Published online Jun 28, 2009. doi: 10.3748/wjg.15.3046
Revised: May 11, 2009
Accepted: May 18, 2009
Published online: June 28, 2009
AIM: To identify and compare the profile of Ca2+ channel subunit expression in INS-1 and rat pancreatic β cells.
METHODS: The rat insulin-secreting INS-1 cell line was cultured in RPMI-1640 with Wistar rats employed as islet donors. Ca2+ channel subunit expression in INS-1 and isolated rat β cells were examined by reverse transcription polymerase chain reaction (RT-PCR). Absolute real-time quantitative PCR was performed in a Bio-Rad iQ5 Gradient Real Time PCR system and the data analyzed using an iQ5 system to identify the expression level of the Ca2+ channel subunits.
RESULTS: In INS-1 cells, the L-type Ca2+ channel 1C subunit had the highest expression level and the TPRM2 subunit had the second highest expression. In rat β cells, the TPRC4β subunit expression was dominant and the expression of the L-type 1C subunit exceeded the 1D subunit expression about two-fold. This result agreed with other studies, confirming the important role of the L-type 1C subunit in insulin-secreting cells, and suggested that non-voltage-operated Ca2+ channels may have an important role in biphasic insulin secretion.
CONCLUSION: Twelve major Ca2+ channel subunit types were identified in INS-1 and rat β cells and significant differences were observed in the expression of certain subunits between these cells.
- Citation: Li F, Zhang ZM. Comparative identification of Ca2+ channel expression in INS-1 and rat pancreatic β cells. World J Gastroenterol 2009; 15(24): 3046-3050
- URL: https://www.wjgnet.com/1007-9327/full/v15/i24/3046.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.3046
Recent theories portray type 2 diabetes mellitus (T2DM) as a heterogeneous disorder. In addition to insulin resistance, clinical studies in humans and animals have documented a variety of defects in β cell function[1], and most researchers agree that both insulin secretion impairment and insulin resistance contribute to the fully established disease[2]. Insulin produced by pancreatic islet β cells efficiently regulates glucose homeostasis in humans and other mammals. These cells are electrically excitable, and couple changes in blood glucose concentration to insulin release via electrical signals.
Glucose-stimulated insulin secretion is biphasic, with about a 10 min first phase and a several hour second phase[3]. Intracellular Ca2+ signals play a pivotal role in β cell function and, as insulin secretion is the most important role of these cells, knowledge of the intricacies of the signals involved in excitation-secretion coupling is important in understanding both normal β cell function and related pathological states. It has been reported that both voltage-dependent and non-voltage-operated Ca2+ channels are involved in these processes. The voltage-dependent Ca2+ channels include L-type, T-type, N-type and R-type channels[45]; the non-voltage-operated Ca2+ channels include ryanodine-sensitive Ca2+ channels[6–8], transient receptor potential channels (TRP)[9–11], and inositol 1,4,5-trisphosphate (IP3)-sensitive channels[12], the latter mobilizing Ca2+ from the endoplasmic reticulum. Impaired first-phase insulin secretion is an early feature of T2DM, whereas second-phase insulin secretion deteriorates with progression of the disease. A genetic study has indicated that polymorphisms in R-type channels in humans are associated with T2DM and impaired insulin secretion[13]. Moreover, most Ca2+ channels consist of various subunits which participate in different physiologic functions in different species or sub-cloned cell lines[12]. For example, T-type Ca2+ channels have little or no expression in rodents, but can be detected in humans[14]. Thus, the above suggests that Ca2+ channel subunits may be suitable candidates as pathogenetic factors in diabetes.
Considering the essential functions of Ca2+ signals for insulin secretion and the various Ca2+ channel subunits in β cells, a systemic identification of Ca2+ channel subunit expression in β cells is necessary to further the understanding of their functions in regulating insulin secretion and to find potential new therapy targets for T2DM. The aim of this study was to detect the expression profile of six voltage-dependent Ca2+ channel subunits, including L-type (α1C, D, S and 1F subunits), R-type (α1E subunit), and N-type (α1B subunit), and nine non-voltage-operated Ca2+ channel subunits including Ryr1, Ryr2, TRPC1, TRPC4α, TRPC4β, TRPM2, IP3R1, IP3R2 and IP3R3, in an INS-1 cell line, and to compare the identified subunits with those detectable in rat primary pancreatic β cells. The INS-1 rat cell line was employed as a model of pancreatic β cells because INS-1 cells show susceptibility to glucotoxicity, similar to β cells.
INS-1 cells lines were glucose-responsive and a gift from Professor Tao Xu (Institute of Biophysics, Chinese Academy of Sciences, P. R. China) and were cultured in RPMI-1640 medium containing 11.2 mmol/L glucose, 100 mL/L fetal calf serum, 2 mmol/L L-glutamine, 1 mmol/L sodium pyruvate[15], 100 000 U/L penicillin, 100 mg/L streptomycin, and 50 mmol/L β-mercaptoethanol, in a fully humidified atmosphere containing 50 mL CO2 per liter air at 37°C.
Pathogen-free, inbred, male Wistar rats weighing 300-350 g were used as islet donors. The animals were maintained on standard rat chow and acidified water ad libitum. For islet retrieval, individuals were sacrificed by cervical dislocation and the pancreas was quickly removed. Pancreatic islets were isolated using a standard collagenase digestion[1617]. Briefly, islets were separated from exocrine tissue by centrifugation over a discontinuous dextran gradient after digestion with 0.5 g/L collagenase V (Sigma C9263) for 30 min, and further purification by handpicking under a microscope. Islets were collected and washed twice in phosphate-buffered saline and dispersed as single cells by mechanical shaking in 4 mL of Hank’s solution (Ca2+ and Mg2+ free) before filtering the cell mixture over a 35 &mgr;m pore size filter (BD Falcon) and diluting to 200 mL. This preparation was divided into 20 mL per tube, and cDNA was synthesized from the total isolated RNA, as described below.
To distinguish glucagon-producing-cells from insulin-producing-cells, specific primer pairs were designed from the insulin and glucagon genes in GenBank. The sequences were: insulin-F, AAACAGCACCTTTGTGG TTCTCA; insulin-R, GTGCCACTTGTGGGTCCTCC; glucagon-F, TCGTGGCTG GATTGTTTG; and glucagon-R, TGGCGTTTGTCTTCGTTTAT. The PCR procedure was: the insulin gene at 95°C for 30 s, 59°C for 30 s, 72°C for 30 s, and 35 cycles; and the glucagon gene at 95°C for 30 s, 53°C for 30 s, 72°C for 30 s, and 35 cycles.
Total RNA was extracted from INS-1 cells and rat pancreatic β cells using the RNeasy Mini Kit (Qiagen, Germany) according to the manufacturer’s instructions. One microliter aliquot of the RNA was incubated with Oligo (dT) 18 at 70°C for 5 min, and then put on ice. RNase inhibitor (1 &mgr;L), 100 &mgr;mol/L dNTPs, 0.01 mol/L dithiothreitol, and 200 U of M-MLV reverse transcriptase (Promega, USA) were added to the mixture (20 &mgr;L final volume), incubated at 42°C for 50 min, and then incubated at 85°C for 5 min. Table 1 summarizes the primer pairs used for the amplification of Ca2+ channel subunits, PCR was performed in a standard 50 &mgr;L reaction volume, and the resulting products were visualized by Golden view after 3% agarose gel electrophoresis in TAE buffer (40 mmol/L Tris-acetate, 2 mmol/L EDTA, pH 8.5).
| Primer | Sequence | Temperature (°C) |
| L-α1C (5') | 5'-GTCCATAGTGGGTCGTCT-3' | 50 |
| L-α1C (3') | 5'-TGGTTTGTTCTTGCTTTC-3' | |
| L-α1D (5') | 5'-CAGGGATGCTGTGGAAGT-3' | 51 |
| L-α1D (3') | 5'-TGGGCTGAGAACCTAGACG-3' | |
| L-α-S (5') | 5'-ATGCCAGAGGATGACAACAAC-3' | 55 |
| L-α-S (3') | 5'-CACCCAgAAAgACAATGATgAA-3' | |
| L-α1F (5') | 5'-GACGGCAACTTGGCTTCT-3' | 53 |
| L-α1F (3') | 5'-GCTGGCATGACTGCTGGT-3' | |
| N-α1β(5') | 5'-GCTCGCTCTTCGTCTTCA-3' | 52 |
| N-α1β (3') | 5'-AGGTTCCGTGTCATCCAGT-3' | |
| R-α1E (5') | 5'-ATGTCCCTGAAGATGTATGG-3' | 50 |
| R-α1E (3') | 5'-AACGACCTCAAAGATGCTG-3' | |
| Ryr1 (5') | 5'-GGGCGGAGAATGAGAAAGA-3' | 55 |
| Ryr1 (3') | 5'-CAGGGTCGTCACGGTTGT-3' | |
| Ryr2 (5') | 5'-TGAGTATGCCCATTGAGT-3' | 48 |
| Ryr2 (3') | 5'-CTTTGCTTTAGGCGTGAG-3' | |
| TRPC1 (5') | 5'-GTCAGACATTAAGAGGCTGTG-3' | 50 |
| TRPC1 (3') | 5'-AAGTTGCCAAGTAAAGGGA-3' | |
| TRPC4α (5') | 5'-AATGGTTCTGCCCTGGTG-3' | 50 |
| TRPC4α (3') | 5'-GAAGATTTGGTTTGCGTTT-3' | |
| TRPC4β (5') | 5'-GCAGCATTCCTGGTCTCA-3' | 51 |
| TRPC4β (3') | 5'-GGGCGTGTTTCTCCTTTG-3' | |
| TRPM2 (5') | 5'-GTCATCACCATCGGCATAGC-3' | 56 |
| TRPM2 (3') | 5'-TGTCCAGGCAGGTCAGGTT-3' | |
| IP3R1 (5') | 5'-CAACCGTTACTATGGAAACATC-3' | 54 |
| IP3R1 (3') | 5'-TCAGCCAGGCTCATCTCAC-3' | |
| IP3R2 (5') | 5'-CGATGCCAGGATACGATGT-3' | 54 |
| IP3R2 (3') | 5'-CACCCTTGAAGTACCGATT-3' | |
| IP3R3 (5') | 5'-AGGAGCTGGTGGACGTGAT-3' | 55 |
| IP3R3 (3') | 5'-TGCTTGTTGTGCCTGGAAA-3' |
The expression levels of the subunits were quantified by absolute real-time quantitative PCR with the Bio-Rad iQ5 Gradient Real Time PCR system and all reactions performed in a 25 &mgr;L reaction volume containing 12.5 &mgr;L of 2xSYBR Green Master mix (Bio-Rad, USA), 1 &mgr;L of each primer pair (10 &mgr;mol/L), and 1 &mgr;L of cDNA templates. The products of PCR were cloned into the pGEM-T easy vector using the pGEM-T easy cloning kit (Promega, USA). Five microliters LB-broth cultures containing single colonies were grown up overnight at 37°C with shaking at 200 r/min and the resulting plasmids were purified. Plasmid DNA was solubilized in 50 &mgr;L TE buffer and the sequences of the cloned products confirmed by DNA sequencing. Purified plasmid clones were quantified by Eppendorf Biophotometer and the copy number calculated as follows: 6.02 × 1023 (copies/mol) × DNA amount (g)/[DNA length (bp) × 660 (g/mol per becquerel)].
Based on the copy number and concentration of the plasmid DNA, the precise number of molecules added to subsequent real-time PCR runs was calculated, thus providing a standard for specific cDNA quantification. All samples were prepared as 1:10, 1:100, and 1:1000 dilutions and each reaction at different dilutions performed in triplicate. The standard curve and data analysis were produced using Bio-Rad iQ5 software.
An average of 300-500 islets were produced from each rat, with about 1000 cells per islet and composed of 70% β cells. In the first 10 cDNA templates analyzed, six templates only expressed the insulin gene, three expressed the glucagon gene, and the last one expressed neither. The cDNA whose glucagon gene was positive was considered to be from contamination by alpha islet cells and could be ignored and, thus, the others were chosen as templates for analysis of β cell Ca2+ channel subunit expression.
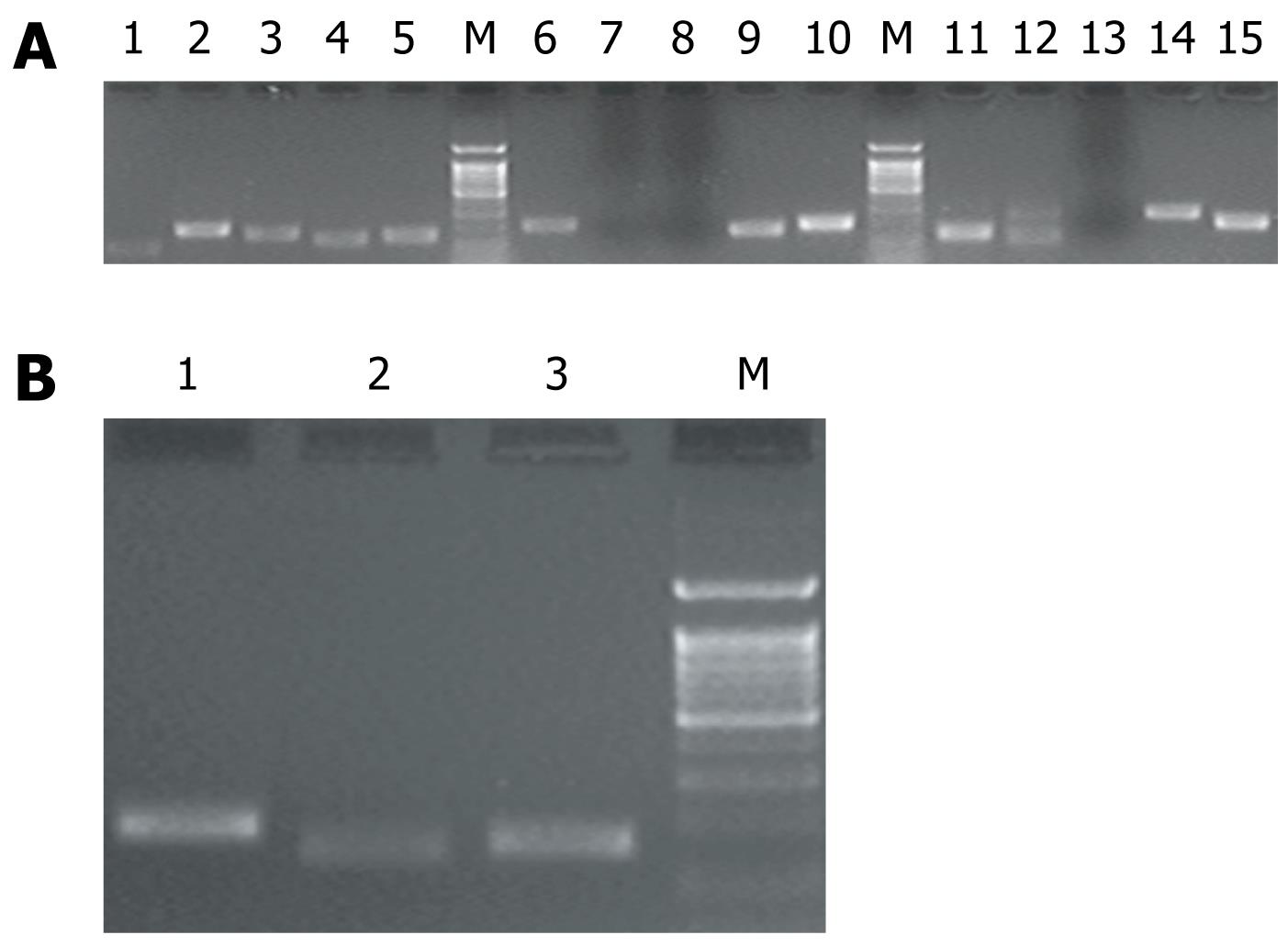
RT-PCR was performed to identify the expression of Ca2+ channel subunits in INS-1 and rat pancreatic β cells. Of the 15 subunit types, 12 types were amplified from INS-1 and rat pancreatic β cells and their identities confirmed (Figure 1A). Three types, not identified in either INS-1 or rat pancreatic β cells, were the L-type α1F, S and Ryr1 subunits. Under the same reaction conditions, these three were identified in cDNA from heart and skeletal muscle because these sources are composed of multiple tissues, including muscles, blood vessels, and nerve fibers (Figure 1B). It was found that 12 subunit types were expressed in INS-1 and rat pancreatic β cells (Table 2): L-type (α1C, α1D subunits), R-type (α1E subunit), and N-type (α1β subunit) preferentially in INS-1 cells; Ryr2, TRPC1, TRPC4α, TRPC4β, TRPM2, IP3R1, IP3R2, and IP3R3 preferentially in β cells.
| Ca2+ channel subunits | Length of PCR production (bp) | INS-1 | β cells |
| L-α1C | 147 | 1.767 × 107± 1.763 × 106 | 3.836 × 106± 1.087 × 106 |
| L-α1D | 197 | 1.046 × 106± 2.074 × 104 | 1.913 × 106± 1.329 × 105 |
| L-α-S1 | 181 | - | - |
| L-α1F1 | 144 | - | - |
| N-α1β | 188 | 1.078 × 104± 6.477 × 102 | 6.832 × 103± 3.220 × 102 |
| R-α1E | 102 | 6.614 × 103± 1.477 × 102 | 2.84 × 103± 1.943 × 102 |
| Ryr11 | 159 | - | - |
| Ryr2 | 250 | 1.367 × 104± 7.213 × 10 | 6.520 × 105± 4.864 × 104 |
| TRPC1 | 101 | 5.631 × 104± 1.904 × 103 | 3.260 × 106± 2.531 ×105 |
| TRPC4α | 196 | 8.033 × 104± 5.405 × 103 | 2.073 × 106± 2.207 × 105 |
| TRPC4β | 165 | 2.226 × 105± 7.672 × 104 | 6.666 × 106± 1.595 × 106 |
| TRPM2 | 130 | 1.028 × 106± 9.696 × 104 | 1.538 × 106± 2.476 × 105 |
| IP3R1 | 159 | 1.189 × 105± 1.650 × 104 | 1.922 × 106± 7.584 × 105 |
| IP3R2 | 191 | 3.339 × 104± 7.945 × 103 | 8.293 × 104± 7.041 × 103 |
| IP3R3 | 130 | 5.613 × 105± 1.009 × 105 | 7.863 × 105± 3.894 × 103 |
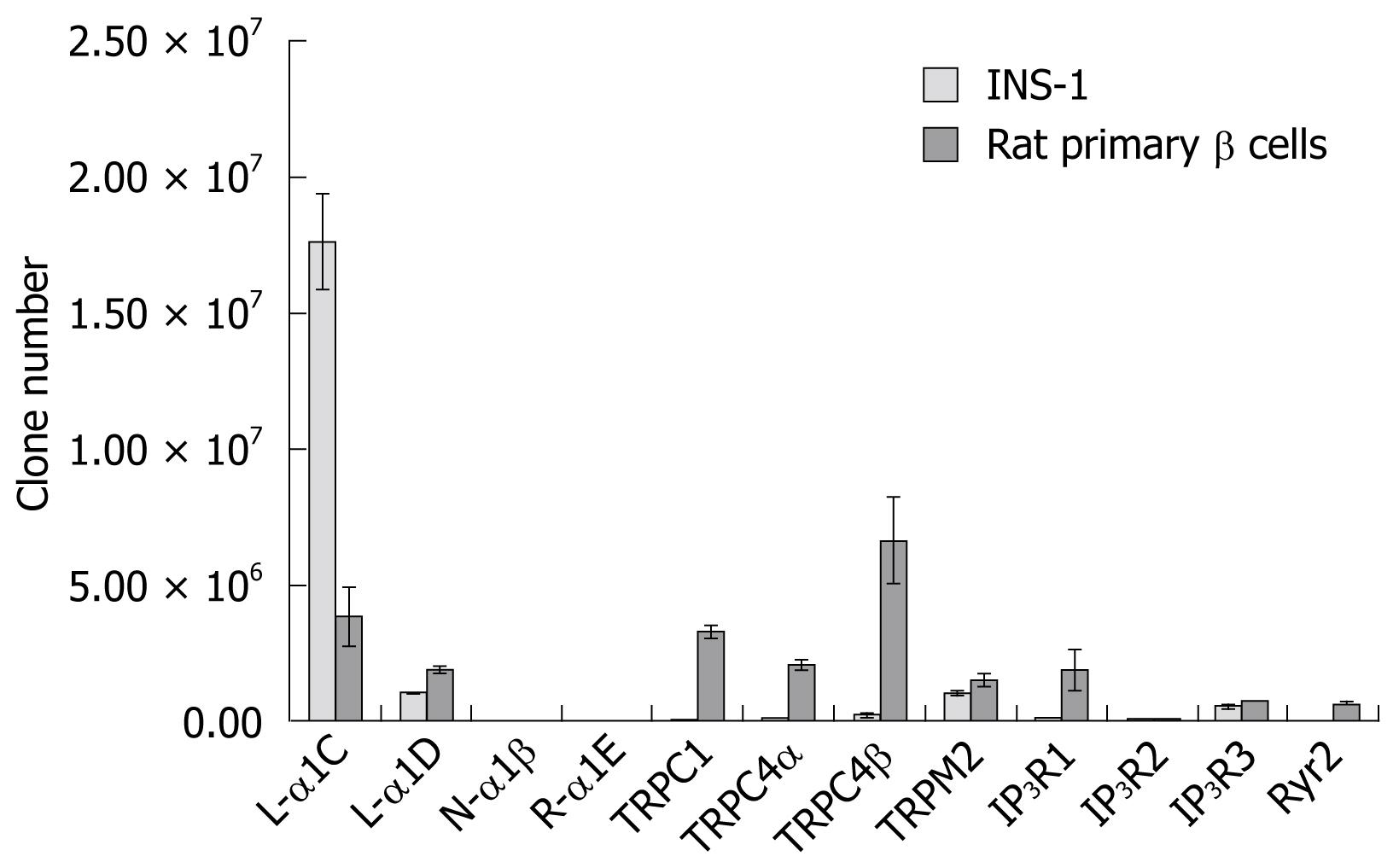
Melt curve analysis of all subunits revealed that there was a single peak at the expected melting temperature for PCR applications. The absolute real-time quantitative PCR analyses showed (Table 2, Figure 2) that, in INS-1, L-type α1C subunits were dominant and were expressed significantly more than other subunits. The R-α1E subunit was expressed at a very low level as in the β cells. In the latter cells, the expression levels of subunits were relatively similar, but the expression of Ryr2, TRPC1, TRPC4α, TRPC4β, TRPM2, and IP3R1 were significantly higher than in INS-1.
As important regulatory factors in insulin secretion, Ca2+ channels have potential as targets for developing new T2DM therapies. Considering the variety of Ca2+ channel subunits present in different cell clones and species, the aim of this study was to perform a systematic identification of Ca2+ channel subunits in INS-1 and rat pancreatic β cells. Collectively, 12 of 15 subunit types were found to be expressed in INS-1 and primary rat islet β cells. The L-type α1C subunit expression exceeded that of α1D 10-fold in INS-1 cells, but only 2-fold in the β cells. These results were similar to recent research in INS-1 832/12 cells which confirmed that α1C subunit expression exceeded that of the α1D subunit by two-fold and that the α1C subunit had a critical role in insulin secretion[18]. The L-type α1C subunit performs a special function in the first phase of insulin secretion and glucose tolerance. In mice, α1C subunit deficiency decreased the whole-cell Ca2+ current by about 45% and abolished the first phase of insulin secretion, resulting in glucose intolerance[19]. In the present study, L-type α1C subunits were dominant among all subunit expressions, which was in accordance with its important biological function in both INS-1 and rat β cells. The L-type α1D subunit’s roles in insulin secretion or proliferation were not confirmed until recently[1820–22].
The expression of the non-voltage-operated Ca2+ channel subunits Ryr2, TRPC1, TRPC4α, TRPC4β, and IP3R1 in rat β cells exceeded those in INS-1 by 10-fold and TRPM2 expression was not significantly different between the β cells and INS-1. These results suggested that non-voltage-operated Ca2+ channels may have a greater role in regulating insulin secretion in comparison with voltage-dependent Ca2+ channels. The expression level of the TRPC4β subunit was dominant in the β cells and, as a member of the TRPC subfamily, TRPC4 shows four protein motifs (M1-M4) characteristic of the TRPC sub-family[23]. Specifically, TRPC4β lacks 84 amino acids in the C-terminus, which corresponds to putative binding sites for calmodulin and IP3 receptors in TRPC4α. The ionic channels formed by TRPC4 appear to be Ca2+-permeable, but there is a considerable discrepancy in the degree of Ca2+ selectivity. Studies with mice lacking TRPC4 suggest an important role for TRPC4 in supporting Ca2+ entry[24]. The defect in Ca2+ entry in TRPC4-/- mice appears to be associated with a reduction in arterial vasorelaxation, vascular permeability in the lung, and neurotransmitter release from thalamic dendrites[25]. Though the expression levels of subunits observed here were not absolutely coupled with their functions, the present results suggested that TRPC4β may be performing some functions in insulin biphasic secretion and that further clarification is needed.
The insulin-secreting INS-1 cell line was established by dispersion of a radiation-induced insulinoma from NEDH rats in 1992[15]. INS-1 cells can respond to glucose and are generally considered to be a β cell model, but an important drawback to this cell line is its polyclonal nature reflected by the presence of glucose-responsive and glucose-unresponsive subpopulations[26]. As was demonstrated here, there was a significant difference in the levels of expression of Ca2+ channel subunits between INS-1 and β cells, which probably reflected differences in the intracellular metabolism and/or secretory pathways. Taken together, these INS-1 cells may not have represented an exclusively insulin-producing β cell line.
The present study systematically identified the expression profile of Ca2+ channel subunits in INS-1 cells and rat pancreatic β cells and quantitatively characterized them by direct comparison. These results will be helpful in advancing the understanding of Ca2+ channel subunits and their roles related to insulin secretion.
The proportion of people with type 2 diabetes has increased throughout the world. It is now recognized that abnormal insulin secretion precedes the onset of type two diabetes. Ca2+ channels have important roles in the progress of insulin secretion by β cells.
Ca2+ channels are composed of several subunits and mainly control Ca2+ influx through different mechanisms because of different patterns of subunit composition. In islet β cells, the expression profile of these subunits has not been systematically investigated. In this study, the authors systematically identified the expression of Ca2+ channels in primary β cells and INS-1, an insulin-secreting rat cell line.
This is the first study to report the expression profile of Ca2+ channel subunits in primary rat pancreatic β cells and INS-1. Furthermore, the real time PCR data suggested that the INS-1 cell line was not an ideal β cell bioelectrical model.
By understanding which types of Ca2+ channels are expressed in rat β and INS-1 cells, this study may have advanced the understanding of the possible functions of various Ca2+ channels in insulin secretion and provided clues to the physiopathology of diabetes.
Ca2+ channels provide pores for the passive diffusion of ions across biological membranes, in particular Ca2+. β cells are the unique cells of insulin production, and the INS-1 is an insulin-secreting cell line established by dispersion of a radiation-induced insulinoma from NEDH rats in 1992.
This is an interesting study, the authors identified the expression profile of Ca2+ channel subunits in the INS-1 cell line and rat pancreatic β cells, by reverse transcription polymerase chain reaction.
| 1. | Cavaghan MK, Ehrmann DA, Polonsky KS. Interactions between insulin resistance and insulin secretion in the development of glucose intolerance. J Clin Invest. 2000;106:329-333. |
| 2. | Mahler RJ, Adler ML. Clinical review 102: Type 2 diabetes mellitus: update on diagnosis, pathophysiology, and treatment. J Clin Endocrinol Metab. 1999;84:1165-1171. |
| 3. | Curry DL, Bennett LL, Grodsky GM. Dynamics of insulin secretion by the perfused rat pancreas. Endocrinology. 1968;83:572-584. |
| 4. | Komatsu M, Yokokawa N, Takeda T, Nagasawa Y, Aizawa T, Yamada T. Pharmacological characterization of the voltage-dependent calcium channel of pancreatic B-cell. Endocrinology. 1989;125:2008-2014. |
| 5. | Ramanadham S, Turk J. omega-Conotoxin inhibits glucose- and arachidonic acid-induced rises in intracellular [Ca2+] in rat pancreatic islet beta-cells. Cell Calcium. 1994;15:259-264. |
| 6. | Gamberucci A, Fulceri R, Pralong W, Bánhegyi G, Marcolongo P, Watkins SL, Benedetti A. Caffeine releases a glucose-primed endoplasmic reticulum Ca2+ pool in the insulin secreting cell line INS-1. FEBS Lett. 1999;446:309-312. |
| 7. | Zhang Q, Köhler M, Yang SN, Zhang F, Larsson O, Berggren PO. Growth hormone promotes Ca(2+)-induced Ca2+ release in insulin-secreting cells by ryanodine receptor tyrosine phosphorylation. Mol Endocrinol. 2004;18:1658-1669. |
| 8. | Bruton JD, Lemmens R, Shi CL, Persson-Sjögren S, Westerblad H, Ahmed M, Pyne NJ, Frame M, Furman BL, Islam MS. Ryanodine receptors of pancreatic beta-cells mediate a distinct context-dependent signal for insulin secretion. FASEB J. 2003;17:301-303. |
| 9. | Togashi K, Hara Y, Tominaga T, Higashi T, Konishi Y, Mori Y, Tominaga M. TRPM2 activation by cyclic ADP-ribose at body temperature is involved in insulin secretion. EMBO J. 2006;25:1804-1815. |
| 10. | Qian F, Huang P, Ma L, Kuznetsov A, Tamarina N, Philipson LH. TRP genes: candidates for nonselective cation channels and store-operated channels in insulin-secreting cells. Diabetes. 2002;51 Suppl 1:S183-S189. |
| 11. | Graham S, Ding M, Sours-Brothers S, Yorio T, Ma JX, Ma R. Downregulation of TRPC6 protein expression by high glucose, a possible mechanism for the impaired Ca2+ signaling in glomerular mesangial cells in diabetes. Am J Physiol Renal Physiol. 2007;293:F1381-F1390. |
| 12. | Dyachok O, Tufveson G, Gylfe E. Ca2+-induced Ca2+ release by activation of inositol 1,4,5-trisphosphate receptors in primary pancreatic beta-cells. Cell Calcium. 2004;36:1-9. |
| 13. | Holmkvist J, Tojjar D, Almgren P, Lyssenko V, Lindgren CM, Isomaa B, Tuomi T, Berglund G, Renström E, Groop L. Polymorphisms in the gene encoding the voltage-dependent Ca(2+) channel Ca (V)2.3 (CACNA1E) are associated with type 2 diabetes and impaired insulin secretion. Diabetologia. 2007;50:2467-2475. |
| 14. | Perez-Reyes E. Molecular physiology of low-voltage-activated t-type calcium channels. Physiol Rev. 2003;83:117-161. |
| 15. | Asfari M, Janjic D, Meda P, Li G, Halban PA, Wollheim CB. Establishment of 2-mercaptoethanol-dependent differentiated insulin-secreting cell lines. Endocrinology. 1992;130:167-178. |
| 16. | van Suylichem PT, Wolters GH, van Schilfgaarde R. The efficacy of density gradients for islet purification: a comparison of seven density gradients. Transpl Int. 1990;3:156-161. |
| 17. | de Haan BJ, Faas MM, Spijker H, van Willigen JW, de Haan A, de Vos P. Factors influencing isolation of functional pancreatic rat islets. Pancreas. 2004;29:e15-e22. |
| 18. | Nitert MD, Nagorny CL, Wendt A, Eliasson L, Mulder H. CaV1.2 rather than CaV1.3 is coupled to glucose-stimulated insulin secretion in INS-1 832/13 cells. J Mol Endocrinol. 2008;41:1-11. |
| 19. | Schulla V, Renström E, Feil R, Feil S, Franklin I, Gjinovci A, Jing XJ, Laux D, Lundquist I, Magnuson MA. Impaired insulin secretion and glucose tolerance in beta cell-selective Ca(v)1.2 Ca2+ channel null mice. EMBO J. 2003;22:3844-3854. |
| 20. | Liu G, Dilmac N, Hilliard N, Hockerman GH. Ca v 1.3 is preferentially coupled to glucose-stimulated insulin secretion in the pancreatic beta-cell line INS-1. J Pharmacol Exp Ther. 2003;305:271-278. |
| 21. | Namkung Y, Skrypnyk N, Jeong MJ, Lee T, Lee MS, Kim HL, Chin H, Suh PG, Kim SS, Shin HS. Requirement for the L-type Ca(2+) channel alpha(1D) subunit in postnatal pancreatic beta cell generation. J Clin Invest. 2001;108:1015-1022. |
| 22. | Platzer J, Engel J, Schrott-Fischer A, Stephan K, Bova S, Chen H, Zheng H, Striessnig J. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell. 2000;102:89-97. |
| 23. | Philipp S, Trost C, Warnat J, Rautmann J, Himmerkus N, Schroth G, Kretz O, Nastainczyk W, Cavalie A, Hoth M. TRP4 (CCE1) protein is part of native calcium release-activated Ca2+-like channels in adrenal cells. J Biol Chem. 2000;275:23965-23972. |
| 24. | Freichel M, Suh SH, Pfeifer A, Schweig U, Trost C, Weissgerber P, Biel M, Philipp S, Freise D, Droogmans G. Lack of an endothelial store-operated Ca2+ current impairs agonist-dependent vasorelaxation in TRP4-/- mice. Nat Cell Biol. 2001;3:121-127. |