Published online Jul 7, 2009. doi: 10.3748/wjg.15.3173
Revised: May 13, 2009
Accepted: May 20, 2009
Published online: July 7, 2009
AIM: To assess the reliability and validity of the translated version of Nepean Dyspepsia Index (NDI) in Chinese patients with documented functional dyspepsia (FD).
METHODS: The translation process included forward translation, back translation, pretest and cross-cultural adaptation. Reliability and validity of the translated version were examined by asking 300 subjects to complete the Chinese version of the NDI. The mean age of subjects was 39.24 years and 68.7% of the subjects were women. Internal consistency analysis with Cronbach’s α was performed to test the reliability. Correlation analysis was used to assess the content validity. Factor analysis and structural equation models were used to assess the construct validity.
RESULTS: The Cronbach’s α coefficients ranged 0.833-0.960, well above the acceptable level of 0.70. Correlation analysis showed that each item had a strong correlation with the corresponding domain, but a weak correlation with other domains. Confirmatory factor analysis indicated that the comparative fit index was 0.94, higher than the acceptable level of 0.90.
CONCLUSION: The Chinese version of the NDI is a reliable and valid scale for measuring health-related quality of life and disease severity in Chinese patients with FD.
- Citation: Tian XP, Li Y, Liang FR, Sun GJ, Yan J, Chang XR, Ma TT, Yu SY, Yang XG. Translation and validation of the Nepean Dyspepsia Index for functional dyspepsia in China. World J Gastroenterol 2009; 15(25): 3173-3177
- URL: https://www.wjgnet.com/1007-9327/full/v15/i25/3173.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.3173
Functional dyspepsia (FD) is a common clinical condition characterized by chronic or recurrent upper abdominal pain associated with a variety of gastrointestinal symptoms in the absence of organic disease[1]. An epidemiological survey in Western countries showed that 19%-41% of the population have symptoms of FD[2]. The report on the incidence of FD in citizens of Tianjin, China, revealed that the number of patients with FD accounts for 23.29% of the total population[3]. Since FD greatly affects the quality of life (QOL) of patients[4–6], and contributes to a higher medical cost[78], it represents an important healthcare problem in modern society. However, treatment of FD is still controversial and no single therapy is uniformly effective, partially due to lack of a reliable evaluation instrument[9]. Many difficulties exist in the single treatment approach to FD[10]. A combined approach, which includes herbal medicine, acupuncture and massage therapy, may be more effective than any single treatment[11].
In addition to symptom evaluation, assessment of QOL has been recognized as a major factor for overall health outcome assessment in FD clinical studies. Nepean Dyspepsia Index (NDI) was designed to measure both symptom scores and impairment of the dyspepsia-specific health-related QOL (H-QOL). Since the development of NDI in 1998[12], it has been used as an outcome assessment in many FD clinical researches[1314] and translated into several languages including French, Dutch, Italian, German, Spanish, and American English[1516]. NDI has proven vital in the diagnosis of FD[12], and its utility has been validated[417]. A number of studies about the effect of FD on QOL in Western patients are available[71819]. However, the effect of FD on the QOL in Asian patients has not received great attention.
Since no validated disease-specific questionnaire is currently available for assessing the impact of FD on the QOL, we translated the original version of NDI (25 items) into Chinese. We also assessed the reliability and validity of this translated version in Chinese patients with documented FD in order to provide researchers, clinicians, and patients with a suitable questionnaire to assess FD.
This study was approved by the Ethics Committee of Chengdu University of Traditional Chinese Medicine. All subjects provided their written consent before participation in this study.
Four hundred and six Chinese outpatients with a suspected diagnosis of FD were screened between March 20, 2007 and June 1, 2007. One hundred and six patients were excluded and the symptom scores for the remaining 300 patients enrolled in our study were 2.970 ± 3.515 for burping/belching, 4.690 ± 3.770 for fullness after eating or slow digestion, 1.800 ± 2.886 for nausea, 2.367 ± 3.421 for inability to finish a regular meal, 1.540 ± 3.038 for bad breath, 3.050 ± 3.575 for bitter/sour tasting fluid coming up into mouth or throat, 0.670 ± 1.855 for vomiting, 5.790 ± 2.939 for discomfort in upper abdomen, 0.786 ± 2.185 for cramps in upper abdomen, 1.306 ± 2.803 for burning sensation in upper abdomen, 5.130 ± 3.567 for pain or ache in upper abdomen, 1.206 ± 2.601 for pressure in upper abdomen, 5.300 ± 3.516 for bloating in upper abdomen, 0.903 ± 2.380 for burning sensation in chest (heartburn), and 0.640 ± 2.045 for pain or ache in chest. The mean age of subjects was 39.24 years and 68.7% of the subjects were women.
The study was performed at Chengdu, Wuhan and Changsha medical centers, which comprise five hospitals in total. Before inclusion in the trial, all patients underwent physical examination, abdominal ultrasonography and upper gastrointestinal endoscopy to exclude organic gastrointestinal cause for their symptoms. Patients at the age of 18 years and older were considered eligible in the study. If their endoscopy was either normal or showed only chronic superficial gastritis, they were diagnosed with FD according to Rome-III criteria[20] with no cholecystitis or cholelithiasis on their sonogram. Patients with a history of upper gastrointestinal surgery, or who were pregnant or lactating, were excluded. Mental deficiency, psychiatric, cardiovascular, hepatic, and renal diseases were the additional exclusion criteria. The investigation was carried out in a quiet environment. Specialists explained each item to the patients so that the patients could understand and complete the questionnaire correctly.
The original 25-item NDI[17] consists of three parts: a symptom checklist that measures the frequency, intensity and level of discomfort of 15 upper gastrointestinal symptoms over the prior 14 d, 25 items designed to assess QOL, and an 11-item questionnaire designed to measure the relevance or importance of the above items.
The QOL includes four domains, namely interference (13 items), know/control (seven items), eat/drink (three items), and sleep/disturb (two items). The scores were determined with a five-point Likert scale which ranges “not at all”-“extremely”. The NDI for QOL score ranges 0-99, higher scores indicate poorer QOL. Symptoms were scored separately from questions representing the QOL. The higher the score of each individual symptom is, the severer the symptom is.
After permission, the original version of NDI was translated into Chinese according to the WHO-QOL methodology of cross-culture adaptation for QOL[21]. Forward translation from the original English version was performed independently by two gastroenterologists with good English. Both forward versions were then reconciled and incorporated into the Chinese version. Back translation was carried out by an English teacher understanding the Chinese language with no knowledge of NDI. The semifinal version was derived from reconciliation of the original, back and forward translations. A pretest, NDI for five patients with FD was performed. The final version was obtained after cross-cultural adaptation.
The reliability of NDI was evaluated for internal consistency using Cronbach’s α. Correlation analyses were used to assess the content validity. Factor analysis and structural equation models were used to assess the construct validity.
All data were analyzed with SPSS 13.0 version. Data derived from descriptive statistical analysis were presented in the form of percentages including categorical variables and calculation of the mean. P < 0.05 was considered statistically significant.
The total score for each symptom on the checklist was calculated by adding its corresponding frequency, severity and level of discomfort. As stated above, higher scores were elicited for symptoms of discomfort, bloating, and pain or ache in upper abdomen, and fullness after eating or slow digestion.
The scores were evaluated using Cronbach’s α coefficient. An α score > 0.7 was considered internally consistent as previously described[22]. The Cronbach’s α coefficient ranged 0.833-0.960 (Table 1).
| Factor | Domain | Cronbach’s α |
| 1 | Interference | 0.944 |
| 2 | Know/control | 0.906 |
| 3 | Eat/drink | 0.833 |
| 4 | Sleep disturb | 0.960 |
The content validity of 25 items and four-field scores were regarded as 29 independent variables. Pearson item-dimension correlation coefficient was employed to evaluate the content validity. Most of the coefficients were higher than 0.6 (P < 0.01, Table 2).
| Item | Domains | |||
| Interference | Know/control | Eat/drink | Sleep disturb | |
| 01 | 0.626 | 0.531 | 0.461 | 0.354 |
| 02 | 0.701 | 0.817 | 0.450 | 0.376 |
| 03 | 0.666 | 0.812 | 0.492 | 0.396 |
| 04 | 0.522 | 0.493 | 0.906 | 0.457 |
| 05 | 0.457 | 0.476 | 0.840 | 0.454 |
| 06 | 0.589 | 0.484 | 0.852 | 0.389 |
| 07 | 0.473 | 0.476 | 0.472 | 0.982 |
| 08 | 0.511 | 0.536 | 0.509 | 0.980 |
| 09 | 0.822 | 0.640 | 0.521 | 0.464 |
| 10 | 0.828 | 0.638 | 0.520 | 0.399 |
| 11 | 0.826 | 0.677 | 0.455 | 0.437 |
| 12 | 0.818 | 0.635 | 0.446 | 0.403 |
| 13 | 0.815 | 0.568 | 0.482 | 0.342 |
| 14 | 0.800 | 0.575 | 0.432 | 0.397 |
| 15 | 0.794 | 0.564 | 0.471 | 0.289 |
| 16 | 0.771 | 0.715 | 0.467 | 0.442 |
| 17 | 0.753 | 0.832 | 0.424 | 0.430 |
| 18 | 0.742 | 0.843 | 0.505 | 0.528 |
| 19 | 0.746 | 0.774 | 0.482 | 0.374 |
| 20 | 0.824 | 0.827 | 0.554 | 0.458 |
| 21 | 0.751 | 0.624 | 0.477 | 0.370 |
| 22 | 0.557 | 0.771 | 0.368 | 0.347 |
| 23 | 0.506 | 0.710 | 0.362 | 0.321 |
| 24 | 0.709 | 0.814 | 0.527 | 0.487 |
| 25 | 0.631 | 0.577 | 0.327 | 0.294 |
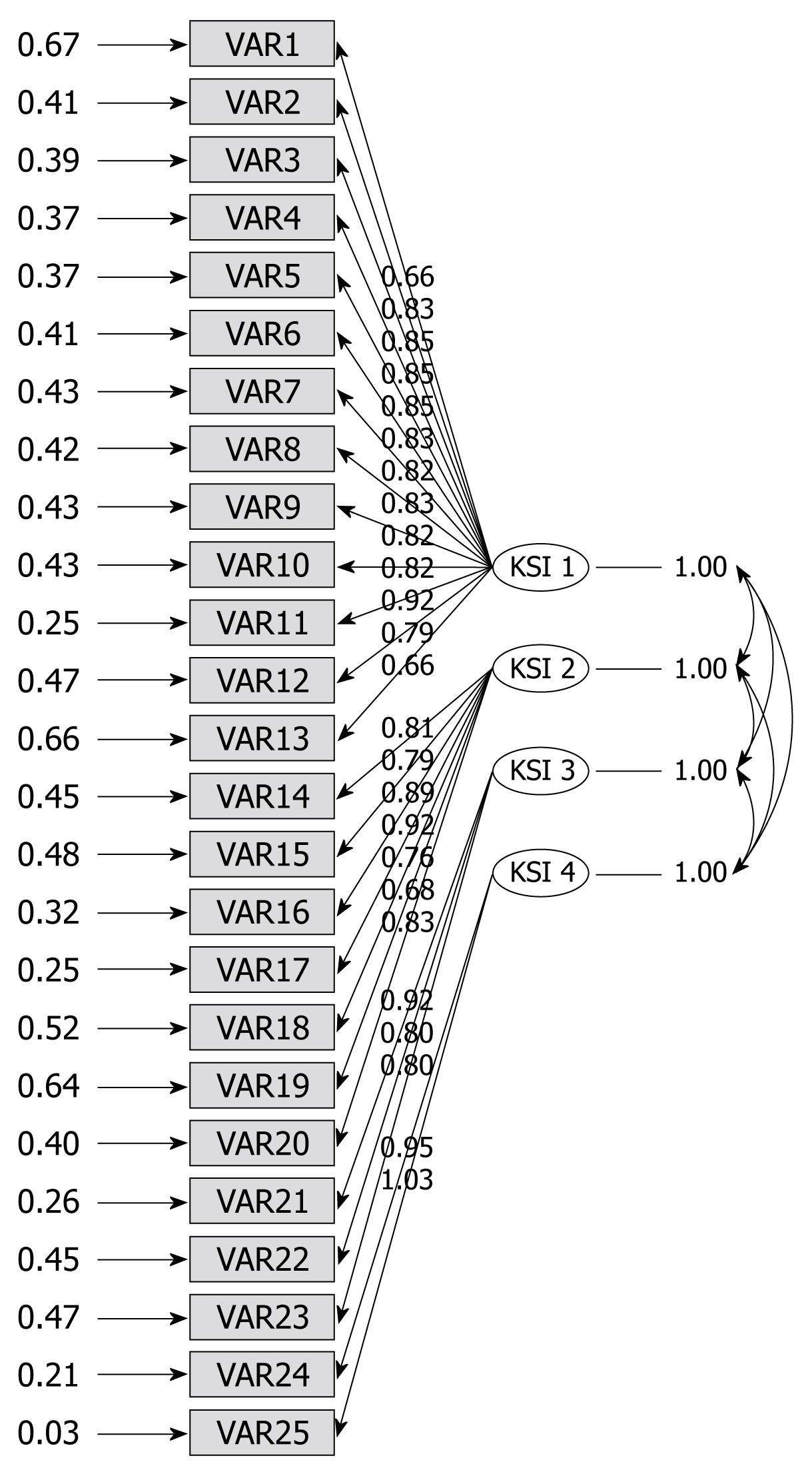
The values of the four preceding factors were above 1.0, and their cumulative factor loading rate was 69.287%. The rotated component matrix showed that component 1 had more loadings on items 1, 9-16, 20, 21 and 25; component 2 had more loadings on items 2, 3, 17-20, 22-24; component 3 had more loadings on items 4-6; component 4 had more loadings on items 7 and 8 (Table 3). The confirmatory factor analysis indicated that degrees of freedom = 269, minimum fit function chi-square = 1703.32 (P < 0.0001), normal theory weighted least chi-square = 1809.13 (P < 0.0001), comparative fit index (CFI) = 0.94, non-normal fit index = 0.94. A structural equation model of construct validity is illustrated in Figure 1.
| Item | Component | |||
| 1 | 2 | 3 | 4 | |
| 22 | 0.777 | 0.101 | 0.154 | |
| 23 | 0.735 | 0.145 | ||
| 19 | 0.725 | 0.352 | 0.201 | |
| 17 | 0.715 | 0.417 | 0.223 | |
| 18 | 0.685 | 0.367 | 0.178 | 0.312 |
| 20 | 0.684 | 0.448 | 0.261 | 0.184 |
| 24 | 0.640 | 0.332 | 0.266 | 0.257 |
| 03 | 0.634 | 0.312 | 0.336 | |
| 02 | 0.612 | 0.413 | 0.263 | |
| 16 | 0.598 | 0.445 | 0.156 | 0.225 |
| 25 | 0.536 | 0.374 | ||
| 13 | 0.206 | 0.853 | 0.200 | |
| 14 | 0.216 | 0.835 | 0.117 | 0.164 |
| 15 | 0.225 | 0.828 | 0.204 | |
| 10 | 0.354 | 0.695 | 0.270 | 0.122 |
| 12 | 0.395 | 0.689 | 0.107 | 0.202 |
| 09 | 0.341 | 0.659 | 0.284 | 0.215 |
| 11 | 0.449 | 0.630 | 0.132 | 0.248 |
| 21 | 0.458 | 0.501 | 0.258 | 0.129 |
| 01 | 0.335 | 0.378 | 0.377 | 0.124 |
| 04 | 0.182 | 0.235 | 0.834 | 0.188 |
| 05 | 0.277 | 0.762 | 0.248 | |
| 06 | 0.171 | 0.399 | 0.713 | |
| 07 | 0.197 | 0.180 | 0.225 | 0.908 |
| 08 | 0.255 | 0.192 | 0.256 | 0.878 |
QOL has received increasing attention as more foci are placed on individual satisfaction as an important health outcome in clinical studies. In addition, QOL is particularly significant in diseases lacking of obvious biological or clinical markers, such as FD[2324]. FD greatly affects the QOL of patients. However, treatment of FD is still controversial and no single therapy is uniformly effective, in part, due to the absence of a reliable evaluation instrument.
From our symptom checklist scores, symptoms with the highest scores were discomfort, bloating, and pain or ache in upper abdomen and fullness after eating or slow digestion, all of which are congruent with the main symptoms of FD according to Rome-III criteria[25]. However, heartburn, a major symptom in the Rome-III criteria, had a lower score in our study, possibly due to lack of patient comprehension or lack of adequate explanation by the investigator.
In clinical trials, Leeds dyspepsia questionnaire (LDQ) and MOS 36-item short-form health survey (SF-36) have been applied as an evaluation instrument for FD[26–28]. LDQ is a valid, reliable and responsive instrument for measuring the presence and severity of dyspepsia, but it lacks of QOL assessment[29]. As it is known that, in addition to symptoms, QOL assessment represents a major part of health outcome assessment in clinical studies of FD. However, as different diseases cause different symptoms which necessitate disease-specific H-QOL instruments. SF-36, a generic QOL measurement, contains a large number of questions, the majority of which are often irrelevant to a particular disease. As a result, it may be insensitive to changes in the relevant items due to interference from the irrelevant items. Thus, the evaluation of FD should contain two aspects, namely symptom measure and disease-specific H-QOL assessment. The NDI addresses both aspects.
The original NDI consists of 42 questions, and is shortened to 25 items, excluding those items with a negative response rate of over 60%. The remaining 25 items represent the clinically relevant QOL concepts in subjects with FD[17]. Our study was based on the revised version of 25 items.
In order to maintain the sensitivity of the original version, our questionnaire considered differences in language, cultural diversities, and life style. After our version of the NDI was established, its reliability and validity were evaluated using Cronbach’s α. We found that all domains were above 0.80, demonstrating that our scale has an excellent reliability. Correlation analysis demonstrated that each item had a strong correlation with the corresponding domain, but a weak correlation with other domains, supporting that our questionnaire is valid. In addition, our results are in accordance with the original NDI design, representing superior construct validity. Confirmatory factor analysis also indicated that the CFI was higher than the acceptable level of 0.90[2530], demonstrating that our scale has a good construct validity.
In the past, FD research in China was limited by the absence of a valid assessment instrument for the disease. Our results suggest that the Chinese version of NDI with excellent psychometrics would promote the clinical study of FD and align its medical course in China with international practice in future.
Our study has some limitations, including lack of extensive demographic characteristics, analysis of multi-dimensional factors (such as education, career, financial and social status), “test-retest” in reliability assessment, and discriminating validity in validity assessment, all of which need to be further studied.
Functional dyspepsia (FD) is a common non-organic disease in the world. However, treatment of FD is still controversial, partially due to lack of a reliable evaluation instrument. Since 1998, the Nepean Dyspepsia Index (NDI) has been designed to diagnose FD and proven to be valid in measuring both symptom scores and impairment of dyspepsia-specific health-related quality of life (H-QOL) in FD patients.
In addition to symptom evaluation, assessment of QOL has been recognized to be more and more important in overall health outcome assessment in FD clinical studies. In this study, the reliability and validity of the Chinese NDI translated from it English version which reflects the dyspepsia-specific H-QOL in Chinese FD patients, were assessed.
Studies on validation of NDI for FD in Western countries are widely available. However, NDI has not been translated and validated in China, since much attention is not paid to the effect of FD on the QOL of patients. This is the first study to translate the original version of NDI (25 items) into Chinese and to assess its reliability and validity in Chinese FD patients.
By validating the translated version of NDI, this study may provide researchers, clinicians, and patients with a suitable questionnaire for the assessment of FD.
Reliability is the extent to which the measurements of a test remain consistent over repeated tests of the same subject under identical conditions. Validity, containing construct, content and convergent validity, refers to the degree to which evidence and theory support the interpretations of test scores entailed by proposed tests. Assessment of the validity of a scale involves evaluation of the scale in relation to the desired conclusion on the basis of prevailing standards.
The authors have made a detail description of how to use the Chinese version of NDI to diagnose FD patients in China. The study is clinically important, because a Chinese version of NDI is needed to diagnose and compare FD patients in China and other countries.
| 1. | Piessevaux H, De Winter B, Louis E, Muls V, De Looze D, Pelckmans P, Deltenre M, Urbain D, Tack J. Dyspeptic symptoms in the general population: a factor and cluster analysis of symptom groupings. Neurogastroenterol Motil. 2009;21:378-388. |
| 2. | Talley NJ, Silverstein MD, Agréus L, Nyrén O, Sonnenberg A, Holtmann G. AGA technical review: evaluation of dyspepsia. American Gastroenterological Association. Gastroenterology. 1998;114:582-595. |
| 3. | Kang XQ, Liu ZW, Xie B, Niu XY, Xiao YY, He C. The incidence of Functional Dyspepsia of parts of citizens in Tianjin. Zhonghua Xiaohua Zazhi. 2002;22:191-192. |
| 4. | Talley NJ, Verlinden M, Jones M. Quality of life in functional dyspepsia: responsiveness of the Nepean Dyspepsia Index and development of a new 10-item short form. Aliment Pharmacol Ther. 2001;15:207-216. |
| 5. | Monés J, Adan A, Segú JL, López JS, Artés M, Guerrero T. Quality of life in functional dyspepsia. Dig Dis Sci. 2002;47:20-26. |
| 6. | El-Serag HB, Talley NJ. Systemic review: the prevalence and clinical course of functional dyspepsia. Aliment Pharmacol Ther. 2004;19:643-654. |
| 7. | Holtmann G, Adam B, Haag S, Collet W, Grünewald E, Windeck T. Efficacy of artichoke leaf extract in the treatment of patients with functional dyspepsia: a six-week placebo-controlled, double-blind, multicentre trial. Aliment Pharmacol Ther. 2003;18:1099-1105. |
| 8. | Meineche-Schmidt V, Talley NJ, Pap A, Kordecki H, Schmid V, Ohlsson L, Wahlqvist P, Wiklund I, Bolling-Sternevald E. Impact of functional dyspepsia on quality of life and health care consumption after cessation of antisecretory treatment. A multicentre 3-month follow-up study. Scand J Gastroenterol. 1999;34:566-574. |
| 9. | Rentz AM, Battista C, Trudeau E, Jones R, Robinson P, Sloan S, Mathur S, Frank L, Revicki DA. Symptom and health-related quality-of-life measures for use in selected gastrointestinal disease studies: a review and synthesis of the literature. Pharmacoeconomics. 2001;19:349-363. |
| 10. | Mönkemüller K, Malfertheiner P. Drug treatment of functional dyspepsia. World J Gastroenterol. 2006;12:2694-2700. |
| 11. | Madisch A, Miehlke S, Labenz J. Management of functional dyspepsia: Unsolved problems and new perspectives. World J Gastroenterol. 2005;11:6577-6581. |
| 12. | Talley NJ, Haque M, Wyeth JW, Stace NH, Tytgat GN, Stanghellini V, Holtmann G, Verlinden M, Jones M. Development of a new dyspepsia impact scale: the Nepean Dyspepsia Index. Aliment Pharmacol Ther. 1999;13:225-235. |
| 13. | Hashash JG, Abdul-Baki H, Azar C, Elhajj II, El Zahabi L, Chaar HF, Sharara AI. Clinical trial: a randomized controlled cross-over study of flupenthixol + melitracen in functional dyspepsia. Aliment Pharmacol Ther. 2008;27:1148-1155. |
| 14. | Talley NJ, Tack J, Ptak T, Gupta R, Giguère M. Itopride in functional dyspepsia: results of two phase III multicentre, randomised, double-blind, placebo-controlled trials. Gut. 2008;57:740-746. |
| 15. | Talley NJ, Verlinden M, Jones M. Can symptoms discriminate among those with delayed or normal gastric emptying in dysmotility-like dyspepsia? Am J Gastroenterol. 2001;96:1422-1428. |
| 16. | Cho YK, Choi MG, Kim SH, Lee IS, Kim SW, Chung IS, Lee SY, Choi SC, Seol SY. [The effect of mosapride on quality of life in functional dyspepsia]. Korean J Gastroenterol. 2004;43:160-167. |
| 17. | Talley NJ, Verlinden M, Jones M. Validity of a new quality of life scale for functional dyspepsia: a United States multicenter trial of the Nepean Dyspepsia Index. Am J Gastroenterol. 1999;94:2390-2397. |
| 18. | Boeckxstaens GE, Hirsch DP, Kuiken SD, Heisterkamp SH, Tytgat GN. The proximal stomach and postprandial symptoms in functional dyspeptics. Am J Gastroenterol. 2002;97:40-48. |
| 19. | Holtmann G, Talley NJ, Liebregts T, Adam B, Parow C. A placebo-controlled trial of itopride in functional dyspepsia. N Engl J Med. 2006;354:832-840. |
| 20. | Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology. 2006;130:1377-1390. |
| 21. | Leplege A, Verdier A. The adaptation of health status measures: methodological aspects of the translation procedure. The international sssessment of health-related quality of life: theory, translation, measurement and analysis. Rapid Communications: Oxford 1995; 93-101. |
| 22. | Hays RD, Anderson R, Revicki D. Psychometric considerations in evaluating health-related quality of life measures. Qual Life Res. 1993;2:441-449. |
| 23. | Halder SL, Locke GR 3rd, Talley NJ, Fett SL, Zinsmeister AR, Melton LJ 3rd. Impact of functional gastrointestinal disorders on health-related quality of life: a population-based case-control study. Aliment Pharmacol Ther. 2004;19:233-242. |
| 24. | Frankhuisen R, Van Herwaarden MA, Heijkoop R, Baron A, Vermeijden R, Smout AJ, Gooszen HG, Samsom M. Functional dyspepsia and irritable bowel syndrome in patients with achalasia and its association with non-cardiac chest pain and a decreased health-related quality of life. Scand J Gastroenterol. 2009;44:687-691. |
| 25. | Hu LT, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Modeling. 1999;6:l-55. |
| 26. | Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473-483. |
| 27. | Kupcinskas L, Lafolie P, Lignell A, Kiudelis G, Jonaitis L, Adamonis K, Andersen LP, Wadström T. Efficacy of the natural antioxidant astaxanthin in the treatment of functional dyspepsia in patients with or without Helicobacter pylori infection: A prospective, randomized, double blind, and placebo-controlled study. Phytomedicine. 2008;15:391-399. |
| 28. | Sararaks S, Azman AB, Low LL, Rugayah B, Aziah AM, Hooi LN, Abdul Razak M, Norhaya MR, Lim KB, Azian AA. Validity and reliability of the SF-36: the Malaysian context. Med J Malaysia. 2005;60:163-179. |
| 29. | Moayyedi P, Duffett S, Braunholtz D, Mason S, Richards ID, Dowell AC, Axon AT. The Leeds Dyspepsia Questionnaire: a valid tool for measuring the presence and severity of dyspepsia. Aliment Pharmacol Ther. 1998;12:1257-1262. |
| 30. | Browne MW, Cudeck R. Alternative ways of assessing model fit. Testing structural equation models. Sage: Beverly Hills 1993; 136-162. |