Published online Aug 21, 2009. doi: 10.3748/wjg.15.3908
Revised: June 30, 2009
Accepted: July 7, 2009
Published online: August 21, 2009
AIM: To determine whether Chinese herbs (CHs) relieve xerostomia (dry mouth) by increasing salivary secretion.
METHODS: The submandibular glands of Wistar rats were surgically isolated and perfused arterially with buffered salt solution. After control perfusion, recording started 5 min prior to the start of stimulation. After fluid secretion was induced by 0.2 μmol/L carbamylcholine (CCh) in the perfusate for 10 min, Chinese herb (CH) was added in the perfusion for 5 min. CCh was then overloaded at 0.2 μmol/L in the perfusion for 20 min. The volume of salivary fluid secretion was recorded by a computer-controlled balance system.
RESULTS: Saliva secretion formed an initial ephemeral peak at 30 s followed by a gradual increase to a sustained level. CH alone induced no or little saliva in all types of CH selected. During perfusion with CH, overloading of CCh promoted fluid secretion in 15 of 20 CHs. This promotion was classified into four patterns, which were eventually related to the categories of CH: Overall sustained phase was continuously raised (Yin-nourishing, fluid production-promoting and heat-clearing agents); The sustained secretion rose to reach a maximum then decreased (Qi-enhancing agent); Sustained secretion rose to reach the highest maximum and was then sustained with a slight decline (swelling-reducing, phlegm-resolving and pus-expelling agents); Stimulation of salivary secretion without any added stimulants. Addition of CCh raised the fluid secretion to reach the highest maximum then sharply decreased to a lower sustained level (blood activating agent).
CONCLUSION: The present findings lead to the conclusion that various CHs have different promotional effects directly on the salivary gland.
- Citation: Murakami M, Wei MX, Ding W, Zhang QD. Effects of Chinese herbs on salivary fluid secretion by isolated and perfused rat submandibular glands. World J Gastroenterol 2009; 15(31): 3908-3915
- URL: https://www.wjgnet.com/1007-9327/full/v15/i31/3908.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.3908
Xerostomia (dry mouth) is caused by salivary hypofunction (reduction in salivary fluid secretion). Severe xerostomia induces speech difficulty, infection of the oral cavity and is associated with systemic disease[1]. Salivary hypofunction is associated with aging and with various diseases including diabetes, hypertension, dehydration, drugs, radiation therapy, autoimmune diseases, irradiation, sarcoidosis and many other renal, neurologic and skin diseases[2]. Xerostomia may be transient and/or reversible as in the case of medications but relatively permanent in the case of autoimmune diseases, damage due to radiation therapy and aging. The present therapeutic procedures for xerostomia are limited and include the supplemental use of artificial saliva and the use of stimulants such as pilocarpine and cevimeline.
Therapeutic use of muscarinic agents is sometimes avoided due to underlying diseases. The use of artificial saliva may bother patients during talking. Clinical research on the gene transfer of aquaporin has recently taken place[34], and is still in progress.
For thousands of years, Traditional Chinese Medicine (TCM) has been used in the treatment of xerostomia. TCM does not follow the western style of diagnosis such as whether the symptom is primary or secondary, but is based on the classical theory-Syndrome Differentiation and Treatment. However, both ancient and contemporary practitioners of TCM have no standard classification of xerostomia symptoms, because all herbalist doctors have their own understanding and clinical experiences of syndrome differentiation and herb-choice. Following a comparison of the types of classifications, we can summarize xerostomia symptoms and classify them as follows: Yin-deficiency and thin fluid-deficiency; Yin-deficiency and dryness-heat; lack of activation due to Qi-deficiency; internal block of blood stasis; accumulation of damp-heat; phlegm accumulating with blood stasis[5–7].
In order to choose herbs, all herbalist doctors obey the principle of therapy “Supply the deficiency, Reduce the excess”. Because all practitioners of Chinese Medicine have their own preferences for particular herbs, overall, they are likely to choose herbs for xerostomia as follows: Qi-enhancing agents, Body fluid-regenerating agents, Yin-nourishing agents, Heat-clearing agents, Dampness-eliminating agents, Blood-activating agents, and Phlegm-resolving agents[8–11].
Several thousand types of Chinese herbs are now in clinical use. By combining our previous findings[12] with reports by other herbalist doctors, we chose the following twenty herbs: Yuzhu (YZ, Rhizoma polygonati odorati[1314]), Shihu (SH, Herba dendrobii[1516]), Shashen (SS, Radix glehniae[1718]), Maimendong (MD, Radix ophiopogonis[1018]), Tianmendong (TD, Radix asparagi[1718]) as Yin-nourishing agents; Gegen (GG, Radix puerarie[5]), Wumei (WM, Fructus mume[1019]) as Body fluid-regenerating agents; Shengdihuang (SD, Radix rehmanniae[1920]), Xuanshen (XS, Radix scrophulariae[1718]), Chishao (CS, Radix paeoniae rubra[521]) as Heat-clearing agents; Huangqi (HQ, Radix astragali[814]), Taizishen (TZS, Radix pseudostellar iae[8]), Gancao (GC, Radix glycyrrhizae[1822]) as Qi-enhancing agents; Danshen (DS, Radix salviae miltiorrhizae[1718]) as Blood-activating agents; Zaojioci (ZJC, Fructus gleditsiae abnormalis[19]), Tianhuafen (THF, Radix trichosanthis[2023]), Ziyuan (ZY, Radix asteris[2425]) as Phlegm-resolving agents; Taoren (TR, Semen persicae[1324]), Chuanshanjia (CSJ, Squama manis[23]) as Body mass-softening and resolving agents; Dandiquionyu (DDQY[26]) as a mixture.
However, the effects of these CHs have not been evaluated quantitatively and their mechanisms have not been widely investigated. In particular, it is unknown whether the effects of these CHs act directly on the salivary glands or indirectly via neural or hormonal controls.
The present study examines whether these CHs have a direct effect on the salivary gland to increase salivary fluid secretion. Effective CHs can induce fluid secretion solely or accelerate fluid secretion by muscarinic agents such as carbachol. For this purpose, we used isolated and arterially perfused submandibular salivary glands from rats. The results provided us with quantitative data on how much CHs can accelerate salivary secretion.
Granular extracts of the Chinese herbs (CHs): YZ, SH, SS, MD, TD, SD, XS, CS, GC, WM, HQ, TZS, GG, DS, TR, ZJC, ZY, THF and CSJ were provided by Tian Jiang Pharmaceutical Co., Ltd., Jiang Yin, China. The preparations were in strict standard control according to the industry standards.
Just before each experiment, the extract equivalent to 10 g of each CH was dissolved in distilled water by ultrasonic wave concussion. After centrifugation at 5000 r/min for 10 min, the supernatant was collected and adjusted to 0.5 g/mL as the CH stock solution. Before use, the stock solution was diluted 100 times into the perfusate to obtain a final concentration of 5 g/L. Then, this CH-containing perfusate was filtered through a 0.22 μm pore size filter (Sterivex-GV, Millipore, MA, USA). The clinical dose of CH ranges from 10 to 50 g/person because the recipe is a mixture of several CHs (each CH dose is 5 g). For experimental convenience, we used an average dose of 25 g/animal for each experiment. Assuming that all CHs will move to the systemic circulation (5 L for 60 kg body weight), the concentration of CHs in the blood will be 5 g/L. We took this as the concentration of CH in the perfusion buffer.
DDQY has been proved effective in promoting saliva secretion. The ingredients of DDQY are as follows: RenShen 5 g, ShengDi 20 g, FuLing 10 g, DanShen 15 g, MaiDong 10 g. All the ingredients were added with 200 mL distilled water and were decocted twice for half an hour each time and the solution after each decoction was mixed and filtered. The final solution obtained was 96.7 mL. For convenience, we blended the solution with distilled water until the CH concentration of the stock solution was 0.5 g/mL. All the above ingredients were provided by JingQuan Group Chinese Traditional Medication Decoction Pieces Co., Ltd., AnHui, China.
Salts, glucose, carbamylcholine chloride (carbachol, CCh) were from Sigma, MO, USA. A fluorine-fiber tube (EXLON™) was purchased from Iwase Co. Ltd. (Atsugi, Japan).
Wistar rats were fed a standard pellet diet and water ad libitum. The rats were anesthetized with pentobarbitone sodium (50 mg/kg body weight, by intraperitoneal injection). The submandibular glands (120-180 mg) were surgically isolated as previously described[12] and the attached sublingual gland was removed after ligation of the feeding arteries, draining vein and sublingual duct. The extralobular main duct from the submandibular gland was cannulated with a fluorine-fiber tube (0.3 mm × 0.5 mm O.D.) for sampling. The artery distal to the glandular branch was cannulated with a stainless steel catheter (26G) connected to the infusion line for perfusion. The vein from the gland was cut free. The gland was isolated and transferred to an organ bath (37°C), where the arterial catheter was connected to the perfusion apparatus. The drained venous effluent was continuously removed.
The glands were perfused arterially at a rate of 2 mL/min using a peristaltic pump (Cole-Palmer) to supply enough oxygen even without a specific oxygen carrier during the secretory period[12]. The perfusion fluid was a buffered salt solution of the following composition (mmol/L): Na+, 145; K+, 4.3; Ca2+, 1.0; Mg2+, 1.0; Cl-, 148.3; glucose, 5.0. This solution was buffered at pH 7.4 with 10 mmol/L HEPES. The solution was prepared from stock solutions for each experiment and placed in a reservoir where it was equilibrated with 100% oxygen at 37°C.
To measure fluid secretion rate, the ductal cannula was filled with perfusate buffer and the tip placed under the water surface in a cup on an electronic balance (minimum digit was 0.1 mg, Shimadzu AEG-220), avoiding any contact with the bottom of the cup. Then, when salivary secretion started, the cumulative secreted mass could be measured. Cumulative weight was automatically measured every 3 s and transferred to a computer. The rate of fluid secretion was calculated from time-differentiation of the cumulative volume of saliva assuming a saliva specific gravity of 1.0.
Following control perfusion for longer than 20 min, recording was started 5 min prior to the start of stimulation. Fluid secretion was induced by the addition of 0.2 μmol/L carbamylcholine (CCh) in the perfusate. Following control stimulation with CCh for 10 min, CCh was removed from the perfusion line by perfusion with control perfusate for 5 min. Then only the CH was added to the perfusion for 5 min. This was to observe the effect of only the CH. After CH perfusion for 5 min, CCh was overloaded at 0.2 μmol/L in the perfusion for 20 min. To observe the accelerative effect of the CH, the dose of CCh used was 0.2 μmol/L to induce a moderate fluid secretion (c.f. 1.0 μmol/L is a supramaximal dose). The digital noise was smoothed by taking the moving average of every 11 data sets.
To reduce the variance among individual glands, the value of the flow rate was shown as mean ± SE. The statistical significance of the changes were assessed by double-tailed Student’s t-test in a comparison between the control values (4.95-5 min) and the data sets obtained at 19.95-20 min, 24.95-25 min, 29.92-30 min and 34.95-35 min, along the time course. The induction of salivary secretion by CH only was determined by a comparison between the data-sets at 9.95-10 min and 14.95-15 min. P-value < 0.1 were considered statistically significant.
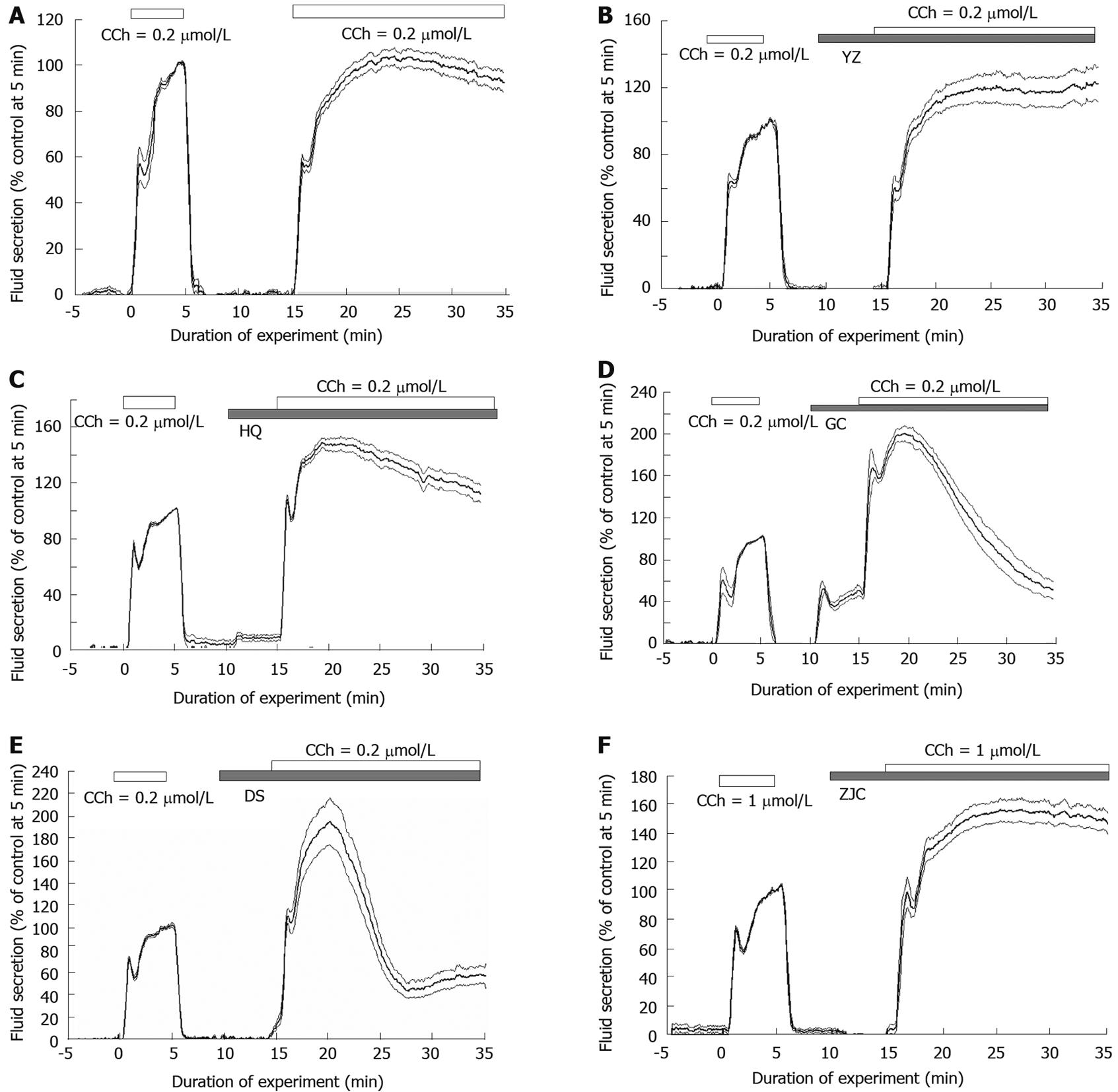
The isolated perfused rat submandibular gland had almost no spontaneous fluid secretion. To standardize secretion in each gland, carbachol (CCh) stimulation was applied for 5 min. This initial phase included an initial transient increase and a pre-sustained stage of fluid secretion around 5 min from the start of CCh stimulation (71.4 ± 8.5 μL/g per min). After washing with CCh for 5 min, CHs were applied for 5 min to check if the single application of the CH could induce fluid secretion. The period of 5 min allows the CHs and secretagogues to fully circulate in the gland. Because physiological neural reflex stimulation was applied to the gland under circulation of CH, we overloaded CCh on the CH circulated gland to determine the promotional effect of CH. Figure 1A shows the CCh-induced fluid secretion without addition of CHs. A single application of CCh showed a sustained plateau phase with a slight hump after the initial phase of secretion. For statistical examination of the promotional effect we compared how much the percentage of fluid secretion changed at 5, 10, 15 and 20 min from the start of the second CCh stimulation. The control stimulation of CCh showed no statistical difference to that of the 5-min value for the first stimulation (Figure 1A, P > 0.05).
YZ, SH, SS, MD and TD induced no secretion on single application (Figure 1B). Pre-loading of YZ did not increase the initial transient response to CCh (Figure 1B and Table 1). The initial transient peak (37.2 ± 12.7 μL/g per min at 16.2 min, n = 6) and the maximal response (68.1 ± 12.4 μL/g per min at 23.7 min) were similar to control values (36.9 ± 7.0 μL/g per min at 1.1 min) and the value at 4.85 min (71.6 ± 7.6 μL/g per min). Pre-loading of MD and TD increased the secretory response to CCh (Table 1). The initial transient peak (50.9 ± 5.4 μL/g per min at 16.2 min, n = 6; 41.4 ± 6.0 μL/g per min at 17.55 min, n = 5) was higher than the control response to CCh (36.9 ± 7.0 μL/g per min at 1.1 min; 36.6 ± 12.5 μL/g per min at 1.55 min), and the maximal response (82.2 ± 6.1 μL/g per min at 23.05 min; 73.2 ± 3.6 μL/g per min at 27.2 min) was also higher than the control response to CCh (72.3 ± 7.7 μL/g per min at 23.05 min; 64.1 ± 10.7 μL/g per min at 5.35 min). During CCh stimulation, fluid secretion was maintained at a higher level. On the other hand, pre-loading of SH did not change the responses to CCh. Pre-loading of SS decreased the responses to CCh (Table 1). The initial transient peak (25.3 ± 5.8 μL/g per min at 16.4 min, n = 6) and the maximal response (43.8 ± 5.3 μL/g per min at 27.35 min) were lower than the control value (45.4 ± 6.1 μL/g per min at 1.1 min) and the value at 5.3 min (70.4 ± 11.5 μL/g per min).
| Time (min) | YZ (n = 12) | SS (n = 12) | MD (n = 12) | TD (n = 10) | ||||
| FS (μL/g per min) | % of FS at 5 min | FS (μL/g per min) | % of FS at 5 min | FS (μL/g per min) | % of FS at 5 min | FS (μL/g per min) | % of FS at 5 min | |
| (1) 4.95-5 | 63.8 ± 11.5 | 100.6 ± 0.5 | 69.4 ± 7.0 | 99.8 ± 0.4 | 68.3 ± 5.9 | 100.3 ± 0.3 | 64.7 ± 5.3 | 100.9 ± 0.4 |
| (2) 19.95-20 | 65.3 ± 8.7 | 112.4 ± 6.3b | 42.4 ± 3.4a | 65.4 ± 6.4a | 77.7 ± 3.8 | 122.0 ± 11.1a | 62.0 ± 2.6 | 103.2 ± 11.6 |
| (3) 24.95-25 | 67.0 ± 7.9 | 118.9 ± 8.1a | 45.2 ± 3.4a | 69.7 ± 6.7a | 80.8 ± 4.1b | 125.4 ± 9.3a | 75.0 ± 2.8b | 124.6 ± 13.9b |
| (4) 29.95-30 | 65.8 ± 7.5 | 117.7 ± 8.8b | 40.6 ± 3.6a | 62.8 ± 7.5a | 80.7 ± 4.7b | 123.6 ± 6.9a | 77.0 ± 2.7a | 127.6 ± 13.5b |
| (5) 34.95-35 | 67.9 ± 7.5 | 122.5 ± 10.2a | 32.3 ± 4.9a | 49.4 ± 8.3a | 78.7± 5.3 | 119.2 ± 6.0a | 75.4 ± 3.5b | 125.3 ± 14.5b |
| (6) 9.95-10 | -0.6 ± 0.4 | -0.9 ± 1.1 | 0.8 ± 1.2 | 1.3 ± 1.7 | 0.1 ± 0.4 | 0.2 ± 0.6 | -0.3 ± 0.5 | -0.1 ± 1.1 |
| (7) 14.95-15 | -0.5 ± 1.4 | -0.9 ± 3.4 | -1.2 ± 0.5 | -1.7 ± 0.5 | -1.3 ± 0.81 | -1.2 ± 1.2 | -0.5 ± 0.3 | -0.8 ± 0.4 |
GG and WM did not induce secretion on a single application. Pre-loading of GG decreased the responses to CCh (Table 2). The initial transient peak (45.5 ± 8.8 μL/g per min at 16.5 min, n = 6) was lower than the control initial transient peak (59.3 ± 7.9 μL/g per min at 1.1 min) The maximal response (68.2 ± 13.4 μL/g per min at 21 min) was also lower than the control response at 5 min (83.0 ± 11.4 μL/g per min). After reaching a maximum, the flow rate was slowly decreased to 52.9 ± 16.0 μL/g per min at 35 min even during sustained stimulation. Thus, GG did not show a promotional effect on CCh-induced fluid secretion. Pre-loading of WM increased the secretory response to CCh (Table 2). The initial transient peak (61.3 ± 16.2 μL/g per min at 16.2 min, n = 6) was higher than the control response (53.9 ± 16.1 μL/g per min at 1.1 min), and the maximal response (89.8 ± 15.6 μL/g per min at 25.95 min) was also higher than the control response (72.3 ± 7.7 μL/g per min at 5.4 min). During CCh stimulation, the fluid secretion was maintained at a higher level (83.2 ± 10.2 μL/g per min at 35 min).
| Time (min) | GG (n = 12) | WM (n = 12) | SD (n = 12) | CS (n = 12) | ||||
| FS (μL/g per min) | % of FS at 5 min | FS (μL/g per min) | % of FS at 5 min | FS (μL/g per min) | % of FS at 5 min | FS (μL/g per min) | % of FS at 5 min | |
| (1) 4.95-5 | 82.9 ± 7.2 | 99.9 ± 0.2 | 66.9 ± 9.7 | 107.0 ± 3.8 | 58.5 ± 5.7 | 97.7 ± 0.3 | 66.4 ± 6.9 | 100.6 ± 0.5 |
| (2) 19.95-20 | 64.0 ± 8.1b | 78.4 ± 6.8a | 80.9 ± 10.1 | 116.2 ± 4.2 | 51.7 ± 6.0 | 86.9 ± 5.1a | 86.0 ± 5.6a | 140.1 ± 10.4b |
| (3) 24.95-25 | 60.4 ± 8.5a | 76.5 ± 8.6a | 88.4 ± 9.9 | 130.0 ± 6.5a | 56.2 ± 4.9 | 95.4 ± 3.2 | 101.2 ± 4.7a | 168.0 ± 14.5b |
| (4) 29.95-30 | 55.4 ± 8.2a | 70.4 ± 8.4a | 85.3 ± 7.3 | 129.3 ± 8.7a | 54.5 ± 3.4 | 94.2 ± 4.4 | 106.7 ± 5.5a | 179.4 ± 17.9b |
| (5) 34.95-35 | 50.8 ± 8.2a | 63.7 ± 8.0a | 83.2 ± 6.6 | 127.5 ± 9.6b | 49.7 ± 3.2 | 85.8 ± 3.4a | 109.6 ± 6.3a | 184.64 ± 18.5b |
| (6) 9.95-10 | 1.6 ± 0.7 | 2.2 ± 1.2 | -0.9 ± 0.3 | -1.1 ± 0.3 | -0.4 ± 0.7 | -1.1 ± 1.1 | -0.9 ± 0.4 | -1.3 ± 0.6 |
| (7) 14.95-15 | -1.7 ± 0.6c | -2.4 ± 1.0c | -0.7 ± 0.2 | -1.1 ± 0.3 | -1.7 ± 1.4 | -3.5 ± 2.3 | 0.6 ± 1.6 | 1.8 ± 3.1 |
SD, XS and CS did not induce secretion on a single application. Pre-loading of SD and XS decreased the responses to CCh (Table 2). The initial transient peak of SD (34.4 ± 7.5 μL/g per min at 16.2 min, n = 5) and XS (42.9 ± 7.9 μL/g per min at 16.2 min, n = 6) were similar to or lower than the control values (34.7 ± 7.3 μL/g per min at 1.15 min; 61.0 ± 13.6 μL/g per min at 1.15 min), whereas the maximal response of both SD and XS (56.9 ± 7.6 μL/g per min at 24.5 min; 61.2 ± 14.2 μL/g per min at 21.5 min) were lower than the control maximal value (72.3 ± 7.7 μL/g per min at 5.1 min; 81.0 ± 15.2 μL/g per min at 5 min). The response gradually declined during stimulation. Thus, SD and XS did not show a promotional effect on CCh-induced fluid secretion. On the other hand, pre-loading of CS increased the secretory response to CCh (Table 2). The initial transient peak (57.9 ± 7.4 μL/g per min at 17.2 min, n = 6) was higher than the control response (44.7 ± 8.5 μL/g per min at 1.25 min), and the response increased 86.0 ± 8.7 μL/g per min at 20 min, which was also higher than the corresponding response to single stimulation with CCh (66.6 ± 10.8 μL/g per min at 5 min). During CCh stimulation, fluid secretion continued to increase to the highest value, 109.2 ± 9.9 μL/g per min at 35 min.
HQ did not induce statistically significant fluid secretion (comparison between the values at 10 min and 12 min). Pre-loading of HQ enhanced the secretory response to CCh (Figure 1C and Table 3). Both the initial transient peak (85.9 ± 6.8 μL/g per min at 16 min, n = 6) and the maximal response (116.9 ± 5.1 μL/g per min at 19.4 min) were higher than the control CCh stimulation (60.9 ± 7.3 μL/g per min at 1 min and 80.3 ± 5.7 μL/g per min at 5.15 min, respectively). After reaching a maximum, fluid secretion declined slowly to a similar level to that of the control response (Figure 1C and Table 3). Taizishen (TZS) did not induce secretion on a single application. Pre-loading of TZS increased the secretory response to CCh (Table 3). Both the initial transient peak (71.1 ± 16.2 μL/g per min at 17.25 min, n = 5) and the maximal response (104.9 ± 13.7 μL/g per min at 24.05 min) were higher than the control CCh stimulation (43.5 ± 6.3 μL/g per min at 1.2 min and 72.2 ± 7.8 μL/g per min at 5.05 min, respectively). After reaching a maximum, fluid secretion declined gradually. Gancao (GC) induced a significant secretion even on a single application (Figure 1D) with an initial transient peak (27.0 ± 4.7 μL/g per min at 11.5 min, n = 5) and sustained secretion (25.6 ± 4.6 μL/g per min at 15 min). Pre-loading of GC promoted the secretory response to CCh (Figure 1D and Table 3). Both the initial transient peak (85.1 ± 13.2 μL/g per min at 16.65 min) and the maximal response (102.3 ± 14.4 μL/g per min at 19.4 min) were higher than the control CCh stimulation (39.0 ± 3.5 μL/g per min at 1.15 min and 62.6 ± 7.8 μL/g per min at 5.15 min, respectively). After fluid secretion increased to reach a value double the level of stimulation without GC, secretion then declined gradually to half the maximal value (24.0 ± 5.3 μL/g per min at 35 min).
| Time (min) | HQ (n = 12) | TZS (n = 10) | GC (n = 10) | DS (n = 12) | ||||
| FS (μL/g per min) | % of FS at 5 min | FS (μL/g per min) | % of FS at 5 min | FS (μL/g per min) | % of FS at 5 min | FS (μL/g per min) | % of FS at 5 min | |
| (1) 4.95-5 | 79.1 ± 3.5 | 99.6 ± 0.1 | 80.3 ± 7.0 | 101.1 ± 0.5 | 51.8 ± 4.3 | 101.7 ± 0.6 | 71.6 ± 8.0 | 101.9 ± 1.8 |
| (2) 19.95-20 | 115.5 ± 3.6a | 146.8 ± 4.4a | 95.7 ± 8.8 | 122.7 ± 7.0a | 101.5 ± 8.9a | 204.4 ± 6.8a | 126.5 ± 11.5a | 194.8 ± 20.4a |
| (3) 24.95-25 | 107.6 ± 4.2a | 137.2 ± 6.0a | 103.6 ± 8.3a | 135.3 ± 9.5a | 69.2 ± 6.2a | 144.0 ± 14.1a | 50.3 ± 10.4 | 75.0 ± 11.7a |
| (4) 29.95-30 | 96.6 ± 3.5a | 123.6 ± 6.0a | 100.5 ± 7.9b | 132.0 ± 11.0a | 38.3 ± 4.7a | 82.3 ± 15.3 | 31.3 ± 3.7a | 50.7 ± 8.1a |
| (5) 34.95-35 | 86.9 ± 3.8 | 112.0 ± 6.1a | 91.7 ± 7.1 | 121.6 ± 10.2b | 24.0 ± 3.5a | 50.0 ± 7.9a | 35.1 ± 3.0a | 56.9 ± 7.3a |
| (6) 9.95-10 | 2.2 ± 2.5 | 2.2 ± 2.6 | -0.2 ± 0.3 | -0.6 ± 0.2 | -0.2 ± 0.2 | -6.3 ± 3.5 | 0.7 ± 0.5 | 1.5 ± 1.0 |
| (7) 14.95-15 | 5.9 ± 2.0 | 7.4 ± 2.3 | -0.4 ± 0.3 | -0.1 ± 0.5 | 25.4 ± 2.7c | 50.5 ± 4.7c | 7.4 ± 1.8c | 12.0 ± 3.1c |
DS induced significant secretion even on a single application (Figure 1E) with a long delay of 3-4 min (8.0 ± 2.9 μL/g per min at 15 min, n = 6) and then increased markedly to a maximum and thereafter declined (data are not shown, as the overload of CCh was started at 15 min in the present series of experiments). Pre-loading of DS promoted the secretory response to CCh (Figure 1E and Table 3). Both the initial transient peak (76.2 ± 11.5 μL/g per min at 16.05 min) and the maximal response (124.0 ± 17.5 μL/g per min at 19.3 min) were higher than the control CCh stimulation (52.1 ± 10.0 μL/g per min at 1.05 min, 72.3 ± 12.6 μL/g per min at 5.15 min) as shown in Figure 1E. Fluid secretion then increased to reach a value double the level of stimulation without DS, and declined gradually to half this value (36.6 ± 6.1 μL/g per min at 35 min).
ZJC did not induce secretion on a single application (Figure 1F). Pre-loading of ZJC increased the secretory response to CCh (Figure 1F, Table 4). The initial transient peak (60.9 ± 7.1 μL/g per min at 16.8 min, n = 5) and the maximal response (99.1 ± 11.8 μL/g per min at 25.3 min) were higher than the control CCh stimulation (45.2 ± 7.0 μL/g per min at 1.2 min, 65.8 ± 12.5 μL/g per min at 5.25 min) as shown in Figure 1F. Fluid secretion tended to decline gradually (93.9 ± 11.8 μL/g per min at 35 min) during stimulation. THF and ZY did not induce secretion on a single application. Pre-loading of THF and ZY (Table 4) increased the secretory response to CCh. The initial transient peak (45.1 ± 9.8 μL/g per min at 16.25 min n = 6; 55.7 ± 11.0 µL/g per min at 16.55 min, n = 6) were higher than the control CCh stimulation (43.3 ± 13.2 μL/g per min at 1.15 min, 45.3 ± 9.3 μL/g per min at 1.2 min); the maximal response (77.0 ± 11.0 μL/g per min at 26.3 min; 86.9 ± 9.7 μL/g per min at 28.95 min) was also higher than the control CCh stimulation (65.4 ± 15.4 μL/g per min at 5.15 min, 63.9 ± 10.6 μL/g per min at 5.3 min). Fluid secretion tended to decline gradually (73.2 ± 10.0 μL/g per min at 35 min; 80.5 ± 8.0 μL/g per min at 35 min) during stimulation.
| Time (min) | ZJC (n = 10) | ZY (n = 12) | TR (n = 10) | CSJ (n = 10) | ||||
| FS (μL/g per min) | % of FS at 5 min | FS (μL/g per min) | % of FS at 5 min | FS (μL/g per min) | % of FS at 5 min | FS (μL/g per min) | % of FS at 5 min | |
| (1) 4.95-5 | 67.0 ± 7.9 | 100.5 ± 0.7 | 63.5 ± 6.4 | 101.7 ± 0.3 | 53.8 ± 4.4 | 102.1 ± 0.8 | 46.2 ± 5.6 | 100.3 ± 0.4 |
| (2) 19.95-20 | 85.9 ± 7.0b | 135.2 ± 6.3a | 70.8 ± 2.9 | 121.0 ± 8.5a | 57.6 ± 4.2 | 111.0 ± 5.9 | 49.8 ± 6.7 | 107.2 ± 2.7a |
| (3) 24.95-25 | 98.3 ± 7.8a | 154.7 ± 7.1a | 83.8 ± 4.7a | 141.0 ± 7.6a | 66.0 ± 5.4b | 126.0 ± 8.5a | 55.4 ± 8.3 | 118.8 ± 8.0a |
| (4) 29.95-30 | 97.1 ± 7.8a | 152.4 ± 6.4a | 85.6 ± 5.6a | 142.9 ± 7.1a | 63.5 ± 5.2 | 121.9 ± 8.1a | 54.1 ± 7.6 | 117.0 ± 8.9b |
| (5) 34.95-35 | 94.1 ± 7.4a | 148.1 ± 6.9a | 80.5 ± 5.0a | 134.3 ± 6.5a | 59.9 ± 5.4 | 114.3 ± 7.2b | 50.8 ± 7.3 | 109.9 ± 9.2 |
| (6) 9.95-10 | 1.6 ± 1.0 | 2.5 ± 1.4 | 1.4 ± 1.5 | 3.5 ± 3.1 | -1.2 ± 0.7 | -2.4 ± 1.5 | 0.5 ± 0.6 | 0.2 ± 0.9 |
| (7) 14.95-15 | 1.0 ± 0.5 | 2.2 ± 1.3 | -0.9 ± 0.3 | -1.5 ± 0.6 | -2.2 ± 1.0 | -4.5 ± 2.0 | 2.3 ± 1.4 | 3.4 ± 1.8 |
TR and CSJ did not induce secretion on a single application. Pre-loading of TR increased the secretory response to CCh (Table 4). The initial transient peak (47.6 ± 8.6 μL/g per min at 16.4 min, n = 5) was higher than the control CCh stimulation (43.5 ± 6.3 μL/g per min at 1.1 min). However, the maximal response (66.1 ± 8.6 μL/g per min at 1.1 min) was similar to the control CCh stimulation (71.8 ± 7.6 μL/g per min at 5.25 min). Fluid secretion was sustained at the same level during stimulation. Pre-loading of CSJ did not increase the secretory response to CCh (Table 4). The initial transient peak (38.3 ± 8.6 μL/g per min at 16.65 min, n = 5) was similar to the control response (39.0 ± 3.5 μL/g per min at 16.65 min), and the maximal response (53.3 ± 12.7 μL/g per min at 25.75 min) was lower than the control response (71.8 ± 7.4 μL/g per min at 5.2 min). Because the control values for fluid secretion in response to CCh were lower in the series using CSJ, the percentage change (118.8 ± 8.0% at 25 min) showed promotion by CSJ (Table 4).
DDQY did not induce secretion on a single application. Pre-loading of DDQY increased the secretory response to CCh. The initial transient peak (40.9 ± 9.8 μL/g per min at 16.35 min, n = 5) and the maximal response (78.0 ± 7.9 μL/g per min at 27.45 min) were higher than the control CCh stimulation (25.9 ± 4.7 μL/g per min at 1.45 min, 51.3 ± 8.7 μL/g per min at 5.25 min). Fluid secretion tended to decline gradually (74.2 ± 4.7 μL/g per min at 35 min) during stimulation.
Disorders of salivary secretion are the result of various causes and mechanisms in modern medicine, while in TCM, saliva is a body fluid and its deficiency is related to the deficiency of Yin-fluid, the vigor of internal heat and the dysfunctional distribution of Yin-fluid. TCM regards the body as a whole, and holds that the normal secretion of saliva needs the generational effect of Qi as well as it’s promotional effect. Once there is dysfunctional secretion of body fluid, the oral cavity cannot be moistened, and new pathologic features such as phlegm and excessive fluid will occur, leading to stagnation, which incurs another secondary injury which we call internal block (e.g. blood stasis),
In the treatment of secretion disorders, Body fluid-regenerating, Yin-nourishing, and Heat-clearing agents are commonly used and are reported in the literature[910]. Qi-enhancing agents are often prescribed simultaneously[8]. In chronic patients with a long disease course, Blood-activating agents are usually used, and sometimes even stasis-resolving[1718], hard mass-softening and block-dispelling agents[1923]. In conclusion, the therapeutic concept of TCM is to identify suitable herbs which can resolve the pathologic status of patients so as to reach a new balance, which is called Syndrome Differentiation and Treatment.
It is interesting to find in our experiments that the promotional patterns of CH on secretion were classified into four patterns, which were eventually related to the categories of CH: overall sustained phase was continuously raised (Yin-nourishing, fluid production-promoting and heat-clearing agents). Although they belong to different CH types, they are closely correlated in the TCM theory, that is, Yin-nourishing and fluid production-promoting agents are similar in function, while heat-clearing agents can reserve body fluid which is reduced by internal heat; Sustained secretion rose to reach a maximum then decreased, this pattern was observed in HQ and TZS. These are Qi-enhancing agents; Sustained secretion rose to reach the highest maximum then was sustained with a slight decline (swelling-reducing, phlegm-resolving and pus-expelling agents). These 3 types of herbs have one thing in common, in that they all aim to dispel pathologic features inside the body. It is possible that they may have a similar promotional pattern; Stimulation of salivary secretion without any added stimulants. The addition of CCh promoted fluid secretion to reach the highest maximum then inhibited secretion to a lower sustained level (blood activating agent DS and GC). GC, a Qi-enhancing herb, is usually used as a conciliatory agent, and is quite different from the common Qi-enhancing agents such as HQ.
The present findings at an organ level lead to the conclusion that various CHs have different promotional mechanisms and target sites. There are scientific essences in the TCM theory and its herb classifications, however further investigations are needed, including dose-dependency of the promotional pattern of each CH.
The isolated and arterially perfused salivary gland can induce fluid secretion either by neural stimulation and /or by arterial application of secretagogues. If there is no stimulation then no secretion occurs. In the present study, SH, XS, GG, SS, and DH did not show any potential in promoting fluid secretion in the salivary gland. This suggests no salivary promotional effects directly on the salivary glands by SH, XS, GG, SS, and DH. However, we have to examine the dose-response of these CHs, then we might expect to see that these CHs have indirect effects on the neural system and/or hormonal system, and indirectly affect salivary fluid secretion.
Fluid secretion by salivary glands has been recognized as the transcellular movement of electrolyte/water at secretory endpiece cells (or acinar cells). Recently, the paracellular component of fluid secretion was also taken into account. The initial fluid secretion in response to CCh is estimated mainly due to transcellular fluid movement, whereas 60%-70% of fluid secretion is due to the paracellular component during the sustained stimulation period 1 min after the start of stimulation[27]. The initial transient peak of fluid secretion is thought to be due mainly to transcellular fluid secretion. Whereas, fluid secretion during the sustained phase depends both on transcellular and paracellular components.
One possible mechanism for the promotion through transcellular movement is activation of receptors to increase cytosolic Ca2+ which is mobilized from Ca2+ store/Ca2+ entry. In addition, the activation of transporters for Cl--entry is possible. Cl- channels allow Cl- release across luminal membrane, and the activation of Na+/K+ ATPase can increase Na+-coupled Cl- entry. The activation of unknown receptors can also play a role in these possible mechanisms.
In paracellular mechanisms, opening of junctional complexes is possible. In addition, increases in unknown driving forces related to paracellular fluid transport needs further study.
Research to clarify the mechanisms of promotion is tightly linked to the search for control points of fluid secretion mechanisms. We could check cytosolic Ca2+ and Na+-coupled Cl--entry in transcellular mechanisms by measuring the ouabain sensitive component of oxygen consumption. We could also determine the junctional complexes in paracellular mechanisms by measuring the secretion of fluorescent dye which cannot enter the cell. These possible mechanisms require further study.
Xerostomia (dry mouth) is caused by salivary hypofunction (reduction in salivary fluid secretion). Traditional Chinese Medicine (TCM) has been used in the treatment of xerostomia. The aim of the present study was to determine if Chinese herbs (CHs) had a direct effect on the salivary gland to increase salivary fluid secretion.
The present therapeutic procedures for xerostomia are limited and include the supplemental use of artificial saliva and the use of stimulants. TCM is an effective therapy with few side effects. However, there is little research on the effects of CHs on salivary secretion. Our study aims to identify the effects of CHs on salivary secretion.
By combining our previous findings with reports by other herbalist doctors, we chose twenty herbs and used isolated and arterially perfused rat submandibular salivary glands to examine if CHs had direct effects on the salivary gland to increase salivary fluid secretion. The results provided us with quantitative data how much CHs could accelerate salivary secretion.
The quantitative results of this study showed how much CHs could accelerate salivary secretion. This information could possibly provide a theoretical guide for choosing herbs to relieve xerostomia in TCM practices.
The manuscript by Murakami et al, describes the results of studies on the effect of several types of traditional Chinese herb remedies, commonly used to relieve the effects of dry mouth disease (xerostomia), on salivary fluid secretion in the perfused rat submandibular salivary gland. It has some merits as it addresses the problem of xerostomia, a condition affecting quite a large segment of the world population including millions of elderly women.
| 1. | Rossie K. Influence of diseases on salivary glands. Biology of the salivary glands. Florida: CRC Press 1993; 201-227. |
| 2. | Sreebny LM, Valdini A, Yu A. Xerostomia. Part II: Relationship to nonoral symptoms, drugs, and diseases. Oral Surg Oral Med Oral Pathol. 1989;68:419-427. |
| 3. | Delporte C, O'Connell BC, He X, Lancaster HE, O'Connell AC, Agre P, Baum BJ. Increased fluid secretion after adenoviral-mediated transfer of the aquaporin-1 cDNA to irradiated rat salivary glands. Proc Natl Acad Sci USA. 1997;94:3268-3273. |
| 4. | Baum BJ, Voutetakis A, Wang J. Salivary glands: novel target sites for gene therapeutics. Trends Mol Med. 2004;10:585-590. |
| 5. | Gu Q, Liu JY. Prof. Zhou Zhongying's experience on the treatment of Sjogren’s Syndrome. Xin Zhongyi. 2002;9:7-8. |
| 6. | Hao WX, Dong ZH. Retrospective study on classification of TCM Syndromes of 106 Cases of Sjogren Syndrome. Zhongyi Zazhi. 2006;7:528-530. |
| 7. | Shi LP. Progress in the treatment of Sjogren’s Syndrome with TCM. Zhongyiyao Daobao. 2007;12:83-85. |
| 8. | Mao JC, Chen XJ, Su L, Gu JH, Chen XY. Clinical effects of Yi-Qi-Jian-Pi therapy on Sjogren’s syndrome. Zhongguo Linchuang Yaoxue Zazhi. 2007;4:231-233. |
| 9. | Wei QH, Fu HW, Jin YL, Yang HT. Comparative study on the method of clearing away heat and activating blood circulation and the method of nourishing Yin and promoting the production of body fluid in treatment of Sjogren Syndrome. Zhongyi Zazhi. 2006;7:509-511. |
| 10. | Chen H, Chen XJ. Yin-producing therapy for Sjogren Syndrome by sour and sweet herbs. Shanghai Zhonyiyaodaxue Xuebao. 2005;3:22-23. |
| 11. | Li JY, Zhao LJ, Huang YY, Chen Y, Cai SQ, Lu XF. Clinical inspecting of treatment for Sjogren Syndrome with Qingkai Ling injection. Zhongguo Zhongyi Jichu Yixue Zazhi. 2002;8:41-42. |
| 12. | Murakami M, Miyamoto S, Imai Y. Oxygen consumption for K+ uptake during post-stimulatory activation of Na+, K(+)-ATPase in perfused rat mandibular gland. J Physiol. 1990;426:127-143. |
| 13. | Wang Q, Liu H, Qiao N. [Effects of traditional Chinese medicine on salivary glands in the patients with head and neck cancer during radiotherapy]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 1998;18:662-664. |
| 14. | Sun YY, Zhang L, Wang XD, Jia B, Ge DY, Liu JX. Preliminary study on the effect of Shengjin oral solution on the Sjogren’s Syndrome animal model. Zhongyao Yaoli Yu Linchuang. 2006;1:51-53. |
| 15. | Wang LM, Chen Y, Chen LZ, Sun JJ, Wu HH, Xu JH. Studies on the pharmacological effects of Dendrobium candicum capsules. Zhongguo Xiandai Yingyong Yaoxue. 2002;4:232-264. |
| 16. | Xu JH, Chen LZ, Li L. Studies on the effects of White Dendrobium (Denbrobium candicum) and American Ginseng (Panax quinquefolius) on nourishing the Yin and promoting glandular secretion in mice and rabbits. Zhongcaoyao. 1995;2:79-80. |
| 17. | Jiang ZX, Jiang LY, Qiu JX, Qian Y, Luo QP. Clinical investigation of traditional chinese medicine treatment of Xerostomia after head and neck cancer postradiotherapy. Jiangxi Yixueyuan Xuebao. 2005;3:24-25. |
| 18. | Jiang ZX, Qian Y, Jiang LY. Effects of chinese herb on salivary glands of patients with head and neck cancer during radiotherapy. Jiangxi Yixueyuan Xuebao. 2006;2:76-77. |
| 19. | Liu PL, Zhou XL, Zhao JF. Curative Effect observation of granule of Shengjin on Primary Sjogren’s Syndrome, A Report of 40 Cases. Shanxi Zhongyi. 2007;6:27-28. |
| 20. | Shen K, Ma HL, Guo SM, Lv LK. Clinial study of treating of Primary Sjogren Syndrome by moistening oral liquid. Hebei Yixue. 2006;2:124-126. |
| 21. | Fu XL, Zhang LT, Liu L. Thoughts and characteristics of Prof. Zhang Minghe in the Syndrome Differentiation and Treatment of Sjogren‘s syndrome. Zhonghua Zhongyiyao Zazhi. 2001;1:52-53. |
| 22. | Li F, Yao JH, Zhang FX, Sun LJ, Sun LJ, Tao JM, Ning XR. Clinical observation on treatment of primary Sjogren Syndrome by compound clycyrrhizin injection. Zhongguo Yaofang. 2007;11:858-859. |
| 23. | Chen DL. 30 cases of treating primary Sjogren’s syndrome (pSS) with the method of removing blood stasis and clearing away heat. Shanxi Zhongyi. 2007;8:1020-1021. |
| 24. | Qian Y, Jin S, Yu ZW. Effect of Zengye Bujin decoction on blood β_2-M in primary Sjogren’s Syndrome patients. Zhongguo Zhongyiyao Xinxi Zazhi. 2003;2:13-14. |
| 25. | Man TT, Wang Y. Clinical observations of 20 cases of treating Sjogren’s syndrome with MaiDongDiShao decoction. Nanjing Zhongyiyaodaxue Xuebao. 2008;1:63-65. |
| 26. | Zhou DH, Zhang QD, Wei MX, Xu Y. Effect of Dan Di Qiong Yu granule on sjogren syndrome mice’s salivary gland. Nanjing Yikedaxue Xuebao. 2005;4:55-56. |
| 27. | Segawa A, Yamashina S, Murakami M. Visualization of 'water secretion' by confocal microscopy in rat salivary glands: possible distinction of para- and transcellular pathway. Eur J Morphol. 2002;40:241-246. |