Published online Jun 21, 2009. doi: 10.3748/wjg.15.2870
Revised: May 7, 2009
Accepted: May 14, 2009
Published online: June 21, 2009
AIM: To examine the effect of alisol B acetate on the growth of human gastric cancer cell line SGC7901 and its possible mechanism of action.
METHODS: The cytotoxic effect of alisol B acetate on SGC7901 cells was measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Phase-contrast and electron microscopy were used to observe the morphological changes. Cell cycle and mitochondrial transmembrane potential (Δψm) were determined by flow cytometry. Western blotting was used to detect the expression of apoptosis-regulated gene Bcl-2, Bax, Apaf-1, caspase-3, caspase-9, Akt, P-Akt and phosphatidylinositol 3-kinases (PI3K).
RESULTS: Alisol B acetate inhibited the proliferation of SGC7901 cell line in a time- and dose-dependent manner. PI staining showed that alisol B acetate can change the cell cycle distribution of SGC7901, increase the proportion of cells in G0-G1 phase and decrease the proportion of S phase cells and G2-M phase cells. Alisol B acetate at a concentration of 30 &mgr;mol/L induced apoptosis after 24, 48 and 72 h incubation, with occurrence rates of apoptotic cells of 4.36%, 14.42% and 21.16%, respectively. Phase-contrast and electron microscopy revealed that the nuclear fragmentation and chromosomal condensed, cells shrank and attachment loss appeared in the SGC7901 treated with alisol B acetate. Apoptosis of SGC7901 cells was associated with cell cycle arrest, caspase-3 and caspase-9 activation, loss of mitochondrial membrane potential and up-regulation of the ratio of Bax/Bcl-2 and inhibition of the PI3K/Akt.
CONCLUSION: Alisol B acetate exhibits an anti-proliferative effect in SGC7901 cells by inducing apoptosis. Apoptosis of SGC7901 cells involves mitochondria-caspase and PI3K/Akt dependent pathways.
-
Citation: Xu YH, Zhao LJ, Li Y. Alisol B acetate induces apoptosis of SGC7901 cells
via mitochondrial and phosphatidylinositol 3-kinases/Akt signaling pathways. World J Gastroenterol 2009; 15(23): 2870-2877 - URL: https://www.wjgnet.com/1007-9327/full/v15/i23/2870.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.2870
Gastric cancer is one of the most common malignancies in mankind and its incidence and mortality rank first in China[1]. Recent data indicate that the mortality of gastric cancer in China is tending to increase and it severely threatens the health and life of people[2]. At present, the management of gastric cancer mainly includes surgery and chemotherapy, but the curative effect of the existing chemotherapeutic drugs is not good enough and they have numerous side effects. Therefore, it has become a focus to search the drugs capable of preventing and treating gastric cancer and other malignancies.
Herbal medicines are an important source of novel agents with pharmaceutical potential. Alisol B acetate is a major ingredient isolated from Alismatis rhizoma and has been used for urological diseases in traditional Chinese medicine. In recent years, the pharmacological characterization of alisol B acetate has been identified and several biological activities have been defined, such as the inhibitory effects on lipopolysaccharide[3], the inhibition of complementary activity[45] and antibody-mediated allergic reaction[6]. Furthermore, it has been demonstrated that alisol B acetate induces cell death in hepatoma and leukemia cells[78]. Later studies have shown that alisol B acetate induces Bax nuclear translocation and apoptosis in human hormone-resistant prostate cancer PC-3 cells[9].
However, no detailed data are available about the role and mechanisms of alisol B acetate in gastric carcinoma. In order to understand the role and mechanisms of alisol B acetate in the treatment of gastric carcinoma, we investigated the effect of alisol B acetate on the growth of human SGC7901 cells and the underlying intracellular signal transduction pathways involved in regulating apoptosis. We found that alisol B acetate-induced apoptosis is accompanied by the modulation of the Bcl-2 family, mitochondrial dysfunction and activation of caspases; in SGC7901 cells, alisol B acetate induced apoptosis via the mitochondrial death pathway; and the apoptosis induced by alisol B acetate was sensitized through inhibition of the PI3K/Akt signaling pathway.
Gastric adenocarcinoma cell line (SGC7901) was obtained from our laboratory. Alisol B acetate was purchased from Wako Pure Chemical Industries (Osaka, Japan). Methyl thiazolyl tetrazolium (MTT), propidium iodide (PI), Tris-HCl, and Triton X-100 were obtained from Sigma Chemical Company (St. Louis, USA). Monoclonal antibodies to Bax, Bcl-2, Apaf-1 caspase-3 and caspase-9 were purchased from Santa Cruz Biotechnology Incorporation (Santa Cruz, CA, USA). PVDF membrane was obtained from Bio-Rad (CA, USA). Phospho-Akt (Thr308), Akt, PI3K antibodies were procured from Cell Signaling Technology (Beverly, MA, USA).
Cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum, 100 U/mL penicillin and 100 &mgr;g/mL streptomycin at 37°C humidified atmosphere containing 5% CO2. The chemical compounds (alisol B acetate) were dissolved in dimethyl sulfoxide (DMSO), and diluted to appropriate concentrations with culture medium. The final concentration of DMSO in the culture medium did not exceed 0.1%.
Cells were plated at a density of 2 × 103/well in 96-well plates. Twenty-four hours later, cells were treated with alisol B acetate at different final concentrations from 10 to 80 &mgr;mol/L for 24 h or at 30 &mgr;mol/L concentration for the indicated time courses. Control cell cultures were treated with DMSO. After addition of test compounds, 20 &mgr;L MTT was added to each well. Four hours later, 100 &mgr;L of DMSO was added to each well after the medium was removed. Finally, absorbance was detected with an enzyme calibrator at 570 nm and cell viability= (A of study group/A of control group) × 100%. Experiments were done in triplicate. There were six wells for each concentration.
Phase-contrast microscopic studies: SGC7901 cells were grown in 35 mm sterile petri plates and treated with various doses of alisol B acetate for 24 h. Morphological changes were observed under phase-contrast microscope.
Electron microscopy: Cells were cultured with alisol B acetate at a concentration of 30 &mgr;mol/L for 24, 48 and 72 h, then fixed with 2% paraformaldehyde/2% glutaraldehyde in 0.1 mol/L phosphate buffer (pH 7.4), followed by 1% osmium tetroxide. After dehydration, thin sections were stained with uranyl acetate and lead citrate for observation under a JEM 100 CX electron microscope (JEOL, Peabody, NY, USA).
Cells were seeded in 6-well plates and treated with alisol B acetate at 30 &mgr;mol/L concentration for 24, 48 and 72 h. DMSO (0.1%)-treated cells served as control. After treatment, media were discarded. The adherent cells were washed with PBS, and 300 &mgr;L trypsin was added for 5 min at room temperature to detach the cells. Then, the cell suspension was centrifuged at 1500 r/min for 5 min at room temperature. Decanting of all the supernatant was followed by adding 1 mL of 70% methanol to the pellet. After incubation at 4°C for at least 12 h, prior to the samples being analyzed by the flow cytometry (FCM) (Becton Dickinson), 1 mL of cold PI stain solution (20 g/mL PI, 20 g/mL RNase A, and 0.1% Triton X-100) was added to the mixture and it was incubated for 15 min in darkness at room temperature. The samples were analyzed by FCM (BD FACS CantoTM). The results were analyzed by Mod Fit LT 3.0 software.
Changes of mitochondrial membrane potential were monitored by determination of the fluorescence of Rhodamine (Rh)-123. Cells were treated with or without alisol B acetate for the indicated time courses. At the end of treatment, the cells were finally harvested. Rh-123 was added to 1× 106 cells in 5 mL complete growth medium to a final concentration of 5 g/L and cells were incubated at 37°C in the dark for 30 min to allow Rh-123 uptake. Rh-123 loaded cells were washed with ice cold PBS and re-suspended in PBS. Changes in mitochondrial transmembrane potential as a result of mitochondrial perturbation were measured after staining with Rh-123[10]. Ten thousand events were examined by a FACSCAN flow cytometer and data were analyzed with the Macintosh Cell Quest software.
After being treated with alisol B acetate for the indicated periods, the cells were washed with PBS and lysed in a buffer containing 20 mmol/L Tris-HCl, 150 mmol/L NaCl, 1% Triton X-100, 1.5 mmol/L MgCl2, 1 mmol/L NaVO3, 100 mmol/L NaF, 10% glycerol, 1mmol/L EGTA, 10 mmol/L sodium pyrophosphate, and 1 mmol/L phenylmethylsulfonyl fluoride, pH 7.5. Cell lysates were centrifuged at 12 000 ×g for 60 min at 4°C. The protein concentrations were determined using Bio-Rad protein assay (Bio-Rad Laboratories, USA). After SDS-PAGE, proteins were transferred to PVDF membranes for 2 h at 80 mA. The PVDF membrane was treated with TBST containing 50 g/L skimmed milk at room temperature for 2 h, followed by incubation with the first antibodies caspase-3, caspase-9, Apaf-1, Bcl-2, Bax, Akt, P-Akt, PI3K, respectively, at 4°C overnight. After being washed with TBST for 30 min, the corresponding secondary antibody was added and incubated at room temperature for 1 h. The membrane was then washed three times for 15 min each with TBST and visualized with diaminobenzidine. Quantification of protein was detected with a Lumivision IMAGER (Aisin Seiki, Aichi). Each value represents the mean of triple experiments, and is presented as the relative density of protein bands normalized to β-actin.
Data were expressed as mean ± SD. Statistical correlation of data was checked for significance by ANOVA and Student’s t test. Differences with P < 0.05 were considered significant. These analyses were performed using SPSS 11.0 software.
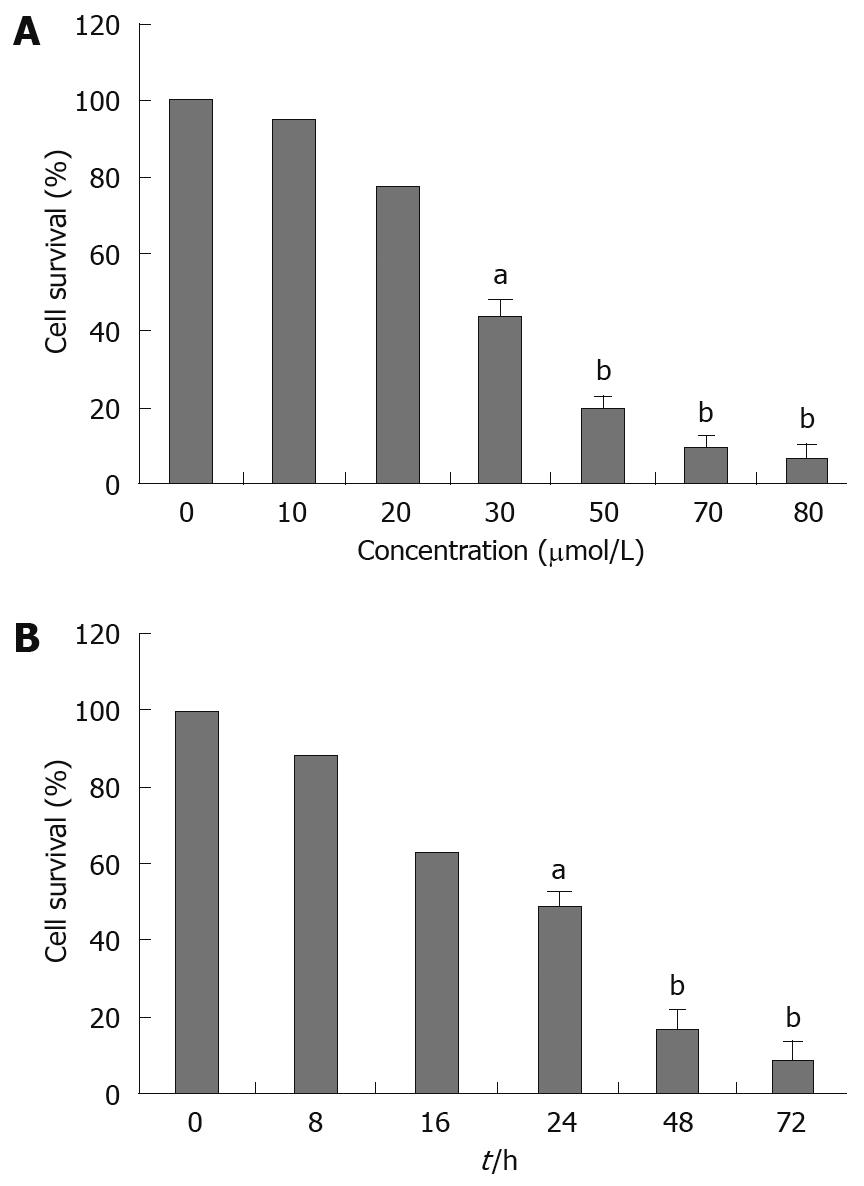
To investigate the growth inhibition effects of alisol B acetate, the cells were treated with various concentrations of alisol B acetate for 24 h and 30 &mgr;mol/L for 8, 16, 24, 48 and 72 h. As shown in Figure 1A, cell viability was decreased remarkably after the cells were treated with 30, 50, 70 and 80 &mgr;mol/L alisol B acetate for 24 h. Only a minor inhibition of SGC7901 cell growth was observed in the presence of 20 &mgr;mol/L alisol B acetate. Growth was inhibited by more than 40% in cells exposed to 30 &mgr;mol/L alisol B acetate after 24, 48 and 72 h. Alisol B acetate had significant growth inhibitory effects on SGC7901 cells in a dose- and time-dependent manner. A concentration of 30 &mgr;mol/L alisol B acetate was used in all further experiments.
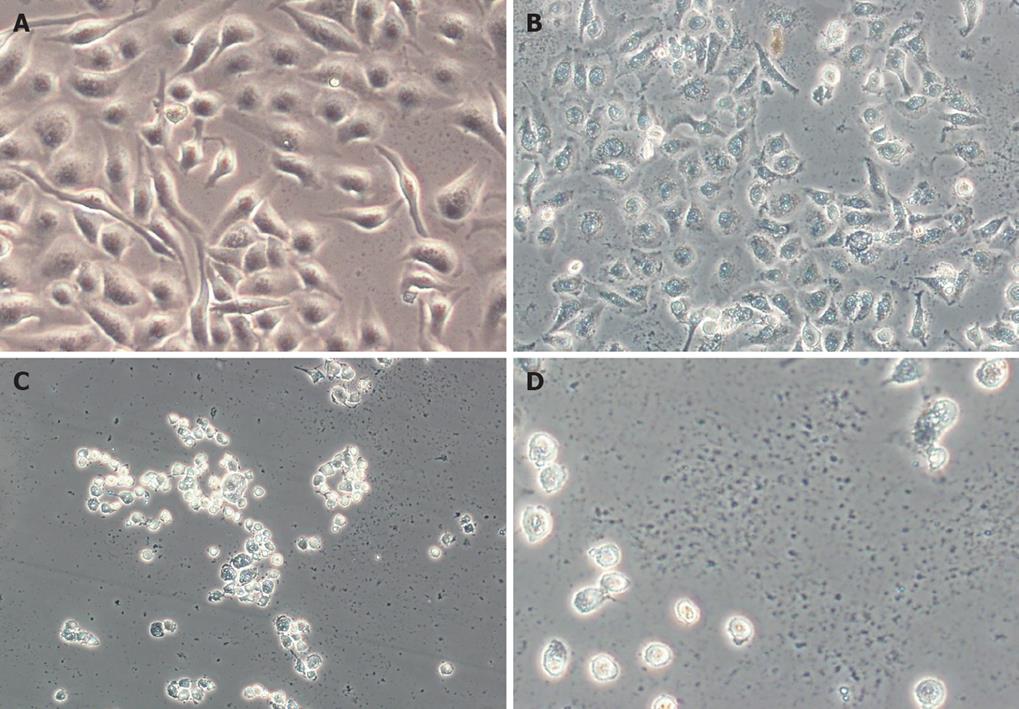
SGC7901 cells were treated with alisol B acetate (0, 30, 50 and 70 &mgr;mol/L) for 24 h, and phase-contrast microscopy revealed that some cells became round, blunt and smaller in size; light refraction was increased; and cells became detached and suspended in the medium, especially with 50 and 70 &mgr;mol/L alisol B acetate. In the control group, cells were regular in morphology and grew fully in patches and confluently, rarely sloughing off (Figure 2).
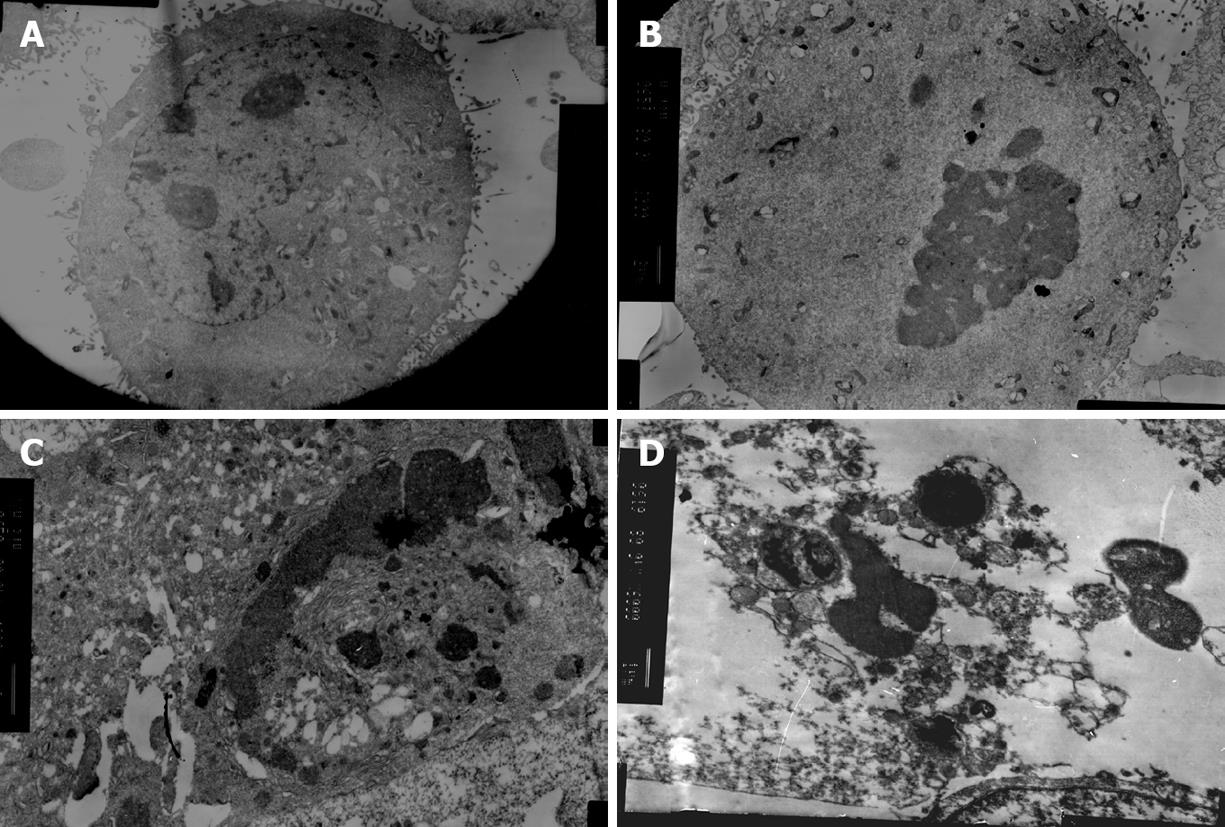
The ultrastructural and morphological changes were also observed under electron microscope. As shown in Figure 3B and C, nuclear fragmentation, chromosome condensation and cell shrinkage were visible. Subsequent formation of apoptotic bodies were also observed (Figure 3D).
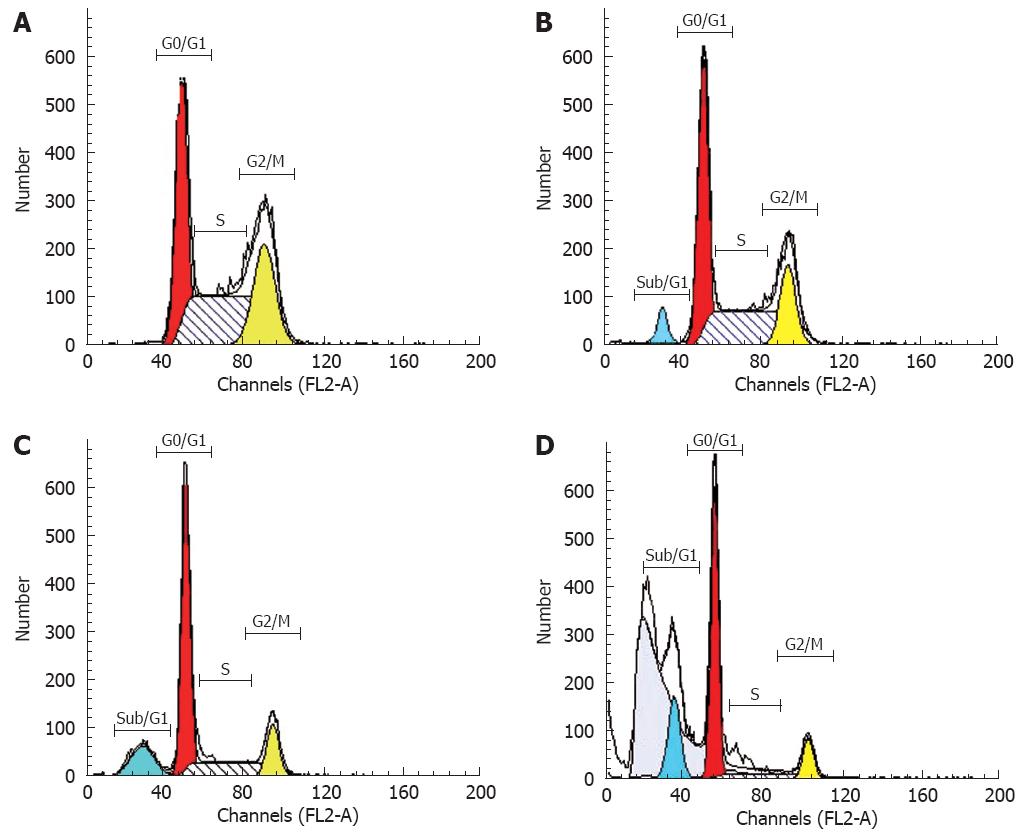
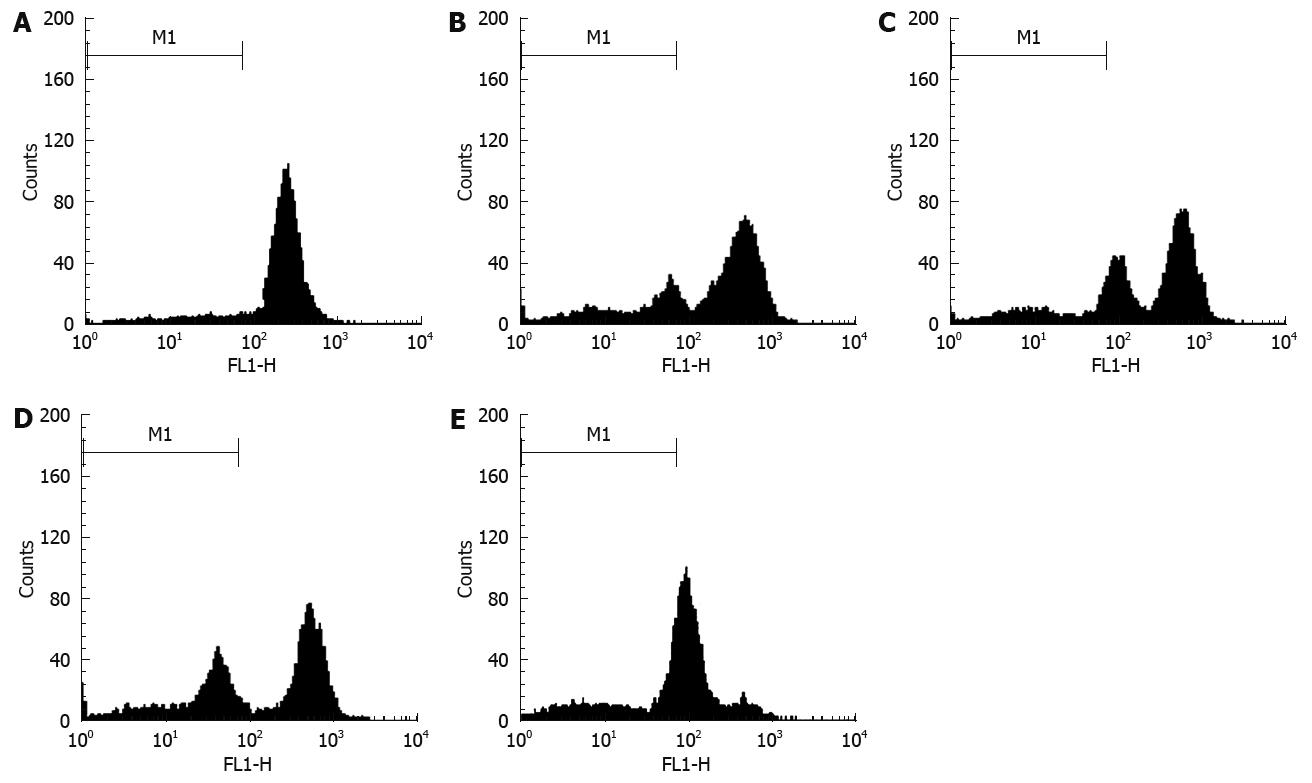
FCM with only PI staining showed (Figure 4) that treatment of SGC7901 cells with 30 &mgr;mol/L alisol B acetate for 72 h resulted in a higher number of cells in the G0/G1 phase (79.61%) compared with the control (40.46%). This increase was coupled with the decreased percentage of cells in S phase. After 72 h treatment, the percentage of S phase in alisol B acetate -treated cells was 16.55%, whereas 48.45% in the control cells. In addition, flow cytometric analysis also revealed the effect of alisol B acetate on the induction of apoptosis. As shown in Figure 4, the percentage of the sub/G1 fraction in alisol B acetate-treated cells was increased in a time-dependent manner, indicative of apoptotic cell death.
Mitochondria played a major role in apoptosis triggered by many stimuli. The early loss of mitochondrial membrane potential is a hallmark of apoptosis[11]. We examined the effect of alisol B acetate on mitochondrial membrane potential by means of the potential sensitive Rh-123. After treatment of SGC7901 cells with 30 &mgr;mol/L alisol B acetate for 12, 24, 48 and 72 h, flow cytometric data revealed that disruption of mitochondrial membrane potential was 17.75%, 29.87%, 43.32% and 70.00%, respectively, while it was only 4.05% in the control group. The data demonstrated that alisol B acetate induced a time-dependent decrease in mitochondrial membrane potential, indicating the participation of mitochondria-related mechanism (Figure 5).
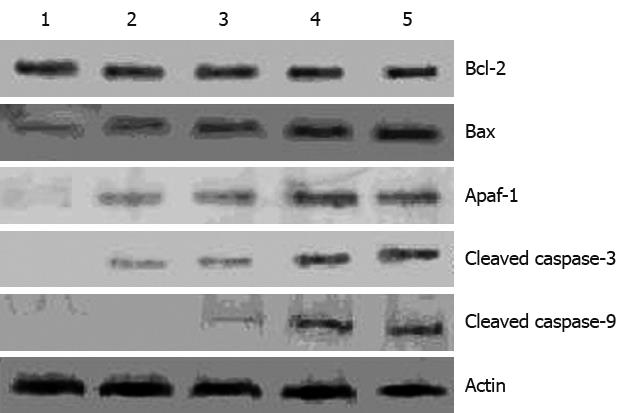
To investigate the mechanism underlying apoptosis induced by alisol B acetate, we tested the effect of this compound on Bcl-2, Bax levels, two important regulators of apoptotic signaling pathways[12]. As shown in Figure 6, Western blotting analysis revealed that alisol B acetate -induced apoptosis did not alter Bcl-2 expression, but resulted in a time-dependent up-regulation of Bax expression, with a maximal up-regulation at 72 h after treatment that was associated with increased levels of Apaf-1, and activated caspases-3 and caspase-9. These results suggest that alisol B acetate induces SGC7901 apoptosis, at least partly, through up-regulation of pro-apoptotic Bax, resulting in up-regulation of the Bax/Bcl-2 ratio and activation of caspase-3 and caspase-9.
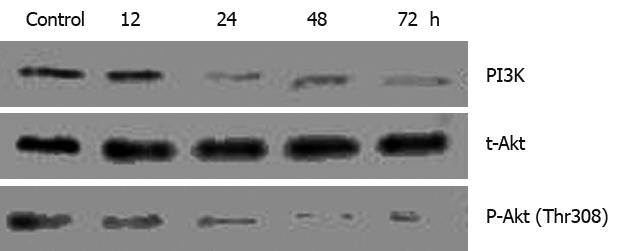
Akt, a major downstream target of PI3K, may be the best-characterized kinase known to promote cellular survival[13] and is dysregulated (mainly overexpressed) in a wide spectrum of human cancers, including gastric, hepatoma, and ovarian cancers[14–16]. To determine whether regulation of the Akt signal pathway is necessary for alisol B acetate-induced apoptosis, we investigated the expression of PI3K and Akt after treatment with 30 &mgr;mol/L Alisol B acetate. As shown in Figure 7, the levels of PI3K and phosphorylated Akt are time-dependently decreased in response to alisol B acetate. PI3K and phosphorylated Akt were rapidly decreased at 24 h, while the total Akt protein levels remained constant during alisol B acetate treatment. Therefore, these results suggested that Akt/PKB is associated with the survival of SGC7901 gastric cancer cells and inhibition of the PI3K/Akt signaling pathway increased the apoptosis induced by the alisol B acetate.
Apoptosis plays an important role in developmental processes, maintenance of homeostasis, and elimination of the damaged cells. Accumulated data indicate that many anticancer drugs can cause the death of tumor cells through the induction of apoptosis[1517]. Alisol B acetate induces cell apoptosis in hepatoma and leukemia cells[78]. However, the effects of alisol B acetate on human gastric cells are still unclear. Therefore, the purpose of the present study was to find out the molecular mechanism of alisol B acetate underlying human gastric cancer cell line SGC7901.
In the present study, we first demonstrated that SGC7901 cells treated with alisol B acetate showed a dose- and time-dependent inhibition of the proliferation. Nuclear fragmentation, chromosome condensation, cell shrinkage and formation of apoptotic bodies were also observed. Flow cytometric analysis revealed that alisol B acetate treatment results in an increase of apoptotic cells. These results suggest that alisol B acetate-induced apoptosis contributes to the growth inhibition of SGC7901 cells.
Mitochondrion plays a critical role in apoptosis induced by some drugs[11]. The mitochondrial death pathway is associated with changes in the permeability of the outer mitochondrial membrane, the collapse of membrane potential[1819]. Mitochondrial membrane permeability is mainly controlled by the Bcl-2 family of proteins, through regulation of the formation of apoptotic protein-conducting pores in the outer mitochondrial membrane[20–22]. Members of the Bcl-2 family proteins can be divided into two subfamilies; one is anti-apoptotic protein such as Bcl-2 and Bcl-XL, the other is pro-apoptotic protein such as Bax, Bad, and Bid[23]. The expression of Bcl-2 and Bax is significantly involved in the balance of pro-apoptotic and anti-apoptotic signals at the mitochondrial level[24]. The ratio of Bax/Bcl-2 is critical for the induction of apoptosis and this ratio determines whether cells will undergo apoptosis[2526]. An increase in the ratio of Bax/Bcl-2 stimulates the release of cytochrome c from mitochondria into the cytosol. The cytosolic cytochrome c then binds to Apaf-1, leading to the activation of caspase-9, caspase-3 and poly (ADP-ribose) polymerase[2728]. Thus, we examined the effect of alisol B acetate on Bax, Bcl-2 and mitochondrial membrane potential. In our experiments, alisol B acetate increased pro-apoptotic Bax expression without affecting Bcl-2 expression, leading to up-regulation of the ratio between pro-apoptotic (Bax) and anti-apoptotic (Bcl-2). This may be responsible for the concomitant execution phase of apoptosis that we observed, which included disruption of mitochondrial membrane potential.
Caspases, a family of cysteine proteases, are integral parts of the apoptotic pathway. Caspase-9 is the apical caspase in the intrinsic or mitochondria-initiated apoptosis pathway and requires the release of cytochrome C from the mitochondria as well as interactions with Apaf-1[18]. Activation of caspase-3 correlated with activation of caspase-9. Caspase-3 in particular, when activated, has many cellular targets[29]. During apoptosis, caspase-3 is one of the key executioners of apoptosis in response to various stimuli[30]. In many studies, it has been determined that a variety of chemotherapeutic agents induce apoptosis through the activation of caspases[15]. Previous studies have observed that alisol B acetate induces apoptosis in human hepatoma Hep3B cells and human hormone-resistant prostate cancer PC-3 cells through an increase of caspase-3 and caspase-9 activity[79]. Consistent with an increase in the ratio of Bax/Bcl-2 and disruption of mitochondrial membrane potential, this study showed that alisol B acetate resulted in a time dependent up-regulation of Apaf-1, and activation of caspase-9 and caspase-3.
According to the present data, alisol B acetate may first increased the ratio of Bax/Bcl-2, which leads to disruption of mitochondrial membrane potential, and then activates mitochondria-mediated downstream molecular events including cytochrome c release and sequential activation of caspase-9 and caspase-3. These results demonstrated that a mitochondrial damage-mediated caspase pathway might be involved in alisol B acetate -induced apoptosis of SGC7901 cells.
The PI3K pathway regulates several cellular processes, including proliferation, growth[31], apoptosis[32] and cytoskeletal rearrangement. Akt, a major downstream target of PI3K, was originally reported as the cellular counterpart of the viral oncogene, which was amplified in gastric adenocarcinoma[14]. Overexpression of Akt isoforms has now been reported in ovarian, breast, prostate, and pancreatic cancers[1633–35]. In addition, Akt activity is increased in cancers where the PTEN tumor suppressor is mutated[36]. In recent years, a number of cancer researchers have focused on the central importance of the PI3K/Akt pathway, and therapeutic strategies that target the PI3K/Akt pathway are now being developed[1537]. PI3K consists of p85 regulatory subunit and a p110 catalytic subunit. Akt is activated by recruitment to the plasma membrane though direct contact of its Plecpstrinhomology domain with PIP3, and phosphorylation at Thr308 and Ser473. Thr308 is phosphorylated by the 3-phosphoinositide-dependent protein kinase PDKI[38]. Through its interaction with proteins in the Bcl-2 family such as BAD, Bax, Bcl-XL and their downstream effectors, Akt provides a survival signal to the cell[39]. Additionally, Akt blocks cytochrome C release from the mitochondria through regulation of Bcl-2[40] and inhibits the catalytic activity of a pro-death protease, caspase 9 through phosphorylation[41].
In our study, we evaluated the effect of alisol B acetate on the PI3K/Akt pathways by measuring the PI3K and total Akt protein and sequential expression levels of phospho-Akt. We observed that alisol B acetate markedly inhibited PI3K and phospho-Akt after 24 h of treatment. No additional reduction in PI3K and phospho-Akt levels was seen when treatment was prolonged to 72 h and the total Akt protein levels remained constant throughout the course of the experiment. Meanwhile, our data showed that alisol B acetate treatment up-regulates Bax protein and down-regulates mitochondrial membrane potential. Furthermore, our data also showed activation of caspase-9 and caspase-3 in alisol B acetate-treated groups. Inhibition of PI3K/Akt signaling pathway is possibly preceded by modulations in Bax protein, leading to mitochondrial damage and activation of caspase-9, caspase-3 in favor of apoptosis.
In conclusion, we have demonstrated that alisol B acetate significantly induces apoptosis. This apoptotic response is associated with the up-regulation of the ratio of Bax/Bcl-2, loss of mitochondrial membrane and caspase activation. Moreover, the inhibition of PI3K/Akt pathway may play an important role in alisol B acetate-induced apoptosis. Therefore, we believe that alisol B acetate might be a promising molecule in cancer chemoprevention or chemotherapy; and further efforts to explore this therapeutic strategy are necessary.
Alisol B acetate is a major ingredient isolated from Alismatis rhizome. In recent years, alisol B acetate has shown various biological activities, including inhibitory effects on lipopolysaccharide, the inhibition of complementary activity, antibody-mediated allergic reaction and anti-tumor effects. However, its molecular mechanisms in the anti-tumor effects have not been well documented.
Studies have confirmed that many Chinese herbs have anti-tumor properties and induce apoptosis. In the process of cell apoptosis induced by drugs, mitochondria plays a great role. phosphatidylinositol 3-kinases (PI3K)/Akt pathway is important in the development and proliferation of various human cancers. In recent years, a number of cancer researchers have focused on the importance of the PI3K/Akt pathway, and therapeutic strategies that target the PI3K/Akt pathway are being developed. In the present work, the authors investigated the effect of alisol B acetate on human SGC7901 cell proliferation, mitochondrial and PI3K/Akt signaling pathways.
The present study shows that alisol B acetate induces apoptosis and decreases proliferation in human gastric cancer cells. It was shown for the first time that alisol B acetate induced human gastric cancer apoptosis by modulation of the Bcl-2 family, loss of mitochondrial membrane potential, activation of caspases and inhibition of PI3K/Akt pathway.
The data of this article demonstrate the anti-cancer properties of alisol B acetate as well as its mechanism of action. By knowing the mechanism of action of alisol B acetate, it may provide a new therapeutic option, as a potential anticancer agent in the treatment of gastric cancer.
PI3K is a major regulator of cell proliferation located in the Akt upstream, and together PI3K and Akt define a signal transduction pathway important in the pathogenesis of many different cancer types. Alisol B acetate is a natural herbal substance.
This is a carefully performed study with novel findings that alisol B acetate has the potential for therapeutic application in gastric cancer. It examines the effect of alisol B acetate on the growth of human gastric cancer cell line and its possible mechanism of action. This paper is well-written. The experiments were well-designed and well-executed.
| 1. | Sun XD, Mu R, Zhou YS, Dai XD, Qiao YL, Zhang SW, Huangfu XM, Sun J, Li LD, Lu FZ. 1990-1992 mortality of stomach cancer in China. Zhonghua Zhongliu Zazhi. 2002;24:4-8. |
| 2. | Sun XD, Mu R, Zhou YS, Dai XD, Zhang SW, Huangfu XM, Sun J, Li LD, Lu FZ, Qiao YL. Analysis of mortality rate of stomach cancer and its trend in twenty years in China. Zhonghua Zhongliu Zazhi. 2004;26:4-9. |
| 3. | Matsuda H, Kageura T, Toguchida I, Murakami T, Kishi A, Yoshikawa M. Effects of sesquiterpenes and triterpenes from the rhizome of Alisma orientale on nitric oxide production in lipopolysaccharide-activated macrophages: absolute stereostructures of alismaketones-B 23-acetate and -C 23-acetate. Bioorg Med Chem Lett. 1999;9:3081-3086. |
| 4. | Lee SM, Kim JH, Zhang Y, An RB, Min BS, Joung H, Lee HK. Anti-complementary activity of protostane-type triterpenes from Alismatis rhizoma. Arch Pharm Res. 2003;26:463-465. |
| 5. | Matsuda H, Tomohiro N, Yoshikawa M, Kubo M. Studies on Alismatis Rhizoma. II. Anti-complementary activities of methanol extract and terpene components from Alismatis Rhizoma (dried rhizome of Alisma orientale). Biol Pharm Bull. 1998;21:1317-1321. |
| 6. | Kubo M, Matsuda H, Tomohiro N, Yoshikawa M. Studies on Alismatis rhizoma. I. Anti-allergic effects of methanol extract and six terpene components from Alismatis rhizoma (dried rhizome of Alisma orientale). Biol Pharm Bull. 1997;20:511-516. |
| 7. | Chou CC, Pan SL, Teng CM, Guh JH. Pharmacological evaluation of several major ingredients of Chinese herbal medicines in human hepatoma Hep3B cells. Eur J Pharm Sci. 2003;19:403-412. |
| 8. | Chen HW, Hsu MJ, Chien CT, Huang HC. Effect of alisol B acetate, a plant triterpene, on apoptosis in vascular smooth muscle cells and lymphocytes. Eur J Pharmacol. 2001;419:127-138. |
| 9. | Huang YT, Huang DM, Chueh SC, Teng CM, Guh JH. Alisol B acetate, a triterpene from Alismatis rhizoma, induces Bax nuclear translocation and apoptosis in human hormone-resistant prostate cancer PC-3 cells. Cancer Lett. 2006;231:270-278. |
| 10. | Scaduto RC Jr, Grotyohann LW. Measurement of mitochondrial membrane potential using fluorescent rhodamine derivatives. Biophys J. 1999;76:469-477. |
| 11. | Liu MJ, Wang Z, Li HX, Wu RC, Liu YZ, Wu QY. Mitochondrial dysfunction as an early event in the process of apoptosis induced by woodfordin I in human leukemia K562 cells. Toxicol Appl Pharmacol. 2004;194:141-155. |
| 12. | Wong WW, Puthalakath H. Bcl-2 family proteins: the sentinels of the mitochondrial apoptosis pathway. IUBMB Life. 2008;60:390-397. |
| 13. | Brazil DP, Hemmings BA. Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem Sci. 2001;26:657-664. |
| 14. | Staal SP. Molecular cloning of the akt oncogene and its human homologues AKT1 and AKT2: amplification of AKT1 in a primary human gastric adenocarcinoma. Proc Natl Acad Sci USA. 1987;84:5034-5037. |
| 15. | Lah JJ, Cui W, Hu KQ. Effects and mechanisms of silibinin on human hepatoma cell lines. World J Gastroenterol. 2007;13:5299-5305. |
| 16. | Cheng JQ, Godwin AK, Bellacosa A, Taguchi T, Franke TF, Hamilton TC, Tsichlis PN, Testa JR. AKT2, a putative oncogene encoding a member of a subfamily of protein-serine/threonine kinases, is amplified in human ovarian carcinomas. Proc Natl Acad Sci USA. 1992;89:9267-9271. |
| 17. | Edderkaoui M, Odinokova I, Ohno I, Gukovsky I, Go VL, Pandol SJ, Gukovskaya AS. Ellagic acid induces apoptosis through inhibition of nuclear factor kappa B in pancreatic cancer cells. World J Gastroenterol. 2008;14:3672-3680. |
| 18. | Martinou JC, Green DR. Breaking the mitochondrial barrier. Nat Rev Mol Cell Biol. 2001;2:63-67. |
| 20. | Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322-1326. |
| 21. | Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647-656. |
| 22. | Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899-1911. |
| 23. | Robertson JD, Orrenius S. Molecular mechanisms of apoptosis induced by cytotoxic chemicals. Crit Rev Toxicol. 2000;30:609-627. |
| 24. | Cory S, Huang DC, Adams JM. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene. 2003;22:8590-8607. |
| 25. | Tang DG, Porter AT. Target to apoptosis: a hopeful weapon for prostate cancer. Prostate. 1997;32:284-293. |
| 26. | Reed JC. Regulation of apoptosis by bcl-2 family proteins and its role in cancer and chemoresistance. Curr Opin Oncol. 1995;7:541-546. |
| 27. | Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng TI, Jones DP, Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129-1132. |
| 28. | Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132-1136. |
| 30. | Tewari M, Quan LT, O'Rourke K, Desnoyers S, Zeng Z, Beidler DR, Poirier GG, Salvesen GS, Dixit VM. Yama/CPP32 beta, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell. 1995;81:801-809. |
| 31. | Klippel A, Reinhard C, Kavanaugh WM, Apell G, Escobedo MA, Williams LT. Membrane localization of phosphatidylinositol 3-kinase is sufficient to activate multiple signal-transducing kinase pathways. Mol Cell Biol. 1996;16:4117-4127. |
| 32. | Kauffmann-Zeh A, Rodriguez-Viciana P, Ulrich E, Gilbert C, Coffer P, Downward J, Evan G. Suppression of c-Myc-induced apoptosis by Ras signalling through PI(3)K and PKB. Nature. 1997;385:544-548. |
| 33. | Tacheau C, Fontaine J, Loy J, Mauviel A, Verrecchia F. TGF-beta induces connexin43 gene expression in normal murine mammary gland epithelial cells via activation of p38 and PI3K/AKT signaling pathways. J Cell Physiol. 2008;217:759-768. |
| 34. | Wang S, Yang Q, Fung KM, Lin HK. AKR1C2 and AKR1C3 mediated prostaglandin D2 metabolism augments the PI3K/Akt proliferative signaling pathway in human prostate cancer cells. Mol Cell Endocrinol. 2008;289:60-66. |
| 35. | Middleton G, Ghaneh P, Costello E, Greenhalf W, Neoptolemos JP. New treatment options for advanced pancreatic cancer. Expert Rev Gastroenterol Hepatol. 2008;2:673-696. |
| 36. | Yamada KM, Araki M. Tumor suppressor PTEN: modulator of cell signaling, growth, migration and apoptosis. J Cell Sci. 2001;114:2375-2382. |
| 37. | Morgan TM, Koreckij TD, Corey E. Targeted therapy for advanced prostate cancer: inhibition of the PI3K/Akt/mTOR pathway. Curr Cancer Drug Targets. 2009;9:237-249. |
| 38. | Stokoe D, Stephens LR, Copeland T, Gaffney PR, Reese CB, Painter GF, Holmes AB, McCormick F, Hawkins PT. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science. 1997;277:567-570. |
| 39. | Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231-241. |
| 40. | Davies MA, Koul D, Dhesi H, Berman R, McDonnell TJ, McConkey D, Yung WK, Steck PA. Regulation of Akt/PKB activity, cellular growth, and apoptosis in prostate carcinoma cells by MMAC/PTEN. Cancer Res. 1999;59:2551-2556. |