Published online Jan 21, 2009. doi: 10.3748/wjg.15.334
Revised: June 6, 2008
Accepted: June 13, 2008
Published online: January 21, 2009
AIM: To determine the prevalence and characteristics of bile reflux in gastroesophageal reflux disease (GERD) patients with persistent symptoms who are non-responsive to medical therapy.
METHODS: Sixty-five patients (40 male, 25 female; mean age, 50 ± 7.8 years) who continued to report symptoms after 8 wk of high-dose proton pump inhibitor (PPI) therapy, as well as 18 patients with Barrett’s esophagus, were studied. All patients filled out symptom questionnaires and underwent endoscopy, manometry and combined pH-metry and bilimetry.
RESULTS: There were 4 groups of patients: 22 (26.5%) without esophagitis, 24 (28.9%) grade A-B esophagitis, 19 (22.8%) grade C-D and 18 (21.6%) Barrett’s esophagus. Heartburn was present in 71 patients (85.5%) and regurgitation in 55 (66.2%), with 44 (53%) reporting simultaneous heartburn and regurgitation. The prevalence of pathologic acid reflux in the groups without esophagitis and with grades A-B and C-D esophagitis was 45.4%, 66.6% and 73.6%, respectively. The prevalence of pathologic bilirubin exposure in these 3 groups was 53.3%, 75% and 78.9%, respectively. The overall prevalence of bile reflux in non-responsive patients was 68.7%. Pathologic acid and bile reflux was observed in 22.7% and 58.1% of non-esophagitic patients and esophagitic patients, respectively.
CONCLUSION: The high percentage of patients poorly responsive to PPI therapy may result from poor control of duodenogastroesophageal reflux. Many patients without esophagitis have simultaneous acid and bile reflux, which increases with increasing esophagitis grade.
- Citation: Monaco L, Brillantino A, Torelli F, Schettino M, Izzo G, Cosenza A, Martino ND. Prevalence of bile reflux in gastroesophageal reflux disease patients not responsive to proton pump inhibitors. World J Gastroenterol 2009; 15(3): 334-338
- URL: https://www.wjgnet.com/1007-9327/full/v15/i3/334.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.334
As a result of their strong acid suppression, proton pump inhibitors (PPIs) have been used to treat most patients with gastroesophageal reflux disease (GERD)[1–5]. Acid reflux is the main risk factor for GERD, with pH-metry being the standard method used in the diagnosis of GERD. Many patients with typical GERD symptoms, however, have been found to have a negative pH-metry[6]; these patients have been found to differ in symptoms, response to medical therapy, and endoscopy results from patients with positive pH-metry.
Although the role of acid reflux in GERD has been established, and links between acid and bile reflux have been found, less is known about the role of bile in the pathogenesis of esophageal mucosal damage. Thus, the incidence of GERD, its clinical impact, etiology, evolution and therapeutic implications cannot be determined directly. This limitation, however, was improved by the introduction of bilimetry in clinical practice[7]. This method uses spectrophotometric analysis to measure the presence of bilirubin in the refluxate, thus providing a direct and reliable measurement of bile reflux.
The combination of pH-metry and bilimetry has increased the sensitivity and accuracy of GERD diagnosis and has shown that increased bile reflux is correlated with increased severity of esophagitis[89]. Moreover, other authors showed that a high percentage of GERD patients, poorly responsive to PPI therapy, had mixed acid and bile reflux, or isolated bile reflux[10]. To expand these investigations, we evaluated the prevalence and characteristics of bile reflux in GERD patients with persistent symptoms who were non-responsive to medical therapy.
Of 230 patients with heartburn and regurgitation evaluated between January, 2002 and July, 2006, 65 (40 male, 25 female; mean age, 50 ± 7.8 years) continued to report symptoms after 8 wk of high-dose PPI therapy (40 mg esomeprazole bid). In addition, 18 patients with Barrett’s esophagus were included. All patients were administered symptom questionnaires and underwent endoscopy, perfused esophageal manometry and combined 24-h esophago-gastric pH-bilimetry.
The presence of esophagitis was classified according to the Los Angeles Classification[11]. The presence of hiatal hernia was determined and esophageal biopsies were used to diagnose Barrett’s esophagus.
Manometric evaluation was made without sedation after 1 wk of pharmacologic wash-out and an overnight fast. An 8-channel manometric device (Menfis Biomedical Inc. Bologne, Italy) connected to a low compliance hydro-pump (Arndorfer Medical Specialties, Greendale, Wisconsin, USA) was used. The 8 open tip (4 radial and 4 longitudinal) manometric probe was inserted through the nose into the stomach and lower esophageal sphincter (LES) parameters (pressure, length and postdeglutitive relaxation) were evaluated by a rapid and stationary pull-through technique. Esophageal motor activity (amplitude and duration of waves, percentage of peristaltic and simultaneous post-deglutitive sequences) was evaluated with stationary pull-through after 20 wet and dry swallows.
We performed this test after 1 wk of pharmacologic wash-out. We used a two channel portable recorder (Menfis Biomedical Inc., Bologna, Italy) connected to two glass pH-metric probes (Telemedicine srl., Naples Italy), which were introduced through the nose without any sedation, and placed 5 cm above and 10 cm below the upper and the lower edge of the LES, respectively. The percentage of total time of exposure to pH < 4 (normal value < 4.2%) was determined.
Bilimetric evaluation was performed simultaneously with pH-metry. We utilized a portable recorder (BILITEC 2000, Sinectics Medical Inc.) connected to two optic-fiber probes placed 5 cm above and 10 cm below the upper and the lower edge of the LES, respectively. The percentage of total time of esophageal bilirubin absorbance > 0.14 (normal value < 7%) was determined.
The ethics committee of the Second University of Naples approved our study and verbal consent was obtained from the study participants.
Values are expressed as mean ± SD. Data were compared using Student’s t-test, Fischer’s exact test, or the Chi-square test wherever appropriate. A P-value less than 0.05 was considered statistically significant.
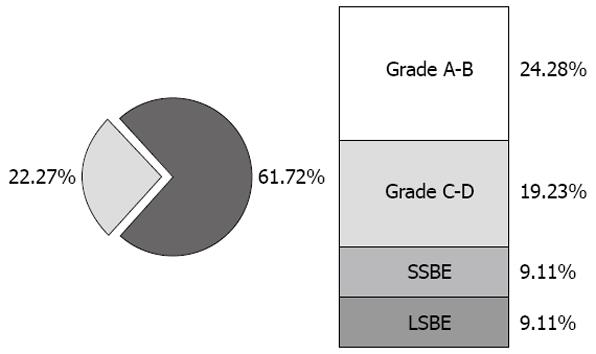
Endoscopic evaluation divided the 83 patients into 4 groups. Group I consisted of 22 (26.5%) non-esophagitic patients, Group II consisted of 24 patients (28.9%) with grade A-B esophagitis, Group III consisted of 19 patients (22.8%) with grade C-D esophagitis, and Group IV consisted of 18 patients (21.6%) with Barrett’s esophagus; of the latter, nine had short segment Barrett’s esophagus (SSBE) and nine had long segment Barrett’s esophagus (LSBE, Figure 1). Of the 83 patients, 61 (73.4%) had a hiatal hernia.
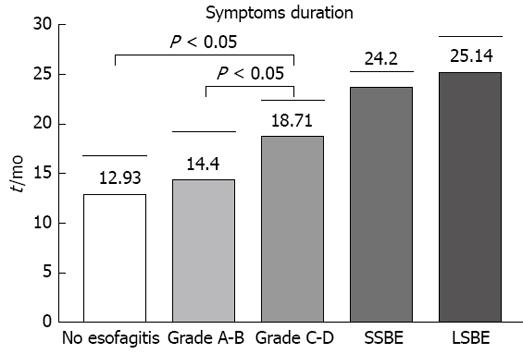
The analysis of the symptoms questionnaire showed that 71 patients (85.5%) had heartburn, 55 (66.2%) had regurgitation, and 44 (53%) had simultaneous heartburn and regurgitation. Twelve patients (14.4%) reported nocturnal cough and 7 (8.4%) reported chest pains. Analysis of symptom scores showed no significant between group differences. In contrast, symptom history was significantly higher in Group III than in Groups I and II, but not between Barrett’s patients (Figure 2).
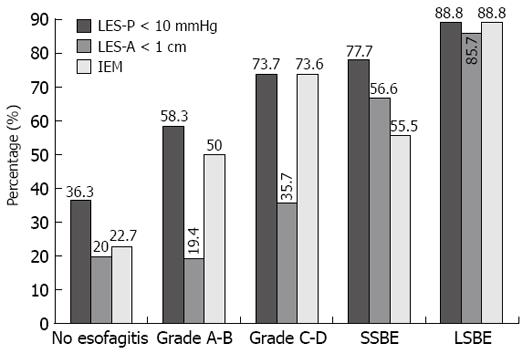
Hypotonic LES was observed in 8 of 22 (36.3%) Group I, 14 of 24 (58.3%) Group II and 14/19 (73.7%) Group III patients (Group I vs Group III: P = 0.0279). Hypotonic LES was also present in 8 of 9 (88.8%) LSBE and 7 of 9 (77.7%) SSBE patients. Mean LES-P in Group I (13.91 ± 4.8 mmHg) was significantly higher than in Groups II (9.2 ± 2.2 mmHg, P < 0.001), and III (8.6 ± 2.8 mmHg, P < 0.001) and in SSBE (9.2 ± 3.4 mmHg, P = 0.0056) and LSBE (7.1 ± 1.6 mmHg, P < 0.001) patients.
Five of the 22 (22.7%) patients in Group I showed ineffective esophageal motility, increasing to 50% (12/24) in Group II and to 73.6% (14/19) Group III. In comparison, 5 of 9 SSBE (55.5%) and 8 of 9 (88.8%) LSBE patients showed ineffective esophageal motility (Figure 3).
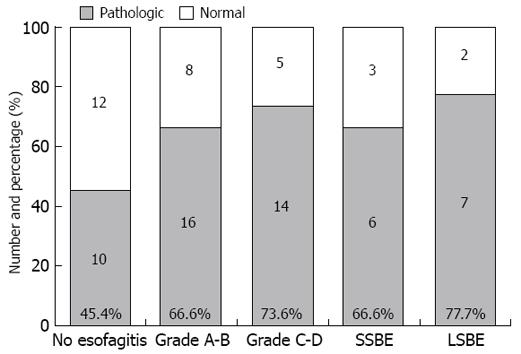
The prevalence of pathologic acid reflux increased relative to esophagitis, from 45.4% (10/22) in Group I, to 66.6% (16/24) in Group II and 73.6% (14/19) in Group III. Six of 9 (66.6%) SSBE and 7 of 9 (77.7%) LSBE patients showed pathologic pH-metry (Figure 4).
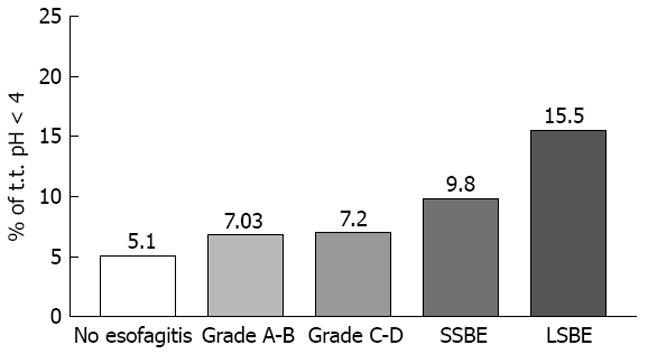
Relative time at pH < 4 was 5.1 ± 2.7% in non-esophagitic (Group I) patients, increasing to 7.03 ± 3.6% (P > 0.05) in Group II and 7.2 ± 0.24% (P > 0.05) in Group III. In contrast, both the SSBE (9.8 ± 5.1%, P = 0.0022) and LSBE (15.5 ± 7.7%, P < 0.0001) groups had significantly more time at pH < 4 than did Group I (Figure 5).
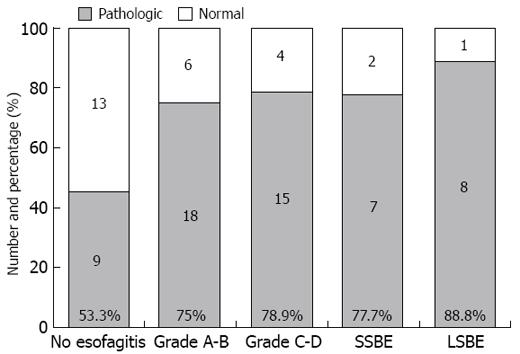
Pathologic bilirubin exposure was observed in 9 of 22 (53.3%) Group I, 18 of 24 (75%) Group II and 15 of 19 (78.9%) Group III patients, as well as in 7 of 9 (77.7%) SSBE and 8 of 9 (88.8%) LSBE patients (Figure 6). The global prevalence of patients non-responsive to PPI therapy was 68.7% (57/83).
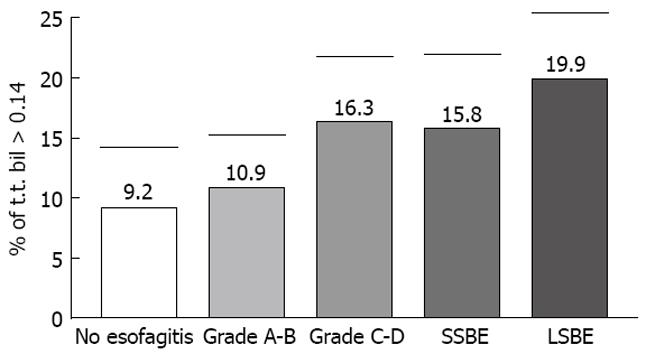
Mean time of bile absorbance > 0.14 in all patients was 16.9 ± 4.6%, 9.2 ± 5.2% in Group I, 10.9 ± 4.6% in Group II, 16.3 ± 6.3% in Group III, 15.8 ± 6.7% in SSBE and 19.9 ± 6.2% in LSBE (Figure 7).
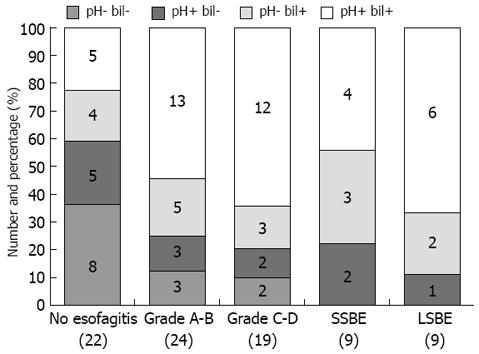
The analysis of combined pH-metry and bilimetry showed that 8 of 22 (36.4%) non-esophagitic patients and only 5 of 43 (11.6%) esophagitic patients [3 of 24 (12.5%) in Group II and 2 of 19 (10.5%) in Group III] had both values within the normal range. None of the 18 Barrett’s patients had normal esophageal exposure to acid and bile.
Pathological bilimetry associated with normal pH-metry was observed in 4 of 22 (18.2%) non-esophagitic and 8 of 43 (18.6%) esophagitic patients [5 of 24 (20.8%) in Group II and 3 of 19 (15.8%) in Group III], as well as in 3 of 9 (33.3%) SSBE and 2 of 9 (22.2%) LSBE patients.
Conversely, pathological pH-metry associated with normal bilimetry was observed in 5 of 22 (22.7%) non-esophagitic and 5 of 43 (11.6%) esophagitic patients (3 of 24 (12.5%) in Group II and 2 of 19 (10/5%) in Group III), as well as in 2 of 9 (22.2%) SSBE and 1 of 9 (11.1%) LSBE patients.
Pathologic bilimetry and pathologic pH-metry were observed in 5 of 22 (22.7%) non-esophagitic and 25 of 43 (58.1%) esophagitic patients [13 of 24 (54.2%) in Group II and 12 of 19 (66.1%) in Group III], as well as in 4 of 9 (44.4%) SSBE and 6 of 9 (66.7%) LSBE patients (Figure 8).
Although the introduction of PPIs has improved outcomes in GERD patients, a significant number of patients treated with a high dosage of PPIs (40 mg bid) show no improvements in symptoms or esophagitis. Of patients who do not respond to PPI therapy, however, only 37% show pathological pH-metry results[10]. In contrast, the combination of pH-metry and bilimetry showed pathological results in 70% of patients, thus improving the sensitivity of detection of reflux by 35%. These outcomes are important for the management of GERD patients, in that the constant presence of GERD symptoms, as documented by pH-metry, are probably caused by the incomplete acid-secretion control of the PPI drugs. The presence of biliary reflux, as documented by bilimetry, suggests that the PPIs are unable to inhibit bile secretion. Persistent symptoms in patients without any documented evidence of acid and/or bile reflux suggests that these patients may be suffering from a less common disease, such as hypersensitive esophagus, or that these symptoms arise from psychiatric causes[12].
In this study, we analyzed patients non-responsive to an 8 wk course of high-dosage PPI therapy. In addition to assessing the presence of both acid and bile reflux, we assessed the features and duration of symptoms and the esophageal motor pattern. Our overall goal was to identify characteristics that could be linked to the persistent symptoms and typical lesions of GERD. We found that a high percentage of patients (36%) were poorly responsive to PPI therapy. When associated with a long symptom history, this characteristic showed a strong correlation with the presence of esophagitis. Compared with patients without esophagitis, those with esophagitis showed a significantly longer history of symptoms, but there was no differences in severity[1314].
Functionally, manometric analysis has shown that hypotonic and short lower esophageal sphincter was correlated with esophagitis, with hypotonic and short LES having a strong influence on the natural history of GERD[1516]. We found that these manometric alterations were present in only 36% of patients without esophagitis, increasing to 60% in patients with grade A-B esophagitis and to 70% in patients with grade C-D esophagitis. Moreover, in agreement with findings showing that effective esophageal motility (non peristaltic sequences and waves with amplitude < 15 mmHg) is important[1617], we found that ineffective esophageal motility, while infrequent in non-esophagitic patients (22%), increased to 50% in patients with grade A-B esophagitis and to 73% in patients with grade C-D esophagitis.
Our findings also showed that a high percentage of GERD patients poorly responsive to PPIs have biliary reflux. We found that a high percentage (53.3%) off non-esophagitic GERD patients had pathologic bile reflux, increasing to 70% in esophagitic patients. In addition, the percentage of total time of bile absorbance > 0.14 was associated with esophagitis severity.
It is important to emphasize that patients with severe GERD (i.e. presence of esophagitis and/or Barrett’s esophagus) showed a significant increase in simultaneous bile and acid reflux relative to that in non-esophagitic GERD patients. Thus, in these patients, the esophageal mucosa is simultaneously exposed to the harmful effects of gastric and duodenal juice, with increased damage correlated with increased exposure. Similarly, animal models have shown that simultaneous exposure of the esophageal mucosa to both acid and bile reflux results in greater mucosal damage than exposure to isolated acid or bile reflux[17]. Moreover, while taurocholate does not cause mucosal damage at neutral pH, it does so at acid pH, as evidenced by ionic permeability studies[18].
On the contrary, the presence of a pathologic bile test without pathologic acid reflux, which was quite common in non-esophagitic patients and those with Grade A-B esophagitis, was observed in only 30% of patients with grade C-D esophagitis. This shows how the evolution of GERD to a more severe grade is influenced not only by acid reflux, but also by the association of acid reflux with duodenogastroesophageal reflux disease.
The available literature suggests that proton pump inhibitors (PPIs) are less efficacious in normalizing duodeno gastroesophageal reflux disease (DGERD), compared with their effect on acid reflux, in contrast to reflux surgery that has shown to adequately suppress both esophageal acid and bile exposure.
This study clearly shows that the high percentage of patients poorly responsive to PPI therapy may result from poor control of DGERD. Many patients without esophagitis have simultaneous acid and bile reflux, which increases with increasing esophagitis grade.
Laparoscopic anti-reflux surgery seems to be the treatment of choice, being effective in suppressing both acid and bilirubin exposure.
In this manuscript, the authors ascertained that many PPI-resistant GERD patients have simultaneous acid and bile reflux, which increases with increasing esophagitis grade. The study was well performed and the conclusion was clear.
| 1. | Tytgat G. Long-term GERD management: the individualized approach. Drugs Today (Barc). 2006;42 Suppl B:23-29. |
| 2. | van Pinxteren B, Numans ME, Bonis PA, Lau J. Short-term treatment with proton pump inhibitors, H2-receptor antagonists and prokinetics for gastro-oesophageal reflux disease-like symptoms and endoscopy negative reflux disease. Cochrane Database Syst Rev. 2006;3:CD002095. |
| 3. | Orel R, Brecelj J, Homan M, Heuschkel R. Treatment of oesophageal bile reflux in children: the results of a prospective study with omeprazole. J Pediatr Gastroenterol Nutr. 2006;42:376-383. |
| 4. | Sarela AI, Hick DG, Verbeke CS, Casey JF, Guillou PJ, Clark GW. Persistent acid and bile reflux in asymptomatic patients with Barrett esophagus receiving proton pump inhibitor therapy. Arch Surg. 2004;139:547-551. |
| 5. | Netzer P, Gut A, Brundler R, Gaia C, Halter F, Inauen W. Influence of pantoprazole on oesophageal motility, and bile and acid reflux in patients with oesophagitis. Aliment Pharmacol Ther. 2001;15:1375-1384. |
| 6. | Patti MG, Diener U, Tamburini A, Molena D, Way LW. Role of esophageal function tests in diagnosis of gastroesophageal reflux disease. Dig Dis Sci. 2001;46:597-602. |
| 7. | Bechi P, Pucciani F, Baldini F, Cosi F, Falciai R, Mazzanti R, Castagnoli A, Passeri A, Boscherini S. Long-term ambulatory enterogastric reflux monitoring. Validation of a new fiberoptic technique. Dig Dis Sci. 1993;38:1297-1306. |
| 8. | Felix VN, Viebig RG. Simultaneous bilimetry and pHmetry in GERD and Barrett's patients. Hepatogastroenterology. 2005;52:1452-1455. |
| 9. | Osugi H, Kaseno S, Takada N, Takemura M, Kisida S, Okuda E, Ueno M, Tanaka Y, Fukuhara K, Kinoshita H. [Clinical significance of ambulatory intraesophageal bilirubin monitoring in diagnosis of gastroesophageal reflux]. Nippon Rinsho. 2000;58:1823-1826. |
| 10. | Tack J, Koek G, Demedts I, Sifrim D, Janssens J. Gastroesophageal reflux disease poorly responsive to single-dose proton pump inhibitors in patients without Barrett's esophagus: acid reflux, bile reflux, or both? Am J Gastroenterol. 2004;99:981-988. |
| 11. | Lundell LR, Dent J, Bennett JR, Blum AL, Armstrong D, Galmiche JP, Johnson F, Hongo M, Richter JE, Spechler SJ. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999;45:172-180. |
| 12. | Lee YC, Wang HP, Chiu HM, Liao SC, Huang SP, Lai YP, Wu MS, Chen MF, Lin JT. Comparative analysis between psychological and endoscopic profiles in patients with gastroesophageal reflux disease: a prospective study based on screening endoscopy. J Gastroenterol Hepatol. 2006;21:798-804. |
| 13. | Maekawa T, Kinoshita Y, Okada A, Fukui H, Waki S, Hassan S, Matsushima Y, Kawanami C, Kishi K, Chiba T. Relationship between severity and symptoms of reflux oesophagitis in elderly patients in Japan. J Gastroenterol Hepatol. 1998;13:927-930. |
| 14. | Okamoto K, Iwakiri R, Mori M, Hara M, Oda K, Danjo A, Ootani A, Sakata H, Fujimoto K. Clinical symptoms in endoscopic reflux esophagitis: evaluation in 8031 adult subjects. Dig Dis Sci. 2003;48:2237-2241. |
| 15. | Iwakiri K, Hayashi Y, Kotoyori M, Sugiura T, Kawakami A, Sakamoto C. The minimum pressure of the lower esophageal sphincter, determined by the rapid pull-through method, is an index of severe reflux esophagitis. J Gastroenterol. 2004;39:616-620. |
| 16. | Somani SK, Ghoshal UC, Saraswat VA, Aggarwal R, Misra A, Krishnani N, Naik SR. Correlation of esophageal pH and motor abnormalities with endoscopic severity of reflux esophagitis. Dis Esophagus. 2004;17:58-62. |
| 17. | Oh DS, Hagen JA, Fein M, Bremner CG, Dunst CM, Demeester SR, Lipham J, Demeester TR. The impact of reflux composition on mucosal injury and esophageal function. J Gastrointest Surg. 2006;10:787-796; discussion 796-797. |