, Leach SD, Rich T, Cleary KR, Evans DB, Curley SA. Neoadjuvant chemoradiation for extrahepatic cholangiocarcinoma. Am J Surg. 1997;174:605-608; discussion 608-609. |
| 249. | Abu-Hamda EM, Baron TH. Endoscopic management of cholangiocarcinoma. Semin Liver Dis. 2004;24:165-175. |
| 250. | Paik WH, Park YS, Hwang JH, Lee SH, Yoon CJ, Kang SG, Lee JK, Ryu JK, Kim YT, Yoon YB. Palliative treatment with self-expandable metallic stents in patients with advanced type III or IV hilar cholangiocarcinoma: a percutaneous versus endoscopic approach. Gastrointest Endosc. 2009;69:55-62. |
| 251. | Levy MJ, Baron TH, Gostout CJ, Petersen BT, Farnell MB. Palliation of malignant extrahepatic biliary obstruction with plastic versus expandable metal stents: An evidence-based approach. Clin Gastroenterol Hepatol. 2004;2:273-285. |
| 252. | Davids PH, Groen AK, Rauws EA, Tytgat GN, Huibregtse K. Randomised trial of self-expanding metal stents versus polyethylene stents for distal malignant biliary obstruction. Lancet. 1992;340:1488-1492. |
| 253. | Soderlund C, Linder S. Covered metal versus plastic stents for malignant common bile duct stenosis: a prospective, randomized, controlled trial. Gastrointest Endosc. 2006;63:986-995. |
| 254. | Kaassis M, Boyer J, Dumas R, Ponchon T, Coumaros D, Delcenserie R, Canard JM, Fritsch J, Rey JF, Burtin P. Plastic or metal stents for malignant stricture of the common bile duct? Results of a randomized prospective study. Gastrointest Endosc. 2003;57:178-182. |
| 255. | Cheung KL, Lai EC. Endoscopic stenting for malignant biliary obstruction. Arch Surg. 1995;130:204-207. |
| 256. | Wagner HJ, Knyrim K, Vakil N, Klose KJ. Plastic endoprostheses versus metal stents in the palliative treatment of malignant hilar biliary obstruction. A prospective and randomized trial. Endoscopy. 1993;25:213-218. |
| 257. | De Palma GD, Pezzullo A, Rega M, Persico M, Patrone F, Mastantuono L, Persico G. Unilateral placement of metallic stents for malignant hilar obstruction: a prospective study. Gastrointest Endosc. 2003;58:50-53. |
| 258. | De Palma GD, Galloro G, Siciliano S, Iovino P, Catanzano C. Unilateral versus bilateral endoscopic hepatic duct drainage in patients with malignant hilar biliary obstruction: results of a prospective, randomized, and controlled study. Gastrointest Endosc. 2001;53:547-553. |
| 259. | Becker CD, Glättli A, Maibach R, Baer HU. Percutaneous palliation of malignant obstructive jaundice with the Wallstent endoprosthesis: follow-up and reintervention in patients with hilar and non-hilar obstruction. J Vasc Interv Radiol. 1993;4:597-604. |
| 260. | Stoker J, Laméris JS. Complications of percutaneously inserted biliary Wallstents. J Vasc Interv Radiol. 1993;4:767-772. |
| 261. | Fumex F, Coumaros D, Napoleon B, Barthet M, Laugier R, Yzet T, Le Sidaner A, Desurmont P, Lamouliatte H, Letard JC. Similar performance but higher cholecystitis rate with covered biliary stents: results from a prospective multicenter evaluation. Endoscopy. 2006;38:787-792. |
| 262. | Park do H, Kim MH, Choi JS, Lee SS, Seo DW, Kim JH, Han J, Kim JC, Choi EK, Lee SK. Covered versus uncovered wallstent for malignant extrahepatic biliary obstruction: a cohort comparative analysis. Clin Gastroenterol Hepatol. 2006;4:790-796. |
| 263. | Smith AC, Dowsett JF, Russell RC, Hatfield AR, Cotton PB. Randomised trial of endoscopic stenting versus surgical bypass in malignant low bileduct obstruction. Lancet. 1994;344:1655-1660. |
| 264. | Shepherd HA, Royle G, Ross AP, Diba A, Arthur M, Colin-Jones D. Endoscopic biliary endoprosthesis in the palliation of malignant obstruction of the distal common bile duct: a randomized trial. Br J Surg. 1988;75:1166-1168. |
| 265. | Prat F, Chapat O, Ducot B, Ponchon T, Fritsch J, Choury AD, Pelletier G, Buffet C. Predictive factors for survival of patients with inoperable malignant distal biliary strictures: a practical management guideline. Gut. 1998;42:76-80. |
| 266. | Sunpaweravong S, Ovartlarnporn B, Khow-ean U, Soontrapornchai P, Charoonratana V. Endoscopic stenting versus surgical bypass in advanced malignant distal bile duct obstruction: cost-effectiveness analysis. Asian J Surg. 2005;28:262-265. |
| 267. | Ohnishi H, Asada M, Shichijo Y, Iijima N, Itobayashi E, Shimura K, Suzuki T, Yoshida S, Mine T. External radiotherapy for biliary decompression of hilar cholangiocarcinoma. Hepatogastroenterology. 1995;42:265-268. |
| 268. | Cameron JL, Pitt HA, Zinner MJ, Kaufman SL, Coleman J. Management of proximal cholangiocarcinomas by surgical resection and radiotherapy. Am J Surg. 1990;159:91-97; discussion 97-98. |
| 269. | Bruha R, Petrtyl J, Kubecova M, Marecek Z, Dufek V, Urbanek P, Kodadova J, Chodounsky Z. Intraluminal brachytherapy and selfexpandable stents in nonresectable biliary malignancies--the question of long-term palliation. Hepatogastroenterology. 2001;48:631-637. |
| 270. | Ishii H, Furuse J, Nagase M, Kawashima M, Ikeda H, Yoshino M. Relief of jaundice by external beam radiotherapy and intraluminal brachytherapy in patients with extrahepatic cholangiocarcinoma: results without stenting. Hepatogastroenterology. 2004;51:954-957. |
| 271. | Golfieri R, Giampalma E, Renzulli M, Galuppi A, Vicenzi L, Galaverni MC, Cappelli A. Unresectable hilar cholangiocarcinoma: multimodality approach with percutaneous treatment associated with radiotherapy and chemotherapy. In Vivo. 2006;20:757-760. |
| 272. | Takamura A, Saito H, Kamada T, Hiramatsu K, Takeuchi S, Hasegawa M, Miyamoto N. Intraluminal low-dose-rate 192Ir brachytherapy combined with external beam radiotherapy and biliary stenting for unresectable extrahepatic bile duct carcinoma. Int J Radiat Oncol Biol Phys. 2003;57:1357-1365. |
| 273. | Kuvshinoff BW, Armstrong JG, Fong Y, Schupak K, Getradjman G, Heffernan N, Blumgart LH. Palliation of irresectable hilar cholangiocarcinoma with biliary drainage and radiotherapy. Br J Surg. 1995;82:1522-1525. |
| 274. | Bowling TE, Galbraith SM, Hatfield AR, Solano J, Spittle MF. A retrospective comparison of endoscopic stenting alone with stenting and radiotherapy in non-resectable cholangiocarcinoma. Gut. 1996;39:852-855. |
| 275. | Alden ME, Mohiuddin M. The impact of radiation dose in combined external beam and intraluminal Ir-192 brachytherapy for bile duct cancer. Int J Radiat Oncol Biol Phys. 1994;28:945-951. |
| 276. | Gaba RC, Lewandowski RJ, Kulik LM, Riaz A, Ibrahim SM, Mulcahy MF, Ryu RK, Sato KT, Gates V, Abecassis MM. Radiation lobectomy: preliminary findings of hepatic volumetric response to lobar yttrium-90 radioembolization. Ann Surg Oncol. 2009;16:1587-1596. |
| 277. | Ibrahim SM, Mulcahy MF, Lewandowski RJ, Sato KT, Ryu RK, Masterson EJ, Newman SB, Benson A 3rd, Omary RA, Salem R. Treatment of unresectable cholangiocarcinoma using yttrium-90 microspheres: results from a pilot study. Cancer. 2008;113:2119-2128. |
| 278. | Choi CW, Choi IK, Seo JH, Kim BS, Kim JS, Kim CD, Um SH, Kim JS, Kim YH. Effects of 5-fluorouracil and leucovorin in the treatment of pancreatic-biliary tract adenocarcinomas. Am J Clin Oncol. 2000;23:425-428. |
| 279. | Patt YZ, Jones DV Jr, Hoque A, Lozano R, Markowitz A, Raijman I, Lynch P, Charnsangavej C. Phase II trial of intravenous flourouracil and subcutaneous interferon alfa-2b for biliary tract cancer. J Clin Oncol. 1996;14:2311-2315. |
| 280. | Ducreux M, Rougier P, Fandi A, Clavero-Fabri MC, Villing AL, Fassone F, Fandi L, Zarba J, Armand JP. Effective treatment of advanced biliary tract carcinoma using 5-fluorouracil continuous infusion with cisplatin. Ann Oncol. 1998;9:653-656. |
| 281. | Nehls O, Klump B, Arkenau HT, Hass HG, Greschniok A, Gregor M, Porschen R. Oxaliplatin, fluorouracil and leucovorin for advanced biliary system adenocarcinomas: a prospective phase II trial. Br J Cancer. 2002;87:702-704. |
| 282. | Hong YS, Lee J, Lee SC, Hwang IG, Choi SH, Heo JS, Park JO, Park YS, Lim HY, Kang WK. Phase II study of capecitabine and cisplatin in previously untreated advanced biliary tract cancer. Cancer Chemother Pharmacol. 2007;60:321-328. |
| 283. | Furuse J, Okusaka T, Funakoshi A, Yamao K, Nagase M, Ishii H, Nakachi K, Ueno H, Ikeda M, Morizane C. Early phase II study of uracil-tegafur plus doxorubicin in patients with unresectable advanced biliary tract cancer. Jpn J Clin Oncol. 2006;36:552-556. |
| 284. | Kubicka S, Rudolph KL, Tietze MK, Lorenz M, Manns M. Phase II study of systemic gemcitabine chemotherapy for advanced unresectable hepatobiliary carcinomas. Hepatogastroenterology. 2001;48:783-789. |
| 285. | Lee GW, Kang JH, Kim HG, Lee JS, Lee JS, Jang JS. Combination chemotherapy with gemcitabine and cisplatin as first-line treatment for immunohistochemically proven cholangiocarcinoma. Am J Clin Oncol. 2006;29:127-131. |
| 286. | Knox JJ, Hedley D, Oza A, Feld R, Siu LL, Chen E, Nematollahi M, Pond GR, Zhang J, Moore MJ. Combining gemcitabine and capecitabine in patients with advanced biliary cancer: a phase II trial. J Clin Oncol. 2005;23:2332-2338. |
| 287. | André T, Tournigand C, Rosmorduc O, Provent S, Maindrault-Goebel F, Avenin D, Selle F, Paye F, Hannoun L, Houry S. Gemcitabine combined with oxaliplatin (GEMOX) in advanced biliary tract adenocarcinoma: a GERCOR study. Ann Oncol. 2004;15:1339-1343. |
| 288. | Eckel F, Schmid RM. Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br J Cancer. 2007;96:896-902. |
| 289. | Meyerhardt JA, Zhu AX, Stuart K, Ryan DP, Blaszkowsky L, Lehman N, Earle CC, Kulke MH, Bhargava P, Fuchs CS. Phase-II study of gemcitabine and cisplatin in patients with metastatic biliary and gallbladder cancer. Dig Dis Sci. 2008;53:564-570. |
| 290. | Kim JH, Yoon HK, Sung KB, Ko GY, Gwon DI, Shin JH, Song HY. Transcatheter arterial chemoembolization or chemoinfusion for unresectable intrahepatic cholangiocarcinoma: clinical efficacy and factors influencing outcomes. Cancer. 2008;113:1614-1622. |
| 291. | Gusani NJ, Balaa FK, Steel JL, Geller DA, Marsh JW, Zajko AB, Carr BI, Gamblin TC. Treatment of unresectable cholangiocarcinoma with gemcitabine-based transcatheter arterial chemoembolization (TACE): a single-institution experience. J Gastrointest Surg. 2008;12:129-137. |
| 292. | Aliberti C, Benea G, Tilli M, Fiorentini G. Chemoembolization (TACE) of unresectable intrahepatic cholangiocar¬cinoma with slow-release doxorubicin-eluting beads: preliminary results. Cardiovasc Intervent Radiol. 2008;31:883-888. |
| 293. | Ortner MA. Photodynamic therapy in cholangiocarcinomas. Best Pract Res Clin Gastroenterol. 2004;18:147-154. |
| 294. | Berr F, Wiedmann M, Tannapfel A, Halm U, Kohlhaw KR, Schmidt F, Wittekind C, Hauss J, Mössner J. Photodynamic therapy for advanced bile duct cancer: evidence for improved palliation and extended survival. Hepatology. 2000;31:291-298. |
| 295. | Ortner ME, Caca K, Berr F, Liebetruth J, Mansmann U, Huster D, Voderholzer W, Schachschal G, Mössner J, Lochs H. Successful photodynamic therapy for nonresectable cholangiocarcinoma: a randomized prospective study. Gastroenterology. 2003;125:1355-1363. |
| 296. | Wiedmann M, Caca K, Berr F, Schiefke I, Tannapfel A, Wittekind C, Mössner J, Hauss J, Witzigmann H. Neoadjuvant photodynamic therapy as a new approach to treating hilar cholangiocarcinoma: a phase II pilot study. Cancer. 2003;97:2783-2790. |
| 297. | Kiesslich T, Wolkersdörfer G, Neureiter D, Salmhofer H, Berr F. Photodynamic therapy for non-resectable perihilar cholangiocarcinoma. Photochem Photobiol Sci. 2009;8:23-30. |
| 298. | Zoepf T, Jakobs R, Arnold JC, Apel D, Riemann JF. Palliation of nonresectable bile duct cancer: improved survival after photodynamic therapy. Am J Gastroenterol. 2005;100:2426-2430. |
| 299. | Zgodzinski W, Espat NJ. Radiofrequency ablation for incidentally identified primary intrahepatic cholangiocarcinoma. World J Gastroenterol. 2005;11:5239-5240. |
| 300. | Burger I, Hong K, Schulick R, Georgiades C, Thuluvath P, Choti M, Kamel I, Geschwind JF. Transcatheter arterial chemoembolization in unresectable cholangiocarcinoma: initial experience in a single institution. J Vasc Interv Radiol. 2005;16:353-361. |
| 301. | Waggershauser T, Herrmann K, Schalhorn A, Reiser M. [Percutaneous implantation of port-catheter systems for intraarterial chemotherapy of the liver]. Radiologe. 1999;39:772-776. |
| 302. | Prat F, Lafon C, De Lima DM, Theilliere Y, Fritsch J, Pelletier G, Buffet C, Cathignol D. Endoscopic treatment of cholangiocarcinoma and carcinoma of the duodenal papilla by intraductal high-intensity US: Results of a pilot study. Gastrointest Endosc. 2002;56:909-915. |
| 303. | Sirica AE. Cholangiocarcinoma: molecular targeting strategies for chemoprevention and therapy. Hepatology. 2005;41:5-15. |
Peer reviewer: Simon D Taylor-Robinson, MD, Department of Medicine A, Imperial College London, Hammersmith Hospital, Du Cane Road, London W12 0HS, United Kingdom
S- Editor Tian L L- Editor Logan S E- Editor Lin YP