Published online Jul 21, 2009. doi: 10.3748/wjg.15.3417
Revised: June 2, 2009
Accepted: June 9, 2009
Published online: July 21, 2009
AIM: To study the dynamic computed tomography (CT) features of hepatic angiomyolipoma (AML) in patients with or without tuberous sclerosis complex (TSC).
METHODS: The clinical information, CT findings and histopathological results of hepatic AML were analyzed retrospectively in 10 patients.
RESULTS: Hepatic AML was prone to occur in female patients (7/10), and most of the patients (8/10) had no specific symptoms. All tumors presented as well-defined, unenveloped nodules in the liver. Six patients with sporadic hepatic AML had a solitary hepatic nodule with a definite fat component. Non-fat components of the hepatic lesions were enhanced earlier and persistently. Prominent central vessels were noted in the portal venous phase in three patients. In four patients with hepatic AML and TSC, most of the nodules were within the peripheral liver. Seven fat-deficient nodules were found with earlier contrast enhancement and rapid contrast material washout in two patients. Lymphangioleiomyomatosis was found in one patient.
CONCLUSION: Imaging features of hepatic AML are characteristic. Correct diagnosis preoperatively can be made in combination with clinical features.
- Citation: Yang B, Chen WH, Li QY, Xiang JJ, Xu RJ. Hepatic angiomyolipoma: Dynamic computed tomography features and clinical correlation. World J Gastroenterol 2009; 15(27): 3417-3420
- URL: https://www.wjgnet.com/1007-9327/full/v15/i27/3417.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.3417
Hepatic angiomyolipoma (AML) is a rare, benign mesenchymal neoplasm that consists of varying numbers of smooth muscle cells, thick-walled blood vessels, and mature adipose tissue[1]. It can occur sporadically or in conjunction with tuberous sclerosis. It is often asymptomatic and found incidentally on imaging studies for unrelated disease. The imaging features of hepatic AML are diverse as a result of the various proportions of its three components[12]. It should not be treated surgically because of its benign nature. However, at present, definitive preoperative diagnosis of hepatic AML is a challenge for radiologists and clinicians. We studied the imaging features of hepatic AML in patients with or without tuberous sclerosis complex (TSC).
We studied retrospectively the clinical, radiological and pathological records of 10 patients with hepatic AML, who were referred to our hospital between 2004 and 2007. There were three male and seven female patients with a mean age of 55.1 years (range, 19-78 years). Four were asymptomatic and were found incidentally upon general health examination. Two had complained of upper abdominal pain. Four had symptoms of unilateral flank pain. Previous medical history showed nephrectomy for renal AML in three patients, and recurrent pneumothorax and hydrothorax in one. Physical examination presented multiple miliary russet papilla on the face and several periungual fibromas in four patients. Renal ultrasound demonstrated unilateral renal AML in three patients who had a previous history of nephrectomy, bilateral multiple renal AML in one, and intratumoral hemorrhage in three. Laboratory analysis showed normal liver function and serum α-fetoprotein in all 10 patients.
MSCT was adopted for all 10 patients, using a 16-slice computed tomography (CT) scanner (Lightspeed, GE Healthcare, USA). The scan parameters included a section thickness of 5 mm, pitch of 1.375:1, reconstruction thickness of 1.25 mm, and field of view of 248 mm × 330 mm. Intravenous contrast material [60-120 mL iopamidol (Isovue 370); Bracco Diagnostics, Princeton, NJ, USA] was used in all patients. Enhanced CT was performed in the arterial phase, portal venous phase, and delayed phase with a delay time of 26, 70 and 120 s since the injection of intravenous contrast material, respectively. All imaging studies were reviewed retrospectively by the same radiologist with over 15 years experience in abdominal CT studies.
Ultrasound-guided biopsy of hepatic nodules was adopted in six patients, with an 18-gauge needle. Left hemihepatectomy was performed in two patients. Microscopical examination of the specimens was performed with routine HE staining. Immunohistochemical studies were carried out on the specimens.
There were six patients with sporadic hepatic AML. The diagnosis of hepatic AML was confirmed by liver biopsy in four patients and partial liver resection in two.
Four patients with hepatic AML had associated TSC, the diagnosis of which was based on its revised criteria[3]. The diagnosis of hepatic AML was established by biopsy of the hepatic fat-deficient nodules in two patients. In another two case, the diagnosis of hepatic AML was based on the detection of fatty density in multiple hepatic nodules[4]. In one patient with hepatic AML and TSC, the diagnosis of lymphangioleiomyomatosis (LAM) was based on the typical imaging features of multiple, thin-walled lung cysts, hydrothorax and enlarged periaortic lymph nodes.
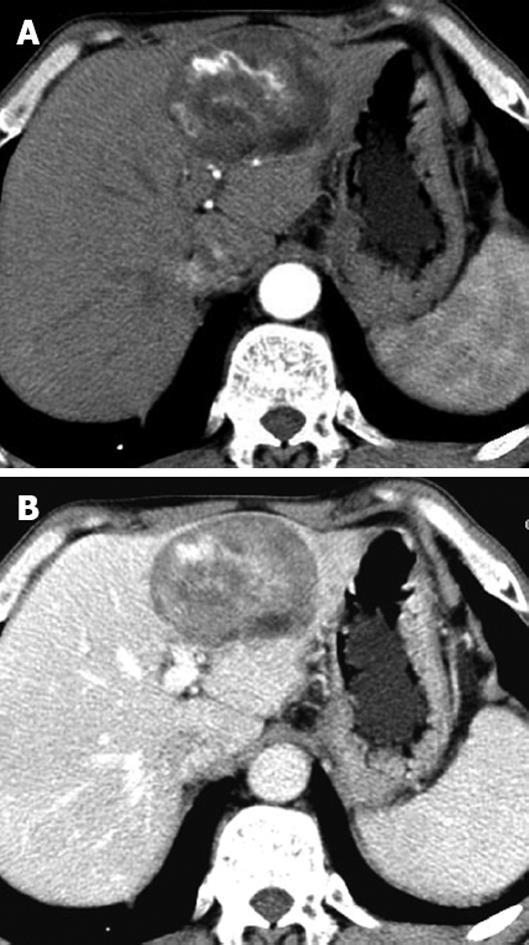
Six patients with sporadic hepatic AML had solitary fat-containing masses with a mean size of 45 mm (size range, 10-80 mm) in the liver. On dynamic contrast-enhanced CT, the non-fat component of the lesions showed heterogeneous hyperdensity in the arterial phase and portal venous phase, and heterogeneous isodensity in the delayed phase, in comparison to the surrounding liver in five patients, (Figure 1A and B). Three had marked intratumoral vessels in the arterial and portal venous phases.
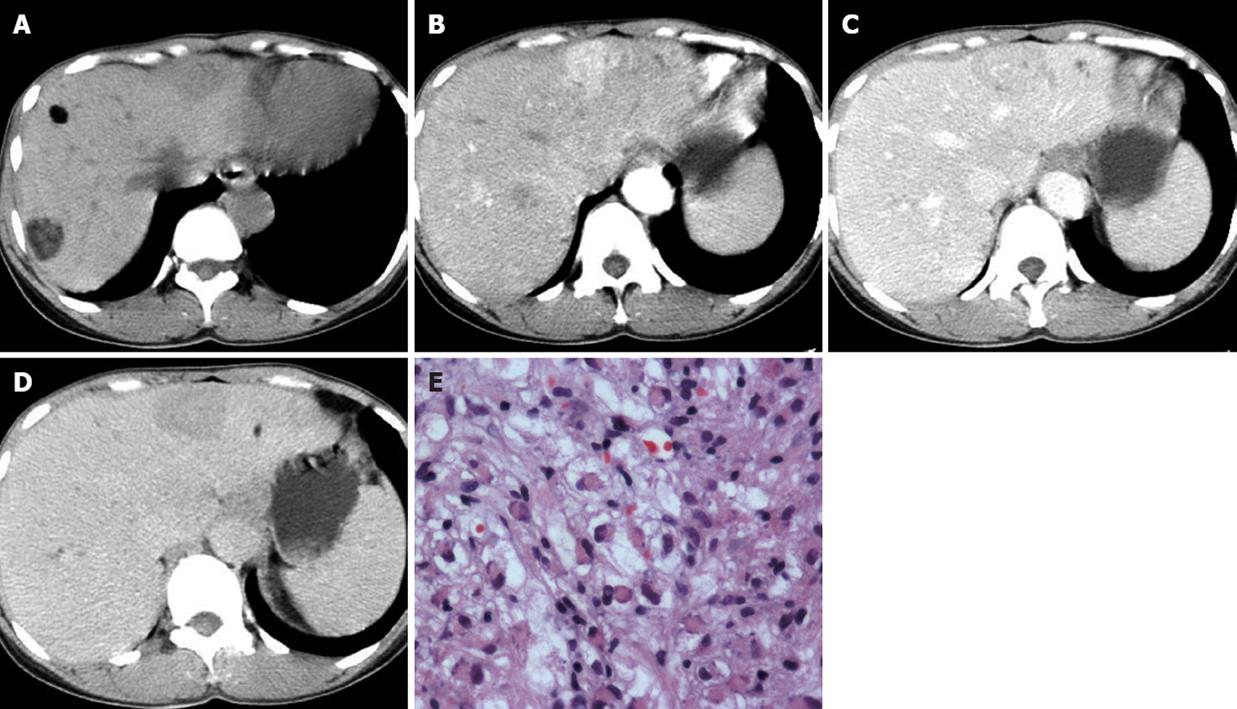
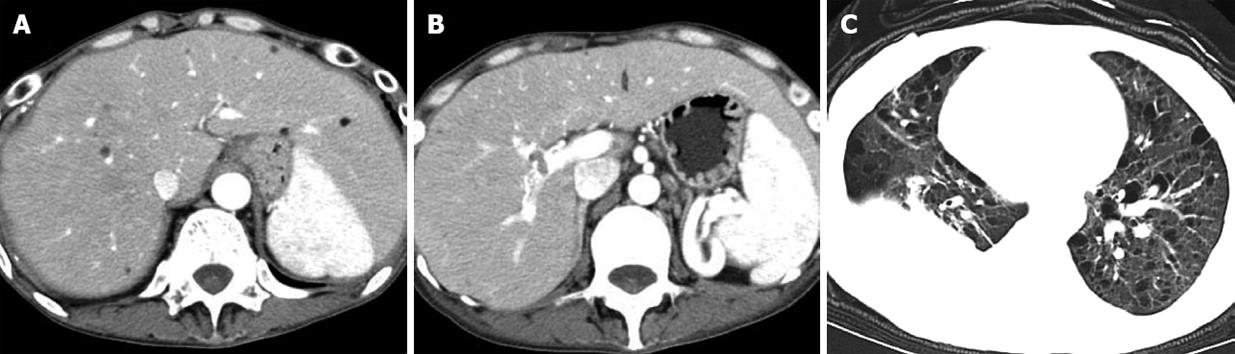
Four patients with hepatic AML and TSC had multiple, well-circumscribed hepatic nodules ranging from 2 to 40 mm (mean size, 8 mm) in the peripheral liver. Most of the nodules contained definite fatty density, and their non-fat components were enhanced mildly to markedly after administration of intravenous contrast material. Seven fat-deficient nodules were found in two patients (Figure 2A). On dynamic contrast-enhanced CT, the non-fat-containing nodules presented with heterogeneous high density in the arterial phase, mild hypodensity in the portal venous phase, and hypodensity in the delayed phase. Distorted intratumoral vessels and dilated feeding arteries were found in two of the seven nodules (Figure 2B-D). Except for multiple fat-containing nodules in the liver parenchyma, one sheet-like lesion in the right portal vein and several enlarged periaortic lymph nodes were noted in one patient (Figure 3A and B). Multiple thin-walled lung cysts and hydrothorax had been demonstrated previously by chest CT (Figure 3C).
Histopathological examination of the specimens showed various amounts of vacuolated lipocytes, blood vessels and scattered epithelioid cells with eosinophilic cytoplasm in all eight patients with liver biopsy or partial liver resection (Figure 2E). Immunohistochemistry demonstrated tumor cells with cytoplasm positive for HMB-45 in all eight patients.
Hepatic AML is an unusual mesenchymal tumor that has no specific symptoms[5]. It often presents as well-demarcated hyperechoic nodules, and can be misinterpreted easily as hepatic cavernous hemangioma upon ultrasound examination[5]. It is demonstrated typically as a well-defined, unencapsulated mass with a fat component upon CT examination. However, fat-deficient hepatic AML has been reported with higher frequency than ever, and is difficult to be differentiated from hepatocellular carcinoma[6]. It is very important to recognize hepatic AML before surgery, because its natural history is benign, and it should not be removed surgically, except for patients who suffer spontaneous rupture[47].
Hepatic AML can occur sporadically or in conjunction with TSC. It has been reported with a frequency of about 13% in patient with TSC[4]. Our study showed that hepatic AML often presents as a solitary mass, with a larger size in sporadic cases, but multiple smaller nodules in patient with TSC. This may be because it is asymptomatic, but growing with time. A long period before discovery may explain the relatively larger size of hepatic AML in patients without TSC. Dynamic contrast-enhanced CT showed sporadic hepatic AML as a hypervascular fat-containing mass with prominent central vessels in the arterial and portal venous phases. This was in accordance with previous studies[15]. In the setting of TSC, hepatic AML often presents as multiple nodules with or without a fat component in the peripheral liver. The enhancement pattern of hepatic AML in TSC varies, and dilated intratumoral vessels are found less often than in sporadic cases. This may be related to their small size and variation in number.
Generally speaking, diagnosis of hepatic AML can be made upon detection of an unenveloped nodule with intratumoral fat, prominent central vessel and no capsule[89]. However, fat-deficient hepatic AML has been reported with increasing frequency in recent years[68]. Our study showed that fat-deficient hepatic AML has the propensity to occur in patients with TSC. Dynamic contrast-enhanced CT showed fat-deficient hepatic AML with earlier contrast enhancement and rapid contrast material washout in our two patients with TSC. The enhancement pattern was similar to that of hepatocellular carcinoma. Although it has been reported that non-fatty hepatic lesions in TSC can be regarded safely as hepatic AML[8]. However, we have reported previously a case of coexisting hepatocellular carcinoma and hepatic AML in a hepatitis B carrier with TSC[10]. This suggests that hepatocellular carcinoma should be considered upon detection of hepatic fat-deficient nodules in hepatitis B carriers with TSC.
LAM has been reported with a frequency of 1% in patients with TSC[11]. It is characterized by disorderly smooth muscle proliferation throughout the bronchioles, perivascular spaces and lymphatic system. Its diagnosis can be made on the detection of multiple, thin-walled lung cysts, pneumothorax, pleural effusion, or lymphadenopathy[12]. In our one TSC patient with hepatic AML and LAM, malignant hepatic AML could not be excluded completely because of the coexistence of intravascular lesions. This patient had an uneventful 2 years follow-up. Therefore, the intravascular lesions may be explained by invasion of LAM that originated from the perivascular space.
In summary, imaging features of hepatic AML are related closely to its clinical setting. Sporadic hepatic AML usually presents as a solitary large nodule with a varying fat component. In the setting of TSC, hepatic AML often presents as multiple small nodules, with or without a fat component, in the peripheral liver. Dynamic CT features of hepatic AML are very characteristic, and correct diagnosis can be made in combination with clinical features. When coexisting intravascular lesions and hepatic AML are found in patients with TSC, LAM should be considered.
Hepatic angiomyolipoma (AML) is a rare benign mesenchymal neoplasm. It can occur sporadically or in conjunction with tuberous sclerosis complex (TSC). Definitive preoperative diagnosis of hepatic AML is important because of its benign nature and it should not be resected. It is still a difficult condition for the radiologist and clinician.
The correlation between hepatic AML and its clinical setting has not been reported often in the literature. The imaging features of hepatic AML vary. Fat-deficient hepatic AML has been reported with increasing frequency in recent times. It is difficult to differentiate from hepatocellular carcinoma.
This study emphasized that imaging features of hepatic AML are related closely to its clinical setting. Multiple hepatic AMLs, with or without a fat component, are characteristic of TSC. When coexisting intravascular lesions and hepatic AML are detected in patients with TSC, lymphangioleiomyomatosis (LAM) should be considered. Hepatocellular carcinoma must be suspected when hepatic non-fatty nodules are detected in hepatitis B carriers with TSC.
This study serves as a reminder of the imaging features of hepatic AML in patient with or without TSC. This study is limited by its retrospective nature and small number of patients, and a large sample study should be undertaken in the future.
TSC is an autosomal dominant genetic disease characterized by the growth of hematomas in multiple organs. LAM is a rare disease that is characterized by disorderly smooth muscle proliferation throughout the bronchioles, perivascular spaces and lymphatic system.
The authors studied the dynamic computed tomography features of hepatic AML in patients with or without TSC. This study was well performed and will be of interest to readers.
| 1. | Prasad SR, Wang H, Rosas H, Menias CO, Narra VR, Middleton WD, Heiken JP. Fat-containing lesions of the liver: radiologic-pathologic correlation. Radiographics. 2005;25:321-331. |
| 2. | Hooper LD, Mergo PJ, Ros PR. Multiple hepatorenal angiomyolipomas: diagnosis with fat suppression, gadolinium-enhanced MRI. Abdom Imaging. 1994;19:549-551. |
| 3. | Roach ES, Gomez MR, Northrup H. Tuberous sclerosis complex consensus conference: revised clinical diagnostic criteria. J Child Neurol. 1998;13:624-628. |
| 4. | Fricke BL, Donnelly LF, Casper KA, Bissler JJ. Frequency and imaging appearance of hepatic angiomyolipomas in pediatric and adult patients with tuberous sclerosis. AJR Am J Roentgenol. 2004;182:1027-1030. |
| 5. | Zhou YM, Li B, Xu F, Wang B, Li DQ, Zhang XF, Liu P, Yang JM. Clinical features of hepatic angiomyolipoma. Hepatobiliary Pancreat Dis Int. 2008;7:284-287. |
| 6. | Yoshimura H, Murakami T, Kim T, Nakamura H, Hirabuki N, Sakon M, Wakasa K, Inoue Y. Angiomyolipoma of the liver with least amount of fat component: imaging features of CT, MR, and angiography. Abdom Imaging. 2002;27:184-187. |
| 7. | Yamada N, Shinzawa H, Makino N, Matsuhashi T, Itasaka S, Takahashi T, Fuyama S. Small angiomyolipoma of the liver diagnosed by fine-needle aspiration biopsy under ultrasound guidance. J Gastroenterol Hepatol. 1993;8:495-498. |
| 8. | Carmody E, Yeung E, McLoughlin M. Angiomyolipomas of the liver in tuberous sclerosis. Abdom Imaging. 1994;19:537-539. |
| 9. | Ren N, Qin LX, Tang ZY, Wu ZQ, Fan J. Diagnosis and treatment of hepatic angiomyolipoma in 26 cases. World J Gastroenterol. 2003;9:1856-1858. |
| 10. | Yang B, Chen WH, Shi PZ, Xiang JJ, Xu RJ, Liu JH. Coincidence of hepatocelluar carcinoma and hepatic angiomyolipomas in tuberous sclerosis complex: a case report. World J Gastroenterol. 2008;14:812-814. |
| 11. | Bonetti F, Chiodera P. The lung in tuberous sclerosis. Pathology of lung tumors. New York: Churchill Livingstone 1997; 225-239. |