Published online Jul 14, 2009. doi: 10.3748/wjg.15.3240
Revised: May 25, 2009
Accepted: June 1, 2009
Published online: July 14, 2009
AIM: To investigate the possible protective effects of carnosol on liver injury induced by intestinal ischemia reperfusion (I/R).
METHODS: Rats were divided randomly into three experimental groups: sham, intestinal I/R and carnosol treatment (n = 18 each). The intestinal I/R model was established by clamping the superior mesenteric artery for 1 h. In the carnosol treatment group, surgery was performed as in the intestinal I/R group, with intraperitoneal administration of 3 mg/kg carnosol 1 h before the operation. At 2, 4 and 6 h after reperfusion, rats were killed and blood, intestine and liver tissue samples were obtained. Intestine and liver histology was investigated. Serum levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT) and interleukin (IL)-6 were measured. Liver tissue superoxide dismutase (SOD) and myeloperoxidase (MPO) activity were assayed. The liver intercellular adhesion molecule-1 (ICAM-1) and nuclear factor κB (NF-κB) were determined by immunohistochemical analysis and western blot analysis.
RESULTS: Intestinal I/R induced intestine and liver injury, characterized by histological changes, as well as a significant increase in serum AST and ALT levels. The activity of SOD in the liver tissue decreased after I/R, which was enhanced by carnosol pretreatment. In addition, compared with the control group, carnosol markedly reduced liver tissue MPO activity and serum IL-6 level, which was in parallel with the decreased level of liver ICAM-1 and NF-κB expression.
CONCLUSION: Our results indicate that carnosol pretreatment attenuates liver injury induced by intestinal I/R, attributable to the antioxidant effect and inhibition of the NF-κB pathway.
- Citation: Yao JH, Zhang XS, Zheng SS, Li YH, Wang LM, Wang ZZ, Chu L, Hu XW, Liu KX, Tian XF. Prophylaxis with carnosol attenuates liver injury induced by intestinal ischemia/reperfusion. World J Gastroenterol 2009; 15(26): 3240-3245
- URL: https://www.wjgnet.com/1007-9327/full/v15/i26/3240.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.3240
Intestinal ischemia reperfusion (I/R) is one of the most common types of cell injury, which is caused by many factors, such as acute blood loss, shock, ileus and multiple trauma. Intestinal I/R not only leads to intestinal damage itself, but also causes severe destruction of remote organs[1–3]. The liver is the first distant organ involved after intestinal I/R[45]. Although the detailed mechanism of liver injury induced by intestinal I/R remains to be elucidated, a variety of inflammatory mediators including intracellular adhesion molecule-1 (ICAM-1) and cytokines, as well as infiltration of neutrophils have been implicated in this process[6–8].
Nuclear factor κB (NF-κB) is one of the most important transcriptional factors, which consists of p50 and p65 subunits. In quiescent cells, it exists as a latent cytoplasmic complex bound to an inhibitor protein, I-κB. In response to an activation signal, I-κB is phosphorylated and degraded through the proteosomal pathway. The free NF-κB complex is then able to translocate into the nucleus and induce expression of various target genes that are critical for cell survival, inflammation and immunity[910]. NF-κB is also activated and plays a major role during the local and systemic inflammatory response following intestinal I/R[11]. Thus, modulating the NF-κB pathway is a new concept and therapeutic option to attenuate intestinal I/R-induced local and remote organ injury[11–13].
Carnosol, a major component of rosemary, is a phenolic diterpene that has potent antioxidant and anti-inflammatory activities[14–16]. Carnosol has been found to suppress NO production and inducible nitric oxide synthase gene expression by inhibiting NF-κB activation[17].
In this study, we investigated the effects of carnosol on liver injury following intestinal I/R and explored the mechanism of its protective action.
Male Wistar rats (Animal Center of Dalian Medical University, Dalian, China) weighing 190-220 g were used in this study, which was approved by the Institutional Ethics Committee. All rats were fed with standard laboratory chow and water, and housed in accordance with institutional animal care guidelines.
Rats were assigned randomly into three experimental groups: control, intestinal I/R and carnosol pretreatment groups (n = 18 in each group). The rats in the control group underwent surgical preparation including isolation of the superior mesenteric artery (SMA) without occlusion; the intestinal I/R group was subjected to 1 h intestinal ischemia and various times of reperfusion after the SMA was isolated and occluded[18]. In the carnosol pretreatment group, surgery was performed as in the intestinal I/R group with intraperitoneal administration of 3 mg/kg carnosol (Cayman Chemical Company, Ann Arbor, MI, USA) 1 h before the operation. Carnosol was dissolved in 3% DMSO before administration. The dose of carnosol administered was determined according to a previous study[19], with modification from our preliminary experiments. The rats in the control and intestinal I/R group were treated with an equal volume of 3% DMSO when needed. At each of the indicated time points (2, 4 and 6 h after reperfusion), six rats were killed randomly from each group, and blood, intestine and liver tissue samples were obtained for further analysis.
The isolated intestine and liver tissues were harvested and fixed in 10% formalin. After being embedded in paraffin, the tissues were stained with hematoxylin and eosin for light microscopy.
The serum levels of AST and ALT were measured with an OLYMPUS AU1000 automatic analyzer (AusBio Laboratories Co., Ltd. Beijing, China). The serum levels of IL-6 were determined using a radioimmunoassay kit (Radioimmunity Institute of PLA General Hospital, Beijing, China) following the manufacturer’s instructions.
The liver tissues were harvested and homogenized immediately on ice in five volumes of normal saline. The homogenates were centrifuged at 1200 r/min for 10 min. The SOD and MPO activity in the supernatant was determined using an assay kit (Nanjing Jincheng Corp., China), according to the manufacturer’s recommendations.
Formalin-fixed, paraffin-embedded liver tissue sections of 4-&mgr;m thickness were stained with SP immunohistochemistry technique for NF-κB. After being dewaxed or washed in PBS, tissue sections were cultured in 3% hydrogen peroxide to eliminate intrinsic peroxidase, and quenched in normal goat serum for 30 min. The sections were then incubated overnight at 4°C with polyclonal rabbit anti-rat NF-κB antibody (NeoMarkers Corp, Jingmei Biotech, Shenzhen, China), followed by addition of anti-rabbit immunoglobulin and streptavidin conjugated to horseradish peroxidase (HRP). Finally, slides were stained with 3,3’-diaminobenzidine (DAB), and hematoxylin was used for counter staining. Brown staining in cytoplasm and nucleus were considered as positive.
Cellular plasma and nuclear protein were extracted from frozen live tissue with a protein extraction kit (Pierce, Rockford, IL, USA). The protein was separated by 10% SDS-PAGE and then electroblotted onto nitrocellulose membranes (Millipore, Bedford, MA, USA) for 30 min. The membranes were then incubated overnight at 4°C with rabbit polyclonal antibody ICAM-1 (Boster Corp., Ltd. Wuhan, China) and NF-κB against rat. The membranes were incubated for 1 h at 37°C with an anti-rabbit IgG conjugated with HRP. The signals were visualized by a DAB assay kit (Fuzhou Maixin Biological Technology Co., Ltd, Fuzhou, China) and analyzed with a gel imaging system (Kodak System EDAS120, Japan).
All data were expressed as mean ± SD. The F test was used to evaluate statistical significance using SPSS 10.0 statistical software (Chicago, IL, USA). P < 0.05 was considered statistically significant.
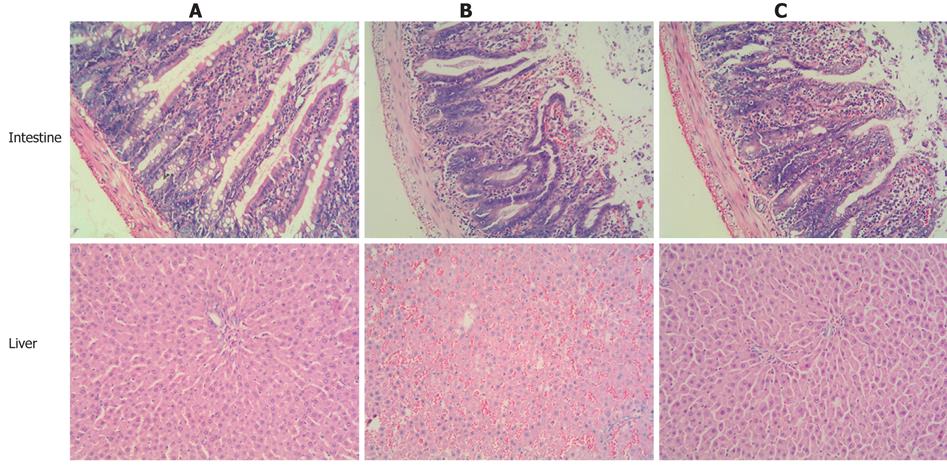
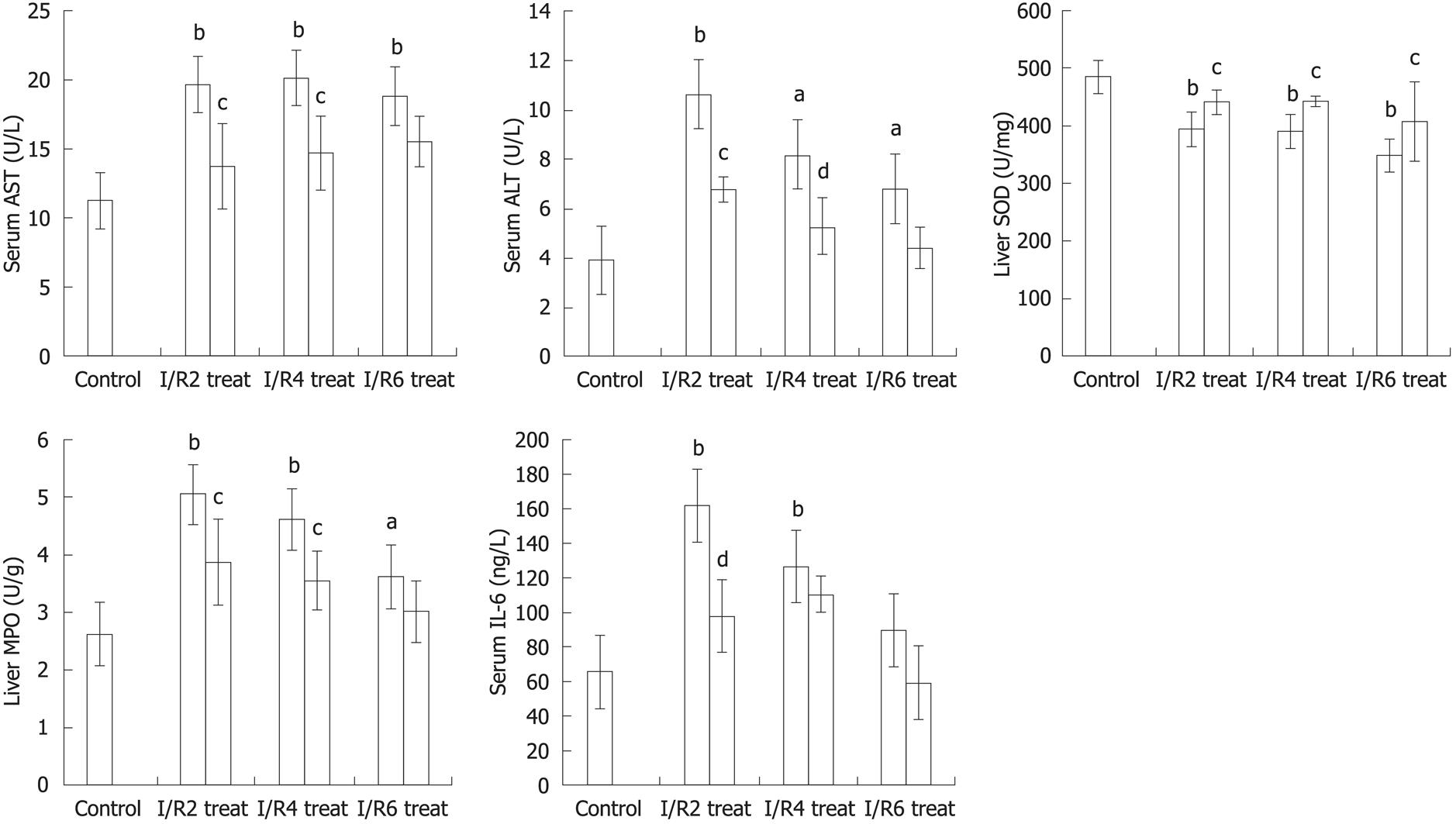
Intestinal I/R induced apparent intestine and liver injury at all the time points (2, 4 and 6 h) after reperfusion, manifested as histological changes in the intestine and liver with edema, hemorrhage and neutrophil infiltration, as well as a significant increase in serum AST and ALT level (P < 0.01, P < 0.05) when compared with the control group. Compared with the I/R group, the intestine and liver pathological damage was improved in the carnosol pretreatment group (Figure 1). In addition, there was a significant difference in liver function between the intestinal I/R and carnosol pretreatment groups (P < 0.05, Figure 2), which indicates that carnosol significantly attenuated the intestinal I/R-induced liver injury.
Compared with the control group, liver tissue SOD level in the I/R group reduced significantly (P < 0.01). SOD level was elevated markedly in carnosol pretreatment (P < 0.05, Figure 2).
Liver neutrophil infiltration was determined by MPO activity. Compared with the control group, liver tissue MPO activity increased significantly after intestinal I/R (P < 0.01). Administration of carnosol reduced MPO activity in liver tissue significantly (P < 0.05, Figure 2).
Compared with the control group, there was a marked increase in serum IL-6 level in the intestinal I/R group at 2 and 4 h of reperfusion (P < 0.01), however carnosol pretreatment decreased serum level of IL-6 significantly at 2 h of reperfusion when compared with the I/R group (P < 0.01, Figure 2). This indicated that carnosol was sufficiently effective to suppress the increase of IL-6 level at early times after reperfusion.
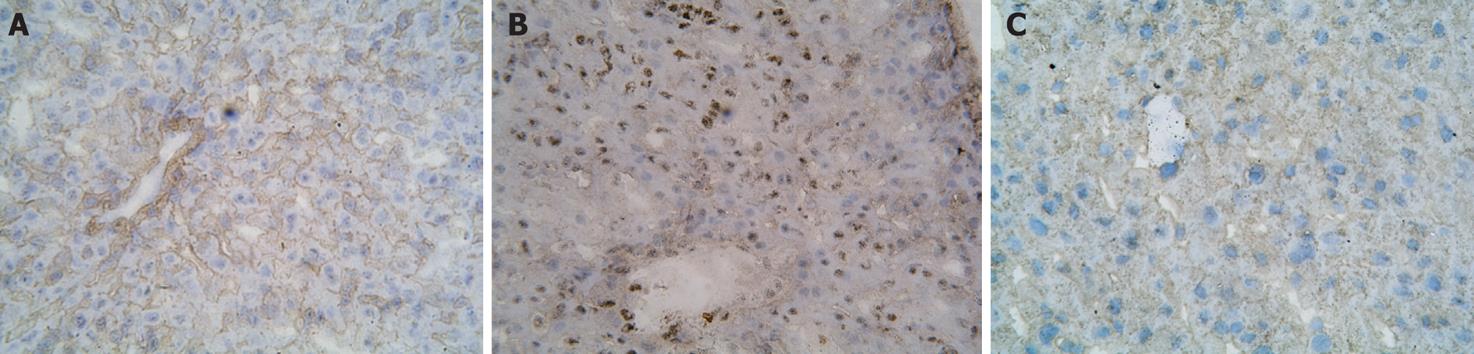
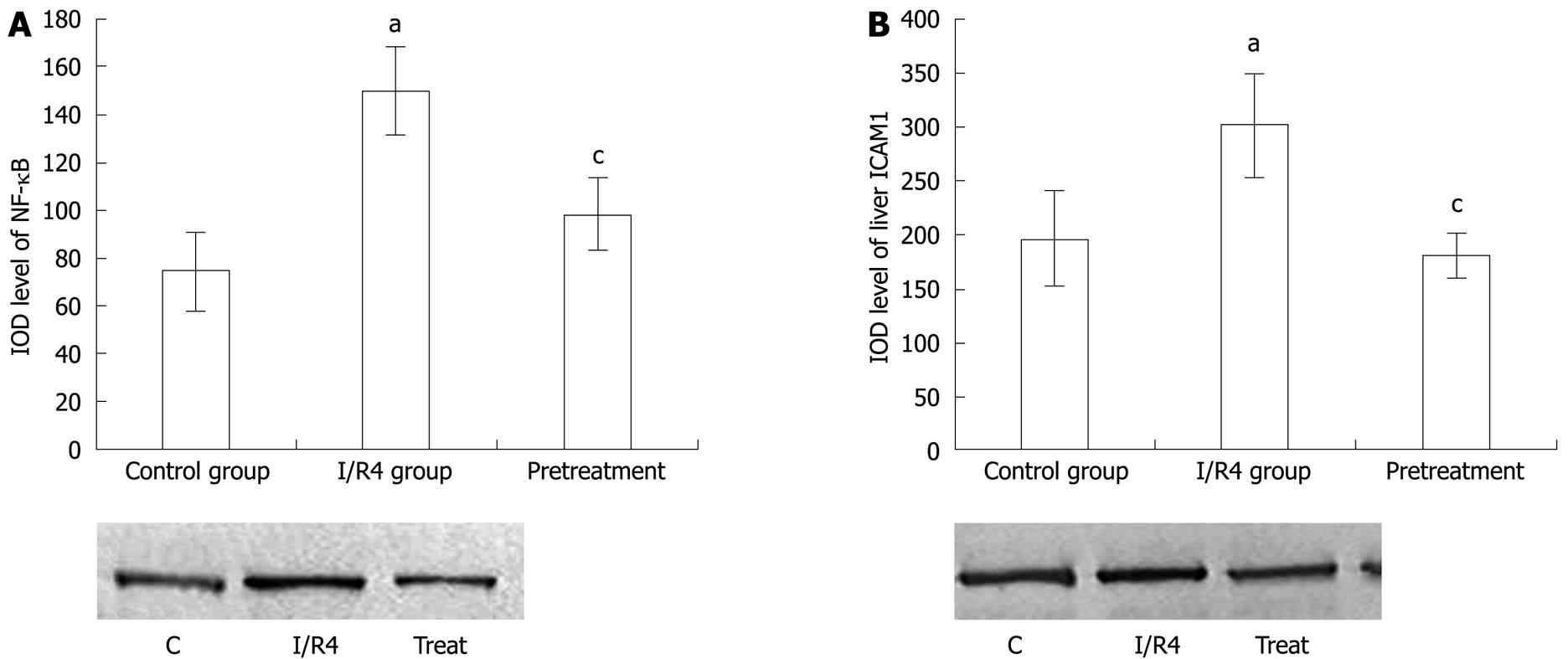
The immunohistochemical study showed that there was little staining of NF-κB p65 in the control group. In comparison, the strong positive expression of NF-κB p65 as brown staining was observed in the nucleus and cytoplasm in the intestinal I/R group. While the positive staining of NF-κB p65 expression was weakened markedly in the carnosol pretreatment group (Figure 3), western blotting showed weak staining for liver NF-κB p65 in the control group. Compared with the control group, IOD level of NF-κB p65 increased markedly in the intestinal I/R group (P < 0.05). After pretreatment with carnosol, the IOD level of NF-κB p65 decreased significantly (P < 0.05, Figure 4A).
Western blotting showed weak positive staining for ICAM-1 in the liver in the control group. However, a significant ICAM-1 protein signal was observed in the intestinal I/R group. Compared with the control group, IOD level of ICAM-1 increased markedly (P < 0.05). This decreased markedly after pretreatment with carnosol (P < 0.05, Figure 4B).
Intestinal I/R is not only necessary to the intestine itself, but involves severe distant tissue dysfunction. Liver is particularly vulnerable to the negative consequences of intestinal I/R because its vasculature is coupled in series with that of the intestine[20]. Although the precise mechanisms of intestinal I/R-induced liver injury have not been elucidated fully, previous research has shown that liver injury associated with intestinal I/R appear to be dependent on leukocyte adhesion and activation. Intestine- and/or liver-derived mediators, such as oxygen radical species, IL-6 and tumor necrosis factor-α, have been suggested as participants in the I/R-induced, leukocyte-mediated liver responses[5721].
Recent studies have implied that the NF-κB pathway is involved in this process. A multitude of signaling factors, including oxidants, inflammatory cytokines, immune stimuli and viruses, can activate the transcriptional factor NF-κB[111221]. In the early stage of intestinal I/R, the gut barrier function is damaged progressively, and bacteria, endogenous endotoxin, bacteriotoxin and reactive oxygen species invade the circulation and induce expression of NF-κB[10]. Since NF-κB activation requires nuclear translocation of the Rel/p65 subunit of NF-κB, nuclear NF-κB p65 level was examined to assess the activation of NF-κB. In the present study, intestinal I/R-induced liver injury manifested as pathological liver injury, significantly increased serum ALT and AST levels, and alterations in the biochemical indicators of oxidative stress in the liver. These changes were parallel to the increased level of serum IL-6 and liver NF-κB p65 expression, which suggests that activation of the NF-κB pathway plays an important part in the pathogenesis of intestinal I/R-induced liver injury. The above observation is also consistent with our previous study[1213].
Extracts of Rosmarinus officinalis L. have been used widely as a preservative in the food industry because of the antioxidant activity of their constituents such as carnosol and carnosic acid[22–24]. Carnosol and carnosic acid are good scavengers of peroxyl radicals and are able to block the formation of the hydroxyl radical generated in non-lipid systems[25]. They have been shown to inhibit non-enzymatic-induced lipid peroxidation in liver microsomes when incubated in the presence of FeCl3[25] or Fe(NO3)3[23]. Carnosol has also been shown to suppress the formation of proinflammatory leukotrienes in rat leukocytes[26]. In addition, inhibition of activation of transcription factor NF-κB has been proposed as one of the important underlying mechanisms of action of carnosol[1617]. Therefore, it is reasonable to postulate that carnosol may exert protective effects in intestinal I/R-induced liver injury.
As expected, the present study showed that pretreatment with carnosol considerably attenuated intestinal I/R-induced liver injury, including reduced liver morphological injury and oxidative stress, as well as decreased serum ALT and AST activity. In addition, carnosol pretreatment resulted in significant down-regulation of the proinflammatory factor IL-6, which in turn, limited activation of circulation leukocytes in the microcirculation of the liver and other tissues. This led to reduced inflammation-mediated tissue injury. Moreover, endothelial adhesion molecules expressed on the surface of endothelial cells, such as ICAM-1, which play an important role in mediating firm adhesion and emigration of activated leukocytes in postcapillary venules, were attenuated after carnosol treatment. The down-regulation of IL-6 and ICAM-1 was consistent with the decreased expression of NF-κB, which indicated that the protective effect of carnosol on intestinal I/R-induced liver injury may be related partly to the inhibition of NF-κB activation.
In conclusion, we showed pretreatment with the natural antioxidant carnosol attenuated liver injury induced by intestinal I/R, attributable to the antioxidant effect and inhibition of the NF-κB pathway.
Carnosol is a phenolic diterpene that has potent antioxidant and anti-inflammatory activities. There have been no reports about its effect on liver injury induced by intestinal ischemia/reperfusion (I/R). The present study investigated the preconditioning effects of carnosol on liver injury induced by intestinal I/R and confirmed the hypothesis that the protective effect of carnosol is mediated via inhibition of nuclear factor κB (NF-κB) activity.
Recently, it has been found that NF-κB plays a major role during the local and systemic inflammatory response following I/R. This study attempted to confirm that modulating the NF-κB pathway is a novel concept and therapeutic strategy for attenuating liver injury caused by intestinal I/R.
The study showed pretreatment with the natural antioxidant carnosol attenuated liver injury induced by intestinal I/R, attributable to the antioxidant effect and inhibition of the NF-κB pathway.
This study indicated that carnosol pretreatment protects liver injury induced by intestinal I/R. The protective effects may be associated with inhibition of NF-κB activation. This may represent a novel and attractive approach to prevent intestinal I/R injury.
NF-κB proteins are a family of transcriptional factors that control the expressions of many genes in immune and acute phase inflammatory responses, cell adhesion, differentiation, oxidative stress responses and apoptosis. Recently, it has been found that NF-κB plays an important role during intestinal I/R.
The authors demonstrated that carnosol attenuated liver injury induced by intestinal I/R in rats. The study is very interesting and the results are sound.
| 1. | Pierro A, Eaton S. Intestinal ischemia reperfusion injury and multisystem organ failure. Semin Pediatr Surg. 2004;13:11-17. |
| 2. | Hassoun HT, Kone BC, Mercer DW, Moody FG, Weisbrodt NW, Moore FA. Post-injury multiple organ failure: the role of the gut. Shock. 2001;15:1-10. |
| 3. | Moore-Olufemi SD, Kozar RA, Moore FA, Sato N, Hassoun HT, Cox CS Jr, Kone BC. Ischemic preconditioning protects against gut dysfunction and mucosal injury after ischemia/reperfusion injury. Shock. 2005;23:258-263. |
| 4. | Horie Y, Ishii H. Liver dysfunction elicited by gut ischemia-reperfusion. Pathophysiology. 2001;8:11-20. |
| 5. | Leister I, Mbachu EM, Post S, Samel ST, Stojanovic T, Gutt CN, Becker H, Markus PM. Vasoactive intestinal polypeptide and gastrin-releasing peptide attenuate hepatic microvasculatory disturbances following intestinal ischemia and reperfusion. Digestion. 2002;66:186-192. |
| 6. | Yao YM, Sheng ZY, Yu Y, Tian HM, Wang YP, Lu LR, Xu SH. The potential etiologic role of tumor necrosis factor in mediating multiple organ dysfunction in rats following intestinal ischemia-reperfusion injury. Resuscitation. 1995;29:157-168. |
| 7. | Kaplan N, Yagmurdur H, Kilinc K, Baltaci B, Tezel S. The protective effects of intravenous anesthetics and verapamil in gut ischemia/reperfusion-induced liver injury. Anesth Analg. 2007;105:1371-1378, table of contents. |
| 8. | Horie Y, Wolf R, Miyasaka M, Anderson DC, Granger DN. Leukocyte adhesion and hepatic microvascular responses to intestinal ischemia/reperfusion in rats. Gastroenterology. 1996;111:666-673. |
| 9. | Lee JI, Burckart GJ. Nuclear factor kappa B: important transcription factor and therapeutic target. J Clin Pharmacol. 1998;38:981-993. |
| 10. | Karin M. The beginning of the end: IkappaB kinase (IKK) and NF-kappaB activation. J Biol Chem. 1999;274:27339-27342. |
| 11. | Souza DG, Vieira AT, Pinho V, Sousa LP, Andrade AA, Bonjardim CA, McMillan M, Kahn M, Teixeira MM. NF-kappaB plays a major role during the systemic and local acute inflammatory response following intestinal reperfusion injury. Br J Pharmacol. 2005;145:246-254. |
| 12. | Tian XF, Yao JH, Li YH, Gao HF, Wang ZZ, Yang CM, Zheng SS. Protective effect of pyrrolidine dithiocarbamate on liver injury induced by intestinal ischemia-reperfusion in rats. Hepatobiliary Pancreat Dis Int. 2006;5:90-95. |
| 13. | Yao JH, Li YH, Wang ZZ, Zhang XS, Wang YZ, Yuan JC, Zhou Q, Liu KX, Tian XF. Proteasome inhibitor lactacystin ablates liver injury induced by intestinal ischaemia-reperfusion. Clin Exp Pharmacol Physiol. 2007;34:1102-1108. |
| 14. | Poeckel D, Greiner C, Verhoff M, Rau O, Tausch L, Hörnig C, Steinhilber D, Schubert-Zsilavecz M, Werz O. Carnosic acid and carnosol potently inhibit human 5-lipoxygenase and suppress pro-inflammatory responses of stimulated human polymorphonuclear leukocytes. Biochem Pharmacol. 2008;76:91-97. |
| 15. | Wijeratne SS, Cuppett SL. Potential of rosemary (Rosemarinus officinalis L.) diterpenes in preventing lipid hydroperoxide-mediated oxidative stress in Caco-2 cells. J Agric Food Chem. 2007;55:1193-1199. |
| 16. | Huang SC, Ho CT, Lin-Shiau SY, Lin JK. Carnosol inhibits the invasion of B16/F10 mouse melanoma cells by suppressing metalloproteinase-9 through down-regulating nuclear factor-kappa B and c-Jun. Biochem Pharmacol. 2005;69:221-232. |
| 17. | Lo AH, Liang YC, Lin-Shiau SY, Ho CT, Lin JK. Carnosol, an antioxidant in rosemary, suppresses inducible nitric oxide synthase through down-regulating nuclear factor-kappaB in mouse macrophages. Carcinogenesis. 2002;23:983-991. |
| 18. | Megison SM, Horton JW, Chao H, Walker PB. A new model for intestinal ischemia in the rat. J Surg Res. 1990;49:168-173. |
| 19. | Sotelo-Félix JI, Martinez-Fong D, Muriel De la Torre P. Protective effect of carnosol on CCl(4)-induced acute liver damage in rats. Eur J Gastroenterol Hepatol. 2002;14:1001-1006. |
| 20. | Turnage RH, Bagnasco J, Berger J, Guice KS, Oldham KT, Hinshaw DB. Hepatocellular oxidant stress following intestinal ischemia-reperfusion injury. J Surg Res. 1991;51:467-471. |
| 21. | Horie Y, Wolf R, Anderson DC, Granger DN. Hepatic leukostasis and hypoxic stress in adhesion molecule-deficient mice after gut ischemia/reperfusion. J Clin Invest. 1997;99:781-788. |
| 22. | Gloire G, Dejardin E, Piette J. Extending the nuclear roles of IkappaB kinase subunits. Biochem Pharmacol. 2006;72:1081-1089. |
| 23. | Haraguchi H, Saito T, Okamura N, Yagi A. Inhibition of lipid peroxidation and superoxide generation by diterpenoids from Rosmarinus officinalis. Planta Med. 1995;61:333-336. |
| 24. | Frankel EN, Huang SW, Prior E, Aeschbach R. Evaluation of antioxidant activity of Rosemary extracts, carnosol and carnosic acid in bulk vegetable oils and fish oil and their emulsions. J Sci Food Agric. 1996;72:201-208. |
| 25. | Aruoma OI, Halliwell B, Aeschbach R, Löligers J. Antioxidant and pro-oxidant properties of active rosemary constituents: carnosol and carnosic acid. Xenobiotica. 1992;22:257-268. |
| 26. | Laughton MJ, Evans PJ, Moroney MA, Hoult JR, Halliwell B. Inhibition of mammalian 5-lipoxygenase and cyclo-oxygenase by flavonoids and phenolic dietary additives. Relationship to antioxidant activity and to iron ion-reducing ability. Biochem Pharmacol. 1991;42:1673-1681. |