Published online Jul 14, 2009. doi: 10.3748/wjg.15.3269
Revised: May 23, 2009
Accepted: May 30, 2009
Published online: July 14, 2009
AIM: To estimate the characteristics of Color Doppler findings and the results of hepatic radionuclide angiography (HRA) in secondary Hodgkin’s hepatic lymphoma.
METHODS: The research included patients with a diagnosis of Hodgkin’s lymphoma with metastatic focal lesions in the liver and controls. Morphologic characteristics of focal liver lesions and hemodynamic parameters were examined by pulsed and Color Doppler in the portal, hepatic and splenic veins were examined. Hepatic perfusion index (HPI) estimated by HRA was calculated.
RESULTS: In the majority of patients, hepatomegaly was observed. Lesions were mostly hypoechoic and mixed, solitary or multiple. Some of the patients presented with dilated splenic veins and hepatofugal blood flow. A pulse wave was registered in the centre and at the margins of lymphoma. The average velocity of the pulse wave was higher at the margins (P > 0.05). A continuous venous wave was found only at the margins of lymphoma. There was no linear correlation between lymphoma size and velocity of pulse and continuous wave (r = 390, P < 0.01). HPI was significantly lower in patients with lymphomas than in controls (P < 0.05), pointing out increased arterial perfusion in comparison to portal perfusion.
CONCLUSION: Color Doppler ultrasonography is a sensitive method for the detection of neovascularization in Hodgkin’s hepatic lymphoma and estimation of its intensity. Hepatic radionuclide angiography can additionally help in the assesment of vascularisation of liver lesions.
- Citation: Stojković MV, Artiko VM, Radoman IB, Knežević SJ, Lukić SM, Kerkez MD, Lekić NS, Antić AA, Žuvela MM, Ranković VI, Petrović MN, Šobić DP, Obradović VB. Color Doppler sonography and angioscintigraphy in hepatic Hodgkin’s lymphoma. World J Gastroenterol 2009; 15(26): 3269-3275
- URL: https://www.wjgnet.com/1007-9327/full/v15/i26/3269.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.3269
The first choice technique for the investigation of liver lesions, including Hodgkin’s hepatic lymphoma, is ultrasound[1]. The diagnosis of this type of lymphoma can be made by non-specific sonographic signs, such as hepatomegaly, irregularity of liver margins, number and appearance of the lesions, echogenity, enlarged retroperitoneal lymph nodes[2], presence of pleural effusion or ascites, etc. Biopsy of focal lesions of the liver controlled by ultrasound in patients with malignant lymphomas is a reliable technique which can replace lymphography and other imaging procedures such as CT and nuclear magnetic resonance. Thrombosis of the portal and splenic vein has been described as a real rare complication of secondary hepatic lymphoma. The use of Color Doppler (CD) in the investigation of vascularisation of focal lesions in the liver brings about new possibilities in differential diagnostics of focal hepatic lesions[2].
In the pre-evaluation and diagnosis of patients with liver tumors, after ultrasonography and Doppler-US and contrast enhanced ultrasonography (CEUS), significant nuclear medicine methods are used: radiocolloids, blood pool, hepatobiliary scintigraphy, angioscintigraphy with radiolabeled microspheres and “first pass” radionuclide angiography[3–6]. These methods precede selective angiography and other invasive methods. CT and nuclear magnetic resonance, although used mainly for the morphological examination of the liver, can be also performed in hepatic blood flow studies[78]. Recently, positron emission tomography has also been included, in combination with other imaging modalities. Nuclear medicine methods are basically founded on the analysis of hepatic radionuclide angiography (HRA), obtained after the “first pass” of the radioactive bolus and registered activity over the liver and other abdominal organs after intravenous injection of 99mTc. As well as methods based on the assessment of total liver blood flow and assessment of shunts the technique of radionuclide angiography and determination of the hepatic perfusion index (HPI) proposed by Sarper[9] is a noninvasive method providing additional information on portal blood flow.
The aim of our examination within this study was the evaluation of characteristics of Color Doppler findings and the results of HRA in secondary Hodgkin’s hepatic lymphoma. Another aim was the estimation of reliability of CD ultrasound (US) in respect of neovascularization and level of intensity of vascularisation of the focal liver change.
The study included 25 patients with a diagnosis of Hodgkin’s lymphoma who had secondary focal lesions in the liver and 30 controls. Controls were patients with different complaints, examined in the Clinical Center of Serbia, who underwent, among other investigations, nuclear medicine examinations (radionuclide ventriculography or another study which required iv injection of 99mTc pertechnetate) and abdominal and cardiology US. All the results, including laboratory analysis were physiological, and showed that they did not have any disease or disorders. All controls were informed that their results would be included in the study.
Focal hepatic lesions had histopathological findings that correlated with our ultrasonographic studies. The ATL Ultramark9 system, and a convex probe with pulsed Doppler and CD of 2.5 MHz (WF50 Hz) were used for ultrasonographic examination. Hemodynamic parameters examined by pulsed Doppler and CD in the portal, hepatic and splenic vein were: diameter, flow, respiratory dependence of flow and collaterals. Examination of vascularisation of the focal hepatic lesion involved detection and measurement of blood flow in the centre of the focal lesion, as well as at its margin and periphery. Counting and recording for each specific focal change was performed by detection of the CD signal at a certain depth of scanning per cm2. The CD signal was created by reflection of an ultrasonic beam from the small blood vessels. Examination was repeated several times for every focal lesion, since the number of CD signals varied while breathing or changing the angle of the ultrasonic beam. An average of 5 planes of scanning were used for every hepatic change. Flow at the margins and in the center of every focal lesion was measured by combination of Color and pulsed Doppler. The results were statistically analyzed using Wilcoxon’s, Kruskal-Wallis and χ2 tests.
HRA was performed in patients with Hodgkin’s hepatic lymphoma and controls after bolus injection of 740 MBq 99mTc-pertechnetate, (1 min, 1f/s), using a ROTA scintillation camera and MicroDelta computer. The arterial-hepatic and portal-venous phase of HRA were separated at the moment when maximal activity over the left kidney region of interest (ROI) was registered. The HPI was calculated according to Sarper’s method[9]. Thus, the HPI reflects the value of the relative portal contribution to the liver blood flow. As well as the mean values (mean) and standard deviation (SD), statistical analyses included t tests, U tests, one and two way analysis of variance and a multiple range test.
The final diagnoses were based on the clinical findings, results of the functional and laboratory analysis, US and Doppler US, angiography, biopsy with histopathology and other clinical examinations. Consent was obtained from each patient, and the study protocol conformed to the established ethical guidelines. Written consent was obtained from all patients, according to the regulations of the School of Medicine Ethical Committee.
Hepatomegaly existed in 23 patients (91%). Hyperechoic appearance of node was present in 11% of cases, hypoechoic in 55% and heterogeneous mixed echoic structure in 34%. Solitary tumor nodes were found in the liver in 19 patients (48%) and multiple nodes existed in 16 patients (52%). The smallest tumor node was 1.5 cm, and the biggest was 14 cm. There was a diffuse infiltration of the liver in 2 patients. Solitary tumor knots were most frequently localized in the right lobe (36%), between right and left lobe (53%) and in the left lobe of liver (11%). The position at the right liver lobe was frequently followed by irregular margins of the liver and pleural effusion at the right side. Ascites was present in 4 patients (16%). Retroperitoneal lymph nodes were found in 16 patients (64%). Splenomegaly was detected in 16 (64%). The spleen consisted of heterogeneous echoes with bigger or smaller knots in 10 patients (40%).
Dilated splenic veins were present in 40% of cases and hepatofugal blood flow in 32%. Hepatofugal flow in the splenic vein was observed in 16% of the patients, while the others had normal hepatopetal flow. A spontaneous splenorenal shunt and collateral blood flow was detected in 12% of patients with mass splenomegaly. A hepatofugal direction of flow existed in the portal vein in 50% of patients. Hemodynamic parameters in patients with lymphomas and controls are presented in Table 1. Thus, the diameter of the portal vein was significantly (P < 0.05) bigger than standard values and ranged from 1.1-2.4 cm. However, the average flow velocity in the portal vein was not significantly different (P > 0.05) from that in the controls. The volume of blood flow through the portal vein was 600-1200 mL/min which was not significantly different from normal values (P > 0.05). Flow velocity in the hepatic artery was 15-55 cm/s, which was significantly different from the values in the control group (P < 0.05).
| Hemodynamic parameters | Statistical parameters | |||||
| Unit | n | Mean | SD | CV | Med | |
| Patients with secondary Hodgkin’s hepatic lymphoma | ||||||
| Diameter portal vein | cm | 25 | 1.7 | 0.4 | 26 | 1.7 |
| Velocity portal vein | cm/s | 25 | 18.0 | 22.0 | 18 | 13.0 |
| Volume of blood flow portal vein | mL/min | 25 | 859.0 | 574.0 | 69 | 643.0 |
| Velocity hepatic artery | cm/s | 25 | 40.0 | 16.0 | 38 | 40.0 |
| Control group | ||||||
| Diameter portal vein | cm | 30 | 1.0 | 0.1 | 10 | 1.0 |
| Velocity portal vein | cm/s | 30 | 17.0 | 2.0 | 13 | 17.0 |
| Volume of blood flow portal vein | mL/min | 30 | 781.0 | 164.0 | 23 | 763.0 |
| Velocity hepatic artery | cm/s | 30 | 38.0 | 5.0 | 14 | 36.0 |
In 3 patients without nodules in the liver visible on US examination, slight perfusion disorders were detected by Doppler-US. The volume of portal blood flow was slightly increased (1000-1200 mL/min), without any impact on the statistical calculation.
Two types of spectral curved lines were found: pulse-arterial wave and continuous venous wave, and velocities are presented in Table 2. A pulse wave was registered in the centre and at the margins of lymphoma and its velocity ranged from 80-140 cm/s. The average velocity of the pulse wave at the margins was a little higher (P > 0.05) than in the center of the lesion. A continuous venous wave was found only at the margins of the lymphoma and its maximum velocity was 35 cm/s. There was no linear correlation between lymphoma size and velocity of pulse and continuous waves (r = 390, P < 0.01).
| Wave | Tumor center | Tumor margin | ||||
| Mean | SD | Med | Mean | SD | Med | |
| Pulse (cm/s) | 80 | 23 | 84 | 84 | 25 | 88 |
| Continuous wave (cm/s) | 25 | 9 | 24 | |||
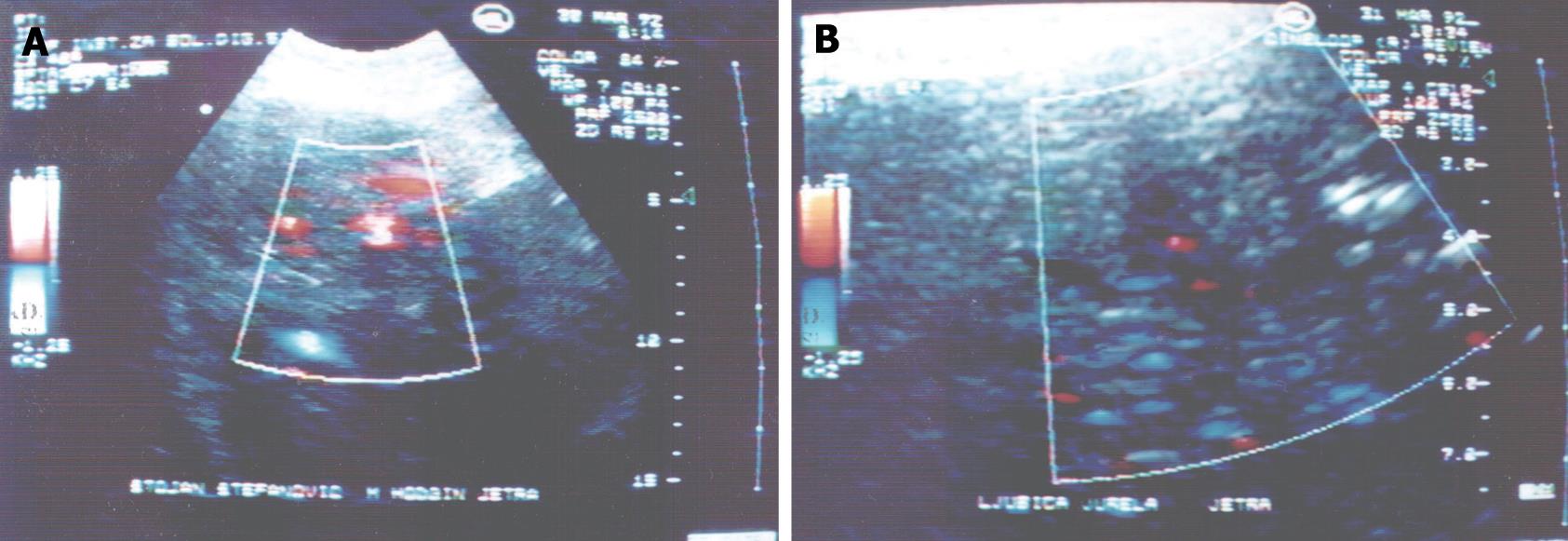
The CD signal could not be found within the parts of the focal lesion where there was no flow in small blood vessels or where the flow could not be detected by the CD system. The CD signal in hypoechoic lesions varied from 0.5-0.9 of blood vessels/cm2. Maximum values of the number of CD signals were noticed in lesions 3-5 cm in size and they ranged from 1-3 blood vessels/cm2 of area in hypoechoic zones. In the case of hyperechoic lesions the result was 0.5-0.6 vessels/cm2. Comparing number of CD signals in vivo to number of blood vessels in histological tissue, we could not find any relevant differences between these two methods (P > 0.05) (Figure 1).
Results of HRA in patients with secondary Hodgkin’s hepatic lymphoma (Table 3), showed that HPI was significantly lower in patients with lymphomas than in controls (P < 0.05). Thus, arterial hepatic perfusion was increased in patients with secondary hepatic lymphomas, while the venous component of hepatic perfusion was decreased.
| Hemodynamic parameter | Statistical parameters | ||||
| n | Mean | SD | CV | Med | |
| Controls | 30 | 0.66 | 0.07 | 8 | 0.66 |
| Lymphoma patients | 25 | 0.27 | 0.22 | 73 | 0.35 |
Most of the patients with Hodgkin’s hepatic lymphoma had hepatomegaly, mainly with hypoechoic or heterogenous mixed echoic structures, while in a small number of patients it was hyperechoic. The lesions were solitary or multiple, of various sizes. Solitary tumor knots were most frequently localized between the lobes or in the right lobe and the rarest lesions were in the left lobe. Some patients had irregular liver margins with pleural effusion at the right side, while some had ascites. Most of them had enlarged retroperitoneal lymph nodes.
Our results regarding the morphology of lesions, are in accordance with the literature data[1,2], where the non-specific sonographic sign in the majority of secondary hepatic lymphomas is hepatomegaly with irregular, cogged like liver margins. According to our results, secondary hepatic lymphomas appear in solitary or multinodular shapes or as diffuse hepatic infiltration. A rarer echoic structure is the shape of the target lesion, or mixed echoic structure[2]. The appearance of secondary hepatic lymphoma is associated with enlarged spleen, or with retroperitoneal lymphadenopathy or pleural effusion in a great number of cases[1]. Ascites is also very often a finding in patients with secondary hepatic lymphoma[1].
Splenomegaly was detected in half of the patients. Splenic lesions were heterogeneous with bigger or smaller knots in nearly half of the patients. Because of the higher vascular pressure, dilated splenic veins were often present with hepatofugal blood flow in a third of the patients. In some patients, blood flow was in 2 directions. A spontaneous splenorenal shunt and collateral blood flow could be detected in patients with mass splenomegaly. In patients with liver Hodgkin’s lymphomas, changes in the diameter of the splenic vein were due to changes in resistance in the portal vascular system, hilar adenopathy and splenomegaly.
Changes in venous vessels of the portal system in patients with liver Hodgkin’s lymphomas included dilated portal veins (P < 0.05) and changes in flow direction (hepatofugal in 50%) (P < 0.05). However, no significant changes in volume of blood flow or blood flow velocity through the portal vein (P > 0.05) were observed. However, significant differences in blood flow velocity through the hepatic artery were seen (P < 0.05). In 3 patients without nodules in the liver visible on US examination, slight perfusion disorders were detected by Doppler-US. The volume of portal blood flow was slightly increased because of the increased inflow through the portal vein due to splenomegaly. This fact could enable early further investigation and detection of lymphomas in the liver, before the obvious changes in liver tissue occur. These values were not statistically significant.
A pulse wave was registered in the center and a little higher (P > 0.05) at the margins of lymphoma. A continuous venous wave was found at the margins of lymphoma. There was no linear correlation between lymphoma size and velocity of pulse and continuous waves (r = 390, P < 0.01). Our results show intense neovascularization of the focal lesions in the center, as well as on the periphery, which differentiate those tumors from other secondary deposits. The highest pulse wave velocities correspond to arteriovenous shunts.
The CD signal could not be found within the parts of focal lesions with no flow in small blood vessels or where flow could not be detected by the current CD system. The CD signal in hypoechoic lesions varied from 0.5-0.9 of blood vessels/cm2. Estimation of CD signals in vivo in these tumors corresponds to the number of blood vessels in histological tissue. HPI values were significantly lower in patients with lymphomas than in controls (P < 0.05). Thus, arterial hepatic perfusion was increased in patients with secondary hepatic lymphomas, while the venous component of hepatic perfusion was decreased which is usual for malignant lesions.
Various literature results prove the value in Doppler-US in the estimation of characteristics of liver tumors that could help in the early diagnosis, differential diagnosis and appropriate choice of therapy. Although there is much literature data about Doppler-US characteristics and possibilities of differential diagnosis of malignant and benign liver tumors, not many of them deal with hepatic metastases of Hodgkin’s lymphoma. According to Tchelepi et al[10], detecting and characterizing focal liver lesions is one of the most difficult challenges in imaging emphasizing the main strengths being in its ability to definitively characterize common benign lesions (cysts and hemangiomas), safety, low cost, and its ability to guide biopsy. Disadvantages include its inability to image the entire liver in many patients and its inferiority to CT as a means of detecting extrahepatic malignant disease. Sonography is less sensitive than CT or MRI in detecting focal lesions. However, US contrast agents can improve liver lesion detection and characterization. Tscelepi indicated that intraoperative US is the most sensitive imaging modality in detecting focal liver lesions. However, some authors obtained better results with CEUS. Thus, Ernst et al[11] with a new US contrast agent showed that metastatic liver lesions had previously undetected blood flow in the rim of the tumor; hepatocellular carcinoma displayed enhanced signal intensity in the vessels of the rim and in the center of the tumor, while adenoma and focal nodular hyperplasia showed signal enhancement in the central area of the tumor. No signal enhancement was observed in hemangiomas, a focal fatty lesion, or in a carcinoid metastatic lesion. Thus, enhanced CD flow study may aid in the detection of flow signals or sonographic differentiation of hepatic tumors. Also, some authors, after the study of patients with malignant diseases (hepatocellular carcinoma, cholangiocellular carcinoma, metastasis and lymphoma) and those with benign lesions, with color stimulated acoustic emission in the late phase of Levovist enhancement showed a high specificity and sensitivity for differentiation between benign and malignant focal liver lesions[12].
Results of HPI show that arterial hepatic perfusion in Hodgkin’s lymphoma is dominant and that venous-portal inflow is reduced in comparison with standard values. Although there are no precise data from the literature concerning metastatic lymphoma in the liver, the results of other authors regarding the flow in malignant lesions in general are in accordance with our findings. Thus, according to Petrović et al[13] HPI is a sensitive indicator of the presence of malignant liver tumors, but is within normal range in patients with hepatic hemangioma. The results of Dragoteanu et al[14] showed that malignant tumors (primitive or metastases) increase the arterial supply of the liver and decrease the portal flow. However, benign tumors do not change the portal/arterial liver blood flow ratio. However, recently, according to Robinson[15] the sensitivity of US, CT and magnetic resonance techniques for detecting liver metastases assessed in comparison with surgical inspection, intraoperative US and pathological examination, are of uncertain accuracy in detecting very small lesions. With current imaging technology, even with optimum imaging, it is possible to detect only about one-half of metastatic nodules < 1 cm in patients undergoing liver resection and pathological correlation. Robinson emphasized that micrometastases produce alterations in blood flow that may be recognized by radionuclide or Doppler perfusion methods in the very early phase.
Currently, use of CT and MRI to detect hepatic lymphoma as well as its perfusion has been employed. Generally speaking, CT reveals Hodgkin’s lymphoma as homogeneously hypodense clearly delineated nodules. After contrast application the lesions appear hypodense, although a weak enhancement may be detected. However, in the case of the infiltrative form, a diffusely decreased attenuation may be seen, without the possibility of distinguishing it from fatty infiltration. MRI reveals focal hepatic lymphomas are homogeneously hypointense in comparison to the normal liver parenchyma on unenhanced T1-weighted images. However, dynamic imaging after gadolinium contrast application reveals hypointensity on arterial phase images followed by homogeneous delayed enhancement on portal venous phase imaging. However, it is still difficult to differentiate lymphoma, metastases and hepatocellular carcinoma.
Thus, according to Liu et al[16], a plain CT scan identified hypodense lesions which did not display marked enhancement on arterial phase and portal venous phase scans. On delayed phase scan, the border of the lesions became clear, and slight enhancement was observed in the periphery and some partitions of the lesions. According to Vinnicombe et al[17], CT accurately depicts nodal enlargement above and below the diaphragm, has variable sensitivity for intra-abdominal visceral involvement and is generally outstanding in depicting the extent of disease, especially extranodal extension. Despite the advances in CT technology, there are still areas where CT performs less well, even when using an intravenous contrast medium. The results of Earl et al[18] show that although CT scan could occasionally demonstrate disease in nodes, its value was limited by its inability to detect involvement of nodes which were not significantly enlarged. However, CT scan would appear to be the investigation of choice in patients with suspected abdominal relapse because of the more frequent presence of disease in sites not seen on lymphography. Gossmann et al[19] emphasized that fast MRI has considerably reduced imaging time, and it is now considered to be as diagnostic as CT for staging Hodgkin’s disease. The excellent soft-tissue contrast and the lack of exposure to ionizing radiation are the main advantages of MRI imaging, especially with the application of newly developed lymphotropic contrast agents. In addition, Hoane et al[20] concluded that MRI and CT may be equivalent imaging modalities in the detection of nodal involvement, and that MRI has an advantage in its ability to diagnose marrow involvement. According to MRI findings in primary lymphoma of the liver, on T1-weighted imaging the tumor was isointense in one and homogeneously hypointense to the liver parenchyma in the other. On T2-weighted imaging both tumors were homogeneously hyperintense. In one case the margin of the tumor was poorly defined, and portal branches were identified within the tumor, an unusual finding in other liver neoplasms. Additionally, according to Hori et al[21] it is possible to investigate the morphological, hemodynamical and functional nature of focal hepatic lesions and correctly establish a diagnosis of liver tumors based on those findings using dynamic CT and MRI with extracellular contrast material or MRI with liver-specific contrast material.
Some recent papers employ positron emission tomography for the assessment of tumor perfusion. Growth of malignant tumors is dependent on sufficient blood supply, an enhanced microvessel density is seen as part of these reactions and this is associated with increased perfusion as measured by PET[22]. Some authors used PET and CT in the investigation of liver tumor blood flow and metabolism[23].
In comparison to other metastatic liver tumors, Hodgkin’s lymphoma has increased vascularization which we have seen only in primary hepatocellular carcinoma, which can help in differentiating it from other metastatic tumors. The significant impact on assessment of microvascularization can be obtained using contrast enhanced US. The absence of splenomegaly and retroperitoneal lymph nodes can further help in differentiation of hepatocellular carcinoma from lymphoma. In that case, further investigation is needed. Firstly, laboratory analysis can be employed (tumor marker levels, the absence of hepatotropic viruses B and C), as well as estimation of the age group, and anamnestic data (alcohol abuse and former cirrhosis). For an exact diagnosis, radionuclide methods, as well as MDCT and MRI, can be also used.
Liver metastases of different tumors have different flow patterns. According to our results, a special characteristic of Hodgkin’s disease is the demonstration of continuous venous flow at the periphery and pulsed arterial flow both at the margins and at the center of the lesion. Increased vascularization has also been obtained in metastases of carcinomas of the kidneys and suprarenal gland. In the center of the lesion, pulse waves did not register at all, while those registered at the good vascularized tumor margin had slightly lower velocities (68-78 cm/s) in comparison to Hodgkin’s lymphoma. In metastases of colorectal adenocarcinomas, there was no pulse wave registered in the center, while it was registered at the margin in only 50% of patients, with obviously decreased velocities in comparison to Hodgkin’s lymphoma (27 cm/s). A continuous wave registered on the edges in all the patients, which showed decreased velocities (19 cm/s). Metastases of pancreatic and gallbladder adenocarcinomas showed pulse wave velocity on the tumor margin was slightly decreased in comparison to Hodgkin’s lymphoma (65-75 cm/s), while continuous wave velocity in the tumor margins registered in all patients had a similar velocity (28-40 cm/s) to that in Hodgkin’s lymphoma[24]. Another type of lymphoma (secondary non-Hodgkin’s) shows a similar echo type, but different vascular characteristics. Arteriovenous shunts in this type of lymphoma are localized on the edges of the tumor, and thus, the pulse waves registered in this area with CD showed lower values (50-60 cm/s). No continuous wave on the periphery was registered in any patient with non-Hodgkin’s lymphoma, and when registered, velocities were also lower (10-26 cm/s)[24].
However, in order to perform prompt and adequate therapy, diagnosis and monitoring of malignant liver disease should be improved. According to our results, as well as the above mentioned data of other authors, contemporary diagnostic imaging methods mainly provide morphological data about the tissue while perfusion imaging of the liver can improve this limitation. According to some authors[7] liver flow scintigraphy and flow quantification at Doppler US have focused on characterization of global abnormalities. CT and MRI imaging can provide regional and global parameters. However, some shortcomings of these methods need to be overcome (by reduction of radiation doses associated with CT perfusion imaging, improvement of spatial and temporal resolution at MR imaging, accurate quantification of tissue contrast material at MR imaging, and validation of parameters obtained from fitting enhancement curves to biokinetic models, applicable to all perfusion methods).
In conclusion, CD US is a useful method for detection of vascularization in Hodkin’s hepatic lymphoma and estimation of its intensity. Thus, it could help in the early diagnosis and differential diagnosis of malignant liver lesions, including hepatic Hodgkin’s lymphoma. In comparison to other metastatic liver tumors, Hodgkin’s lymphoma has increased vascularization which we have seen only in primary hepatocellular carcinoma, which can help in differentiating it from other metastatic tumors. A significant impact on assessment of microvascularization can be obtained using contrast enhanced US. The presence of splenomegaly and retroperitoneal lymph nodes can further help in differential diagnosis. However, in most cases, further investigation (such as laboratory analysis, estimation of the age group and anamnestic data) is needed. For a more reliable diagnosis, radionuclide methods, MDCT and MRI can be also used.
The aim of the paper was to estimate the characteristics of Color Doppler findings and the results of hepatic radionuclide angiography (HRA) in secondary Hodgkin’s hepatic lymphoma, in order to try to distinguish these entities from other hepatic tumors without using more advanced diagnostic methods.
Possibility of distinguishing liver involvement by Hodgkin’s hepatic lymphoma by estimation of the morphology of the liver by US, as well as estimation of typical flow patterns (pulse and continuous flow) at the center and margins of the lymphoma.
“It seems that a peculiar characteristic of Hodgkin’s disease is the demonstration of continuous venous flow at the periphery and pulsed arterial flow both at the margins and at the center of the lesions. This is interesting and merits further investigation (reviewer’s opinion)”.
By carefully examining the liver, one can, using easily available methods, differentiate benign from malignant lesions, as well as suspect Hodgkin’s hepatic lymphoma.
This paper evaluated of characteristics of Color Doppler finding and the results of radionuclide angiography in secondary Hodgkin’s hepatic lymphoma. This is interesting and merits further investigation.
| 1. | Bhargava SK. Ultrasound differential diagnosis. New Delhi: Jaypee Brothers Medical Publishers (P) Ltd 2007; 235-238. |
| 2. | Brkljačić B. Dopler kvrnih žila. Zagreb: Medicinska naklada 2000; 256-302. |
| 3. | Dragoteanu M, Cotul SO, Tamas S, Piglesan C. Nuclear medicine dynamic investigations of diffuse chronic liver diseases and portal hypertension. Rom J Gastroenterol. 2004;13:351-357. |
| 4. | Dragoteanu M, Balea IA, Dina LA, Piglesan CD, Grigorescu I, Tamas S, Cotul SO. Staging of portal hypertension and portosystemic shunts using dynamic nuclear medicine investigations. World J Gastroenterol. 2008;14:3841-3848. |
| 5. | Artiko VM, Sobić-Saranović DP, Pavlović SV, Perisić-Savić MS, Stojković MV, Radoman IB, Knezević SJ, Vlajković MZ, Obradović VB. Estimation of the relative liver perfusion using two methods of radionuclide angiography in the patients with hemodynamic disorders in the portal system. Acta Chir Iugosl. 2008;55:11-16. |
| 6. | Artiko V, Obradović V, Petrović M, Perisić M, Stojković M, Sobić-Saranović D, Mikić A, Vlajković M, Milovanović J, Vuksanović L. Hepatic radionuclide angiography and Doppler ultrasonography in the detection and assessment of vascular disturbances in the portal system. Hepatogastroenterology. 2007;54:892-897. |
| 7. | Pandharipande PV, Krinsky GA, Rusinek H, Lee VS. Perfusion imaging of the liver: current challenges and future goals. Radiology. 2005;234:661-673. |
| 8. | Annet L, Materne R, Danse E, Jamart J, Horsmans Y, Van Beers BE. Hepatic flow parameters measured with MR imaging and Doppler US: correlations with degree of cirrhosis and portal hypertension. Radiology. 2003;229:409-414. |
| 9. | Sarper R, Fajman WA, Rypins EB, Henderson JM, Tarcan YA, Galambos JT, Warren WD. A noninvasive method for measuring portal venous/total hepatic blood flow by hepatosplenic radionuclide angiography. Radiology. 1981;141:179-184. |
| 10. | Tchelepi H, Ralls PW. Ultrasound of focal liver masses. Ultrasound Q. 2004;20:155-169. |
| 11. | Ernst H, Hahn EG, Balzer T, Schlief R, Heyder N. Color doppler ultrasound of liver lesions: signal enhancement after intravenous injection of the ultrasound contrast agent Levovist. J Clin Ultrasound. 1996;24:31-35. |
| 12. | von Herbay A, Vogt C, Häussinger D. Differentiation between benign and malignant hepatic lesions: utility of color stimulated acoustic emission with the microbubble contrast agent Levovist. J Ultrasound Med. 2004;23:207-215. |
| 13. | Petrović N, Artiko V, Obradović V, Kostić K. [Study of blood flow in liver hemangiomas using radionuclide angiography]. Acta Chir Iugosl. 2001;48:25-29. |
| 14. | Dragoteanu M, Cotul SO, Pîgleşan C, Tamaş S. Liver angioscintigraphy: clinical applications. Rom J Gastroenterol. 2004;13:55-63. |
| 15. | Robinson PJ. Imaging liver metastases: current limitations and future prospects. Br J Radiol. 2000;73:234-241. |
| 16. | Liu FY, Chen D, Shang JB, Wu XM, Zhang XL. [Clinical and imaging diagnosis of primary hepatic lymphoma]. Di Yi Jun Yi Da Xue Xue Bao. 2005;25:1290-1292. |
| 17. | Vinnicombe SJ, Reznek RH. Computerised tomography in the staging of Hodgkin’s disease and non-Hodgkin’s lymphoma. Eur J Nucl Med Mol Imaging. 2003;30 Suppl 1:S42-S55. |
| 18. | Earl HM, Sutcliffe SB, Fry IK, Tucker AK, Young J, Husband J, Wrigley PF, Malpas JS. Computerised tomographic (CT) abdominal scanning in Hodgkin’s disease. Clin Radiol. 1980;31:149-153. |
| 19. | Gossmann A, Eich HT, Engert A, Josting A, Müller RP, Diehl V, Lackner KJ. CT and MR imaging in Hodgkin’s disease--present and future. Eur J Haematol Suppl. 2005;31:83-89. |
| 20. | Hoane BR, Shields AF, Porter BA, Borrow JW. Comparison of initial lymphoma staging using computed tomography (CT) and magnetic resonance (MR) imaging. Am J Hematol. 1994;47:100-105. |
| 21. | Hori M, Murakami T, Kim T, Tomoda K, Nakamura H. CT Scan and MRI in the Differentiation of Liver Tumors. Dig Dis. 2004;22:39-55. |
| 22. | Schmidt K, Hoffend J, Altmann A, Strauss LG, Dimitrakopoulou-Strauss A, Engelhardt B, Koczan D, Peter J, Dengler TJ, Mier W. Angiostatin overexpression in Morris hepatoma results in decreased tumor growth but increased perfusion and vascularization. J Nucl Med. 2006;47:543-551. |
| 23. | Ganeshan B, Miles KA, Young RC, Chatwin CR. In search of biologic correlates for liver texture on portal-phase CT. Acad Radiol. 2007;14:1058-1068. |
| 24. | Monas L. 1996;173-179. |