Published online Jun 14, 2009. doi: 10.3748/wjg.15.2754
Revised: April 29, 2009
Accepted: May 6, 2009
Published online: June 14, 2009
AIM: To generate an adenoviral vector specifically targeting the EphA2 receptor (EphA2R) highly expressed on pancreatic cancer cells in vivo.
METHODS: YSA, a small peptide ligand that binds the EphA2R with high affinity, was inserted into the HI loop of the adenovirus serotype 5 fiber knob. To further increase the specificity of this vector, binding sites for native adenoviral receptors, the coxsackie and adenovirus receptor (CAR) and integrin, were ablated from the viral capsid. The ablated retargeted adenoviral vector was produced on 293T cells. Specific targeting of this novel adenoviral vector to pancreatic cancer was investigated on established human pancreatic cancer cell lines. Upon demonstrating specific in vitro targeting, in vivo targeting to subcutaneous growing human pancreatic cancer was tested by intravenous and intraperitoneal administration of the ablated adenoviral vector.
RESULTS: Ablation of native cellular binding sites reduced adenoviral transduction at least 100-fold. Insertion of the YSA peptide in the HI loop restored adenoviral transduction of EphA2R-expressing cells but not of cells lacking this receptor. YSA-mediated transduction was inhibited by addition of synthetic YSA peptide. The transduction specificity of the ablated retargeted vector towards human pancreatic cancer cells was enhanced almost 10-fold in vitro. In a subsequent in vivo study in a nude (nu/nu) mouse model however, no increased adenoviral targeting to subcutaneously growing human pancreas cancer nodules was seen upon injection into the tail vein, nor upon injection into the peritoneum.
CONCLUSION: Targeting the EphA2 receptor increases specificity of adenoviral transduction of human pancreatic cancer cells in vitro but fails to enhance pancreatic cancer transduction in vivo.
-
Citation: van Geer MA, Bakker CT, Koizumi N, Mizuguchi H, Wesseling JG, Oude Elferink RP, Bosma PJ. Ephrin A2 receptor targeting does not increase adenoviral pancreatic cancer transduction
in vivo . World J Gastroenterol 2009; 15(22): 2754-2762 - URL: https://www.wjgnet.com/1007-9327/full/v15/i22/2754.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.2754
Pancreatic cancer is a devastating disease with a very poor prognosis[1]. The lack of options for curative treatment for pancreatic cancer and other gastrointestinal malignancies warrants a search for novel targets and novel therapies including gene therapy[23]. Adenovirus has been used widely as a gene therapy vector to treat solid tumors. After initial negative results in clinical trials with non-replicating vectors, conditional replicating adenoviral vectors have been tested in clinical trials recently. However, these have also shown disappointing efficacy[45]. Poor transduction efficiency and specificity of adenoviral vectors appears to be a major problem. This seems to be the result of low expression of the primary receptor involved in adenoviral transduction on the tumors, the coxsackie and adenovirus receptor (CAR). Development of targeted vectors to circumvent CAR-mediated entry therefore seem to be required to increase the therapeutic potential of this approach.
Incorporation of ligands that bind to receptors highly expressed on cancer cells in the fiber HI loop enhance adenoviral transduction efficiency[6]. We have shown that incorporation of an RGD peptide improved transduction in pancreatic cancer[7]. Tumor specificity of RGD, however, is limited. Therefore, we decided to introduce the YSA peptide (YSAYPDSVPMMS) in the HI loop (van Geer, in review). YSA is a ligand for the EphA2 receptor (EphA2R) that is highly expressed on pancreatic cancers[8] and other solid tumors[9–11]. Since the YSA peptide has a high affinity for this receptor, it can be used for in vivo delivery of agents to tissues and tumors expressing the EphA2R[12]. In addition, binding of YSA activates the EphA2R and induces its internalization which may enhance adenoviral uptake. Adenoviral HI loop insertion of the YSA peptide increased the transduction specificity and efficiency both in human pancreatic tumor cell lines and in pancreatic tumor resection specimens in vitro (van Geer, in preparation). In vivo however, the presence of the native binding sites in the adenoviral capsid will compromise specific targeting. Even in vitro, YSA-mediated entry could only be proven upon inhibition of CAR-mediated entry. As we aim to target pancreas cancer in vivo we decided to ablate the native adenoviral binding sites of the YSA-targeted vector.
A highly conserved cluster of amino acids on the adenovirus fiber trimer is involved in CAR binding[13]. Site-directed mutagenesis of amino acids in this region was used to identify mutations that affect CAR binding. Mutations in the AB loop[1415], the DE loop[16], and the FG loop[17] of the fiber knob all abolished CAR binding in vitro.
In addition to CAR, binding to integrins can also mediate adenoviral transduction. The presence of integrins on virtually all normal cells will also limit specific transduction of tumor cells. Removal of the integrin-binding motif RGD from the adenoviral penton base indeed enhances tumor specific targeting of adenoviral vectors[18].
To generate an adenoviral vector that targeted pancreatic cancer in vivo we therefore decided to combine HI loop insertion of the YSA peptide with ablation of the binding sites for CAR and integrin. The specificity of this doubly-ablated retargeted vector (Ad/∆F(FG)∆P-YSA) was determined in vitro and subsequently in vivo.
Anti-fiber monoclonal 4D2 antibody (NeoMarkers, Fremont, California, USA); anti-EphA2R clone D7 (Sigma, Saint Louis, USA), synthetic peptide YSA (Eurogentec, Seraing, Belgium), Basement Membrane Matrix (BD Biosciences, Bedford, MA); luciferase activity was determined using a commercial kit (Promega) and a Berthold luminometer.
HEK293 cells, the established PC cell lines Capan-1 and Hs766-T, the mouse pre-adipocyte cell line 3T3-L1 and the mouse hepatoma Hepa 1-6 cell lines were obtained from the American Type Culture Collection Rockville, Maryland; BxPC-3 and MIA PaCa-2 were obtained from Boehringer Ingelheim (Belgium). The pancreatic carcinoma cell lines (p6.3 and p10.5) were obtained from Dr. E Jaffee, Johns Hopkins University School of Medicine, Baltimore, MD, USA. Human umbilical vein endothelial cells (HUVECs, passage 1-3) were isolated as described[19] and cultured in Medium-199 (GIBCO-BRL, Paisley, Scotland), supplemented with 20% (v/v) fetal bovine serum, 50 &mgr;g/mL heparin (Sigma, St Louis, MO, USA), 6-25 &mgr;g/mL endothelial cell growth supplement (ECGS; Sigma), penicillin (100 IU/mL), streptomycin (100 mg/mL) (GIBCO-BRL). Human fibroblasts (passage < 10) were a gift from the department of Genetic and Metabolic diseases AMC, Amsterdam. Fiber-293 cells expressing adenovirus type 5 fiber protein were used as the packaging cell line as previously described. All cells were cultured in Dulbecco’s minimal essential medium (DMEM) with 10% fetal bovine serum (heat inactivated); L-glutamine (2 mmol/L) and penicillin (100 IU/mL), streptomycin (100 mg/mL) all from Cambrex Bio Science, Walkersville. All cell lines were cultured at 37°C in 10% CO2 atmosphere.
The E1-, E3-deleted adenovirus vector AdHM43 was used for propagation of integrin- and CAR-binding mutated adenovirus[20]. The RGD-peptide coding sequence at the penton base was changed from MNDHAIRGDTFATRAE to MNDTSRAE and the FG-loop of the fiber was deleted (T489, A490, Y491, T492). The cytomegalovirus (CMV) immediate-early promoter controlled enhanced green fluorescent protein (eGFP) and the CMV-controlled luciferase gene were inserted between the PI-Sce and Ceu-I sites of the pAdHM43 plasmid (van Geer, in review). Insertion of the YSA peptide (YSAPDSVPMMS) into pAdHM43-CMV-GFP and CMV/Luc was performed by digestion with BstB1 and ligation with 2 annealed primers: YSA forward: CGAAGTACAGCGCCTACCCCGACGGCGTGCCCATGATGT. YSA reverse: CGACATCATGGGCACGCTGTCGGGGTAGGCGCTGTACTT. Clones identified by restriction enzyme analysis and PCR were sequenced to exclude mutations.
Recombinant ablated adenoviral vectors were generated by transfection of HEK 293 adeno fiber-expressing cells with PacI-linearized Ad-CMV-GFP/Luc-YSA. Normal HEK 293 cells were used for the last propagation round as previously described[20]. Adenovirus was purified and concentrated by performing 2 cesium chloride gradients and dialyzing against PBS. Glycerol was added to a final concentration of 10% (v/v) and virus preparations were stored at -80°C.
Modification of viral genomes were verified by PCR and sequencing using the following primers: fiber-forward: CAAACGCTGTTGGATTTATG; fiber-reverse: GTGTAAGAGGATGTGGCAAAT; RGD forward: TTGGATGTGGACGCCTAC; RGD reverse: AGGTGTCGCCGCGAATGGC.
The anti-fiber monoclonal 4D2 antibody (NeoMarkers, Fremont, California) was used to confirm proper trimerization of the fiber[21]. The number of viral genomic copies (gc) was determined by qPCR as previously described[22].
Expression of EphA2R was studied with Western blotting using a 1:1000 dilution of a monoclonal antibody (anti-EphA2R clone D7, Sigma, Saint Louis, USA) and a peroxidase-conjugated anti IgG secondary antibody (1:2500). Cell lysates were prepared in 25 mmol/L Tris HCl pH 7.6, 150 mmol/L NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS containing a cocktail of protease inhibitors (1:250, Roche). The protein concentration was determined with a BCA assay kit (Sigma). Detection was performed using the Lumi-light plus Western Blotting Substrate Kit (Roche, Mannheim, Germany). For receptor localization studies pancreatic tumor cells were gown on cover slips and were fixated 2 d later and stained for EphA2R using a 1:500 of the monoclonal antibody and a 1:2500 peroxidase-conjugated IgG secondary antibody.
5.0 × 104 cells were plated in 48-well plates and allowed to adhere overnight at 37°C. The next day virus was added (500 or 1000 VP/cell) in 2% DMEM. Blocking was performed by pre-incubating the cells with blocking agents in PBS for 20 min at room temperature. EphA2R was blocked with the synthetic peptide YSA (Eurogentec, Seraing, Belgium). For the bi-specific antibody experiments, 500 gc of virus were incubated for 60 min with 2.5 &mgr;L scFv bi-specific antibody (a kind gift from Dr. VW van Beusechem[23]).
1.0 × 107 Capan-1 pancreatic cancer cells were 1:1 diluted with Basement Membrane Matrix (BD Biosciences, Bedford, MA) and injected subcutaneously in both flanks of 6-9 wk old female athymic NMRI nu/nu mice (Harlem). When the tumor nodule reached a diameter of 0.4 to 0.7 cm, mice were injected intravenously (i.v.) or intraperitoneally (i.p.) with 1.0 × 1011 gc of Ad-/∆F(FG)∆P or Ad-/∆F(FG)∆P-YSA in 100 &mgr;L PBS. Blood was sampled by orbital puncture at 10 min after i.v. injection or at 90 min after i.p. injection. Three days after injection, mice were sacrificed and organs were harvested, snap frozen and used to determine luciferase activity according to standard procedures (Promega), using a Berthold luminometer. Luciferase activity was normalized for protein content. Viral DNA in blood was purified as described previously[24] and gc were quantified by qPCR using the primers against the CMV promoter (forward: AATGGGCGGTAGGCGTGTA, reverse AGGCGATCTGACGGTTCACTA). Serum aspartate aminotransferase (ASAT) and alanine aminotransferase (ALAT) levels were determined using standard clinical chemistry methods.
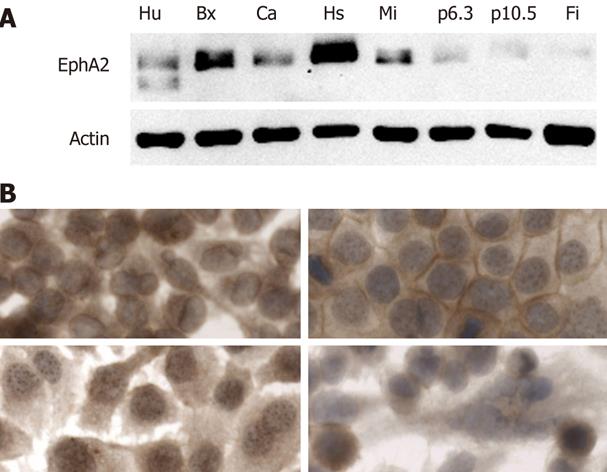
The EphA2R is highly expressed on most solid tumors including pancreatic cancer. To investigate which cell lines are suitable as a model to study targeting, we determined its expression on a panel of established human pancreatic cancer cells and normal human cells such as fibroblasts and endothelial cells. The expression level of the EphA2R varied significantly in human pancreas cancer cell lines (Figure 1A). High expression was seen in Capan1, BxPc3, Hs766T and MIA PaCa-2, while expression in p6.3 and p10.5 was low. Of the normal cells, only human endothelial cells expressed EphA2R. Since this receptor is absent on normal human fibroblasts, we used these as negative control cells.
To investigate whether the EphA2R was accessible, we determined its localization in the cell lines highly expressing this receptor. The localization of this receptor differed between cell lines (Figure 1B). In Capan-1 and BxPc-3 cells, the EphA2R was detected on the membrane while in MIA PaCa-2, it was mostly seen in the cytoplasm. The signal seen in Hs766-T suggests that, in this cell line, the EphA2R is present in nuclear granules. Since, in Capan-1 cells, the presence of the EphA2R on the membrane indicates that it is accessible for YSA binding, we chose to use this cell line for our subsequent studies.
We decided to use the FG loop mutation to ablate CAR binding because it has been well characterized[1725]. By subsequent deletion of the RGD peptide from the penton base we obtained a doubly-ablated vector Ad-/∆F(FG)∆P which lacked both CAR and integrin binding. We inserted the YSA peptide in the HI loop of this doubly-ablated vector and generated Ad-/∆F(FG)∆P-YSA.
The lack of binding to native cellular receptors severely impairs the cellular entry of this doubly-ablated vector. Therefore, it can not be propagated on normal HEK 293 T cells. To overcome this, we used 293T cells that express the adenovirus type 5 fiber protein[20]. Incorporation of the normal fiber expressed in trans into the ablated vector allowed efficient cell entry and vector production. The last round of virus propagation was on normal 293T cells to generate the doubly-ablated vector. Because of impaired cell entry, the infectious particle (ip) titers of ablated vector stocks could not be preformed by standard procedures. To determine the functional titer of these vectors we therefore used a bi-specific antibody[23]. This antibody binds to the adenovirus knob and the epidermal growth factor receptor (EGFR). Upon binding to this antibody, the vector will enter the cells via the EGFR only. Therefore, this antibody allows direct comparison between the ip/gc ratio of the ablated and of the retargeted vectors.
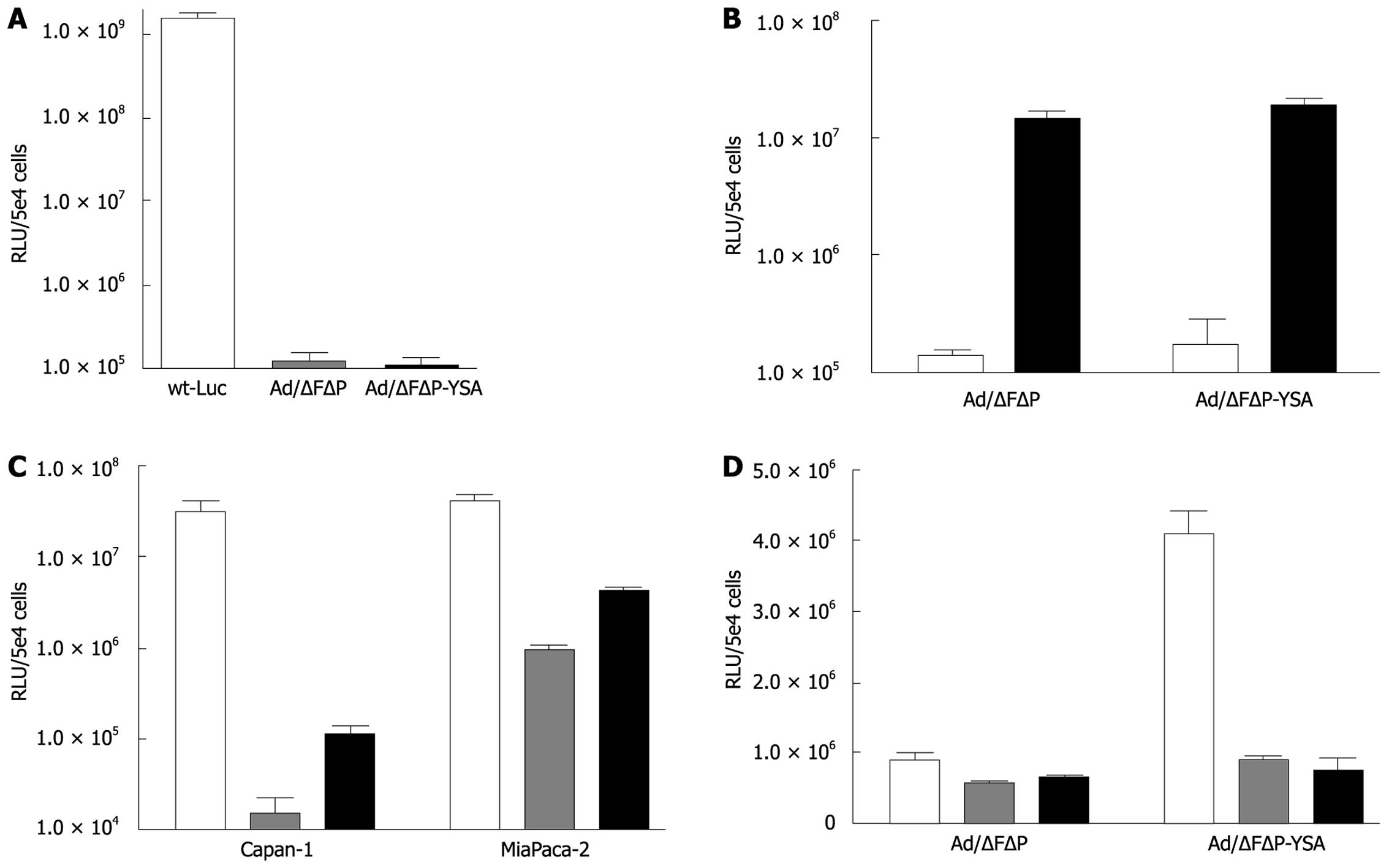
To confirm the absence of CAR- and integrin-mediated entry, we compared the transduction efficiency of wild-type adenovirus type 5 with that of Ad-/ΔFΔP and Ad-/ΔFΔP-YSA on A549 cells that do express CAR but not EphA2R. The 3 to 4 log lower transduction by both ablated vectors confirmed efficient abolition of binding to the native receptors (Figure 2A). As expected, the transduction efficiency of both vectors on normal human fibroblasts that do not express the EphA2R was comparable but very low: 1.42 × 105 RLU for Ad-/∆F(FG)∆P and 1.77 × 105 RLU for Ad-/∆F(FG)∆P-YSA (P = 0.06) (Figure 2B). To confirm viability of the ablated vectors, we incubated both vectors with the bi-specific antibody, which resulted in a 2 log increase of transduction efficiency for both vectors, Ad-/∆F(FG)∆P: 1.5 × 107 RLU and Ad-/∆F(FG)∆P-YSA: 1.9 × 107 RLU. The comparable transduction by both in the presence of the antibody indicated that both had a comparable ip/gc ratio (P = 0.094) and were viable.
To determine if insertion of the YSA peptide was functional, we tested transduction of both vectors on EphA2R-expressing Capan-1 and MiaPaca-2 cells. Although both cell lines do express integrins[7], ablation of native binding sites reduced their transduction by 3 to 1.5 logs. Insertion of the YSA peptide increased gene transfer to Capan-1 cells by 7.6-fold (P = 0.0014) and to MIA PaCa-2 cells by 4.5-fold (P < 0.0001). Insertion of the YSA peptide did not increase transduction in Hs766-T cells which only showed nuclear EphA2R staining (not shown). To confirm that entry of the retargeted vector was mediated by the YSA peptide, we performed competition experiments. As shown in Figure 2D, the 4-fold increased transduction efficiency of Ad-/∆F(FG)∆P-YSA compared to Ad-/∆F(FG)∆P was lost upon pre-incubation with synthetic YSA peptide. This indicated that the increased efficiency of the retargeted vectors was indeed mediated by the inserted YSA peptide. Thus, HI loop insertion of the YSA peptide in an adenovirus that lacks binding to CAR and integrins, resulted in cell entry via the EphA2R. Therefore, this vector appeared suitable for in vivo targeting of pancreatic cancer.
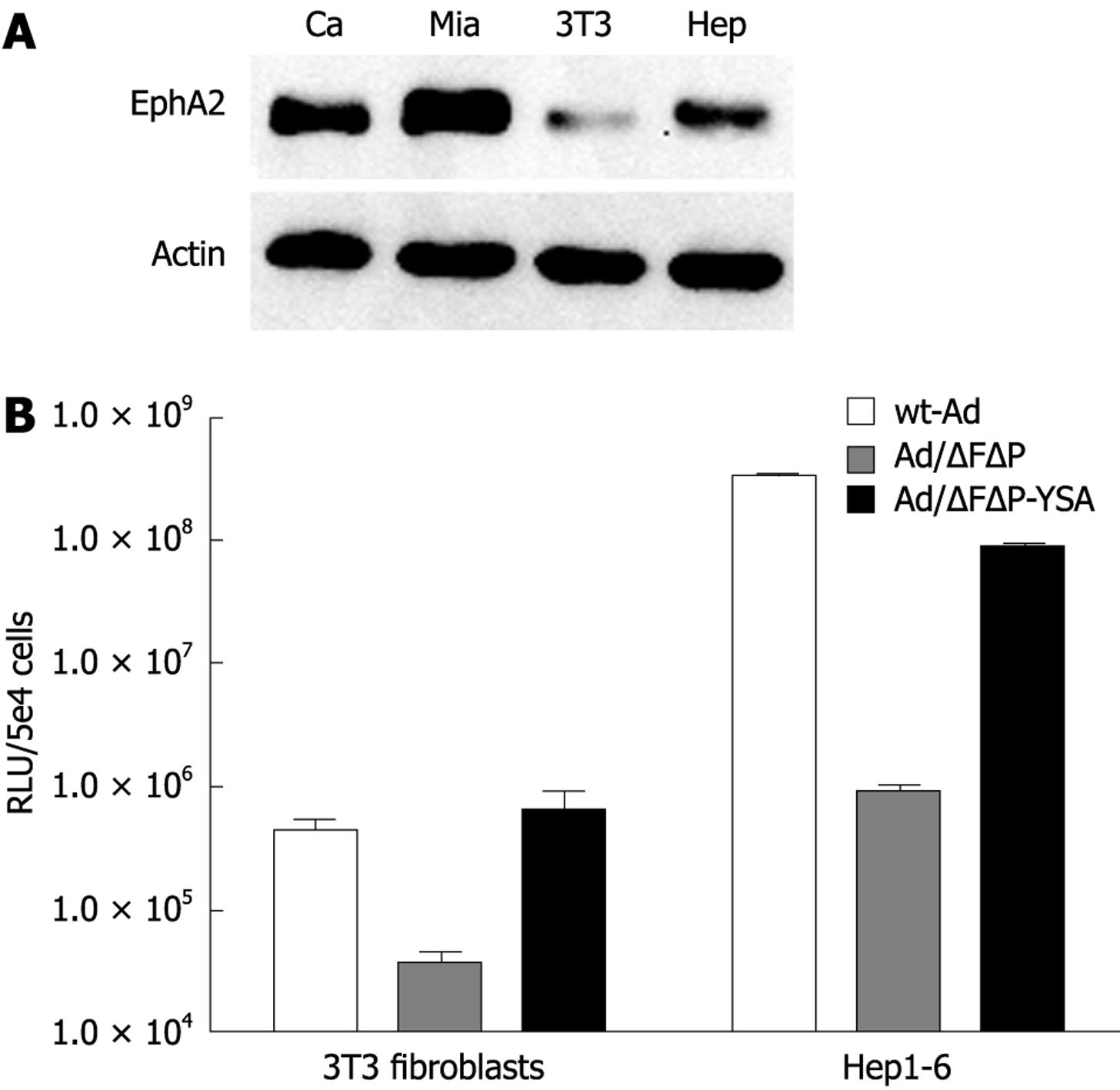
Targeting of the EphA2R by the YSA peptide has only been demonstrated in humans. Binding of the YSA peptide to the murine EphA2R has not been studied. Since binding to EphA2R expressed on activated (murine) endothelial cells will affect targeting of pancreatic cancer in a mouse model, we therefore decided to investigate the transduction efficiency of the YSA-retargeted adenoviral vector to mouse cells expressing the EphA2R. As shown in Figure 3A, mouse 3T3 fibroblasts and hepatoma cells (hepa1-6) both expressed the murine EphA2R, albeit at a lower level than in the human pancreatic cancer cell lines Capan-1 and MIA PaCa-2. Ablation of native cell binding sites reduced adenoviral transduction of these 2 mouse cell lines by 1 to 2 logs (Figure 3B). Insertion of the YSA peptide completely rescued cell entry of the ablated vector. Compared to the ablated vector, gene transfer of the YSA-retargeted vector was enhanced 17-fold in 3T3 cells and 95-fold in Hepa 1-6 cells. This efficient transduction of cells expressing the mouse EphA2R rendered the mouse a suitable model to study the efficiency of YSA-mediated targeting in vivo.
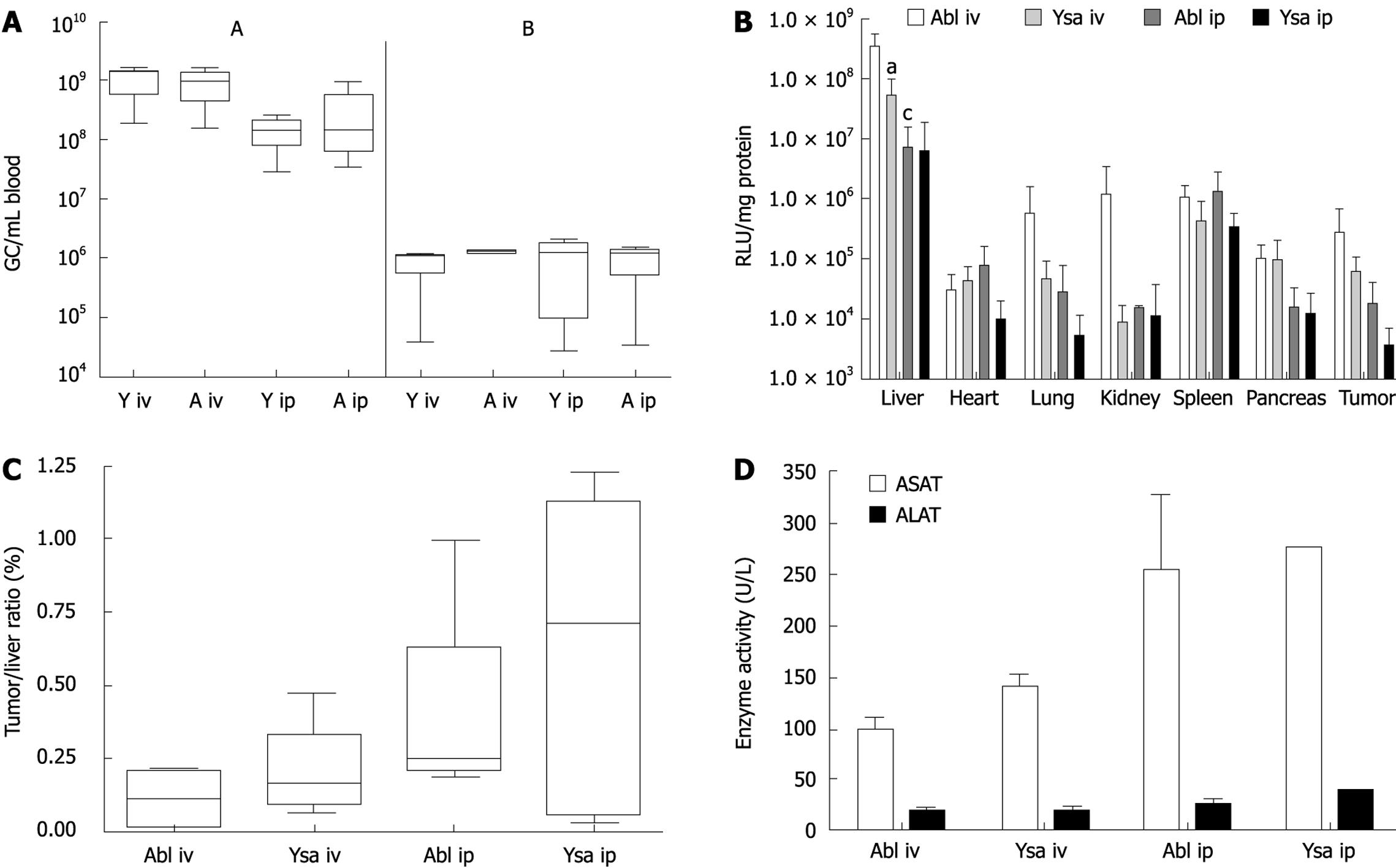
To study in vivo targeting we used a nu/nu mouse model with subcutaneously growing human pancreatic tumor nodules. The virus was injected into mice which had nodules with a diameter of 0.4-0.7 cm, within 3 wk after injection of Capan-1 cells. We administered 1.0 × 1011 gc of ablated [Ad-/∆F(FG)∆P] or of the redirected vector Ad-/∆F(FG)∆P/-YSA, via the tail vein or i.p. Upon i.v. injection the clearance of both vectors was comparable. At 10 min after injection, 9.4 × 108± 6.3 × 108 gc/mL of Ad/∆F(FG)∆P and 1.1 × 109± 5.6 × 108 gc/mL of Ad/∆F(FG)∆P-YSA were present in blood. Since the total blood volume in a mouse is approximately 2.5 mL, the initial concentration of the virus was 4 × 1010 gc/mL. Thus 95% of the injected virus was cleared within 10 min. Based on the literature, we expected a slower clearance after i.p. injection, and determined the amount of virus in blood after 90 min. No significant difference was seen after 90 min between Ad-/∆F(FG)∆P-YSA (1.4 × 108± 8.3 × 107 gc/mL) and Ad-/∆F(FG)∆P (3.2 × 108± 4.3 × 108 gc/mL) (P = 0.3). Thus clearance for both vectors after i.p. injection was also comparable. Clearance of i.p.-injected virus was also efficient since less than 0.5% of the injected dose was present in blood after 90 min.
All mice were sacrificed 72 h after injection. Tissues were harvested and snap frozen in liquid nitrogen to determine adenoviral transduction by determining luciferase expression. After i.v. injection, expression of luciferase in the liver of Ad/∆F(FG)∆P-YSA treated mice was 6.7-fold lower (P = 0.015) than that in mice injected with Ad/∆F(FG)∆P (Figure 4B). Thus, the YSA peptide impaired liver transduction. No significant differences in expression were seen between both vectors in all other tissues. Surprisingly, the amount of luciferase expression in mice injected with Ad-/∆F(FG)∆P-YSA was only 15% of that in mice injected with Ad-/∆F(FG)∆P. This indicated that a large amount of the redirected vector was lost upon i.v. injection. The difference in luciferase expression between both vectors was not seen after i.p. injection. This is due to the almost 50-fold lower luciferase expression in the liver after i.p. injection of Ad-/∆F(FG)∆P (P = 0.0163). For Ad/∆F(FG)∆P-YSA, the luciferase levels in the liver after i.p. injection were only reduced 9-fold and did not reach significance. The lower luciferase expression levels indicated that both vectors were cleared more efficiently without transduction after i.p. injection. To correct for the lower overall expression we chose to use the tumor liver ratio in each animal as an indication of retargeting efficiency. As shown in Figure 4C, no significant targeting of the tumor was seen with the YSA-redirected virus. Furthermore, the route of administration also did not affect tumor targeting.
Adenoviral vectors cause inflammation of the liver. Therefore we determined ALAT and ASAT levels 3 d after injection. Although we did see a 4 to 5-fold increase, no differences were seen between the 2 viral vectors (Figure 4D).
Conditional replicating adenovirus vectors are being developed to treating solid cancers[45]. However, these vectors have only been effective after direct injection into the tumors. As cancer is a systemic disease in virtually all fatal cases, this lack of systemic efficacy presents a major limitation to successful adenovirus-mediated gene therapy. Specific targeting therefore is a prerequisite for efficient eradication of solid cancer cells.
The aim of this study was to target the adenovirus to pancreatic cancers. Effective targeting in vitro to human cancer cells using HI loop insertion of peptide ligands has been reported by other groups. A well known example is the insertion of an integrin-binding RGD peptide that overcomes the poor transduction of human cancer caused by low expression of CAR[72627]. Although integrins are highly expressed on cancer cells, the specificity of RGD targeting in vivo is questionable because of integrin expression in other tissues. Therefore more specific targeting peptides are needed. We compared binding of several ligands to receptors highly expressed on pancreatic cancers for their ability to target the adenovirus to pancreatic cancer cells (van Geer et al[12] in preparation). Of these ligands the YSA peptide appeared the most promising since it provided selective targeting in vitro to the EphA2R. Since YSA targets tissues expressing the EphA2R, we decided to test the specificity this retargeted vector in vivo.
Insertion of a peptide into the HI loop does not shield the native cell binding sites present in the adenoviral capsid. Binding of the retargeted vector to these receptors present on liver cells for instance, will limit specific targeting[17]. Since ablation of these native binding reduces liver transduction and improves specific targeting in vivo[14], we combined YSA targeting with ablation of the native binding sites. We showed that ablation of CAR and integrin binding reduced adenoviral transduction by at least 2 logs in vitro. Insertion of the YSA peptide partly restored the transduction efficiency of the virus, but only on cells that expressed the EphA2R. Addition of synthetic YSA peptide blocked the increase in transduction efficiency. Together these data demonstrated that insertion of YSA enables EphA2R-mediated entry of ablated adenoviral vectors. Other groups have also reported that the loss of infectivity of ablated vectors can be restored by insertion of a targeting ligand in the HI loop[171828]. However, not all retargeted vectors do provide efficient transduction. Insertion of RGD in a vector in which the CAR binding site was ablated in the KO1 mutation did not result in integrin-mediated uptake[29]. Apparently, in addition to preventing CAR binding, this mutation affected other essential steps such as internalization or trafficking of the adenovirus. In conclusion, our data and that of others show that HI loop insertion of a targeting peptide results in specific targeting of CAR/integrin-ablated adenoviral vectors in vitro.
Expression of the EphA2R is also enhanced in several normal tissues including tumor endothelium. Therefore, we investigated whether the YSA peptide also mediated adenoviral transduction via the mouse EphA2R. Since insertion of the YSA peptide increased the transduction of 2 mouse cell lines expressing the EphA2R by 1 to 2 logs, the YSA-retargeted vector was capable of targeting the mouse endothelium. The increase in transduction of mouse cells was stronger than in human pancreatic cancer cell lines while expression of the EphA2R in mouse cells was lower. This discrepancy seems to result from better accessibility of the EphA2R in mouse cells since, in human pancreatic cancer cells, most of the EphA2R was present in the cytosol (Figure 1A). Another possible explanation for this discrepancy is the expression of an inactive EphA2R in cancer cells. Since EphA2R activation impairs survival, cancer cells with an inactive receptor will have a growth advantage[11]. A third explanation could be a higher affinity of the Ad-/∆F(FG)∆P-YSA for the mouse EphA2R. Nevertheless, the efficient transduction of mouse cells expressing EphA2R renders the mouse a good model to study YSA-mediated targeting of pancreatic cancer in vivo.
After intravenous injection, luciferase expression in the liver of the YSA-retargeted vector Ad-/∆F(FG)∆P-YSA was lower than that by the ablated virus (Figure 4B). A decreased transduction of the liver was also reported in other studies in which the FG loop has been mutated. Thus, it seems that FG loop mutations lead to de-targeting of the hepatocytes[17]. In contrast, mutations in the AB loop did not decrease liver transduction[1830]. These studies indicate that liver de-targeting occurred irrespective of the nature of the inserted peptide sequence. In our study, the decreased liver transduction due to YSA-retargeting was not accompanied by an increased transduction of any other tissue tested. Therefore, the increased loss of the redirected virus seems to result from degradation by tissue macrophages, which degrade more than 90% of injected adenoviruses. Since these cells do not express the EphA2R this would appear to be a non-specific effect.
In contrast to the receptor-mediated transduction of hepatocytes, uptake of adenovirus by macrophages depends on the binding of adenovirus fiber to blood factors. This induces uptake of adenovirus, for instance via the scavenger receptor[3132]. Increased binding to blood factors of the mutated FG loop may cause the 10-fold greater loss of re-targeted adenovirus upon i.v. injection by increasing its degradation by macrophages. This may cause a lack of tumor targeting by Ad-/∆F(FG)∆P-YSA in vivo.
Akiyama et al[25] reported that after i.p. injection, a comparable ablated adenoviral vector efficiently entered the blood stream. Furthermore, they showed prolonged blood circulation and absence of hepatocyte transduction. Based on this, i.p. injection seemed a promising approach for systemic targeting. In our study, we could not repeat this observation. At 90 min after injection, less than 1% of the injected dose of vector was still present in the circulation while in contrast they still detected 20%. Increased uptake by macrophages may explain this discrepancy[33]. We used nu/nu mice to study retargeting of adenovirus while Akiyama et al reported a prolonged circulation time in normal mice. Several old studies have reported increased phagocytosis in nu/nu mice compared to normal mice to compensate for their immune defects[3435]. A high dose of adenovirus can saturate the uptake by macrophages residing in the peritoneum and liver, and result in appearance of the virus in the circulation[36]. Apparently, the dose used in this study was too low to saturate the increased macrophage clearance capacity in nu/nu mice. The increased liver enzymes in serum after i.p. injection indicated increased macrophage uptake (Figure 4D). This increase was not observed for this ablated vector in normal mice. The increased uptake by macrophages in nu/nu mice rendered this model less suitable for adenoviral targeting studies[30]. Inhibition of phagocytosis therefore seems to be required to use this model for studying retargeting of ablated adenoviral vectors, e.g., by pre-injection of a small dose of virus[37].
For both administration routes, the luciferase expression in subcutaneously growing Capan-1 tumors was not significantly different between re-targeted and ablated vectors. The transduction of pancreatic cancer by our ablated vector suggests that adenoviral uptake can be mediated by other receptors. The role of additional receptors in Capan-1 cells is in accordance with Havenga et al[38]. They observed that gene transfer in these cells did not correlate with expression levels of CAR and/or integrins. In contrast, transduction by non-ablated adenoviral vectors was strongly impaired by loss of CAR expression[739]. Apparently, removal of native cell binding sites is compensated by other low affinity binding sites, such as heparan sulphate proteoglycans[40]. The expression of the 2 most prominent proteoglycans, glypican-1 and syndecan-1, is indeed enhanced in pancreatic cancers. This may explain the efficient transduction of the tumor by the ablated vector[4142]. Pancreatic cancer therefore seems susceptible to blood factor-mediated transduction by adenovirus as has been reported for herpes virus also[43]. Therefore, ablation of the sites in the fiber knob that bind to blood factors may be required to re-direct the adenovirus to cancer cells in vivo. Furthermore, studies to determine binding to (human) blood components of modified vectors are essential for predicting their in vivo efficacy. Binding to blood factors may explain why ablated vectors with a peptide insertion fail to target tumors following intravenous injection[644], while they do perform properly upon local injection[252845].
In conclusion, we have generated a doubly-ablated virus that targets pancreatic cancer cells via the EphA2R. However, in vivo targeting remains inefficient as yet. Most likely, further modification of the Ad capsid is necessary to prevent binding to blood factors which lowers gene transfer to the liver[3046].
The incidence of pancreatic adenocarcinoma is increasing in the Western world for unknown reasons. Due to its late diagnosis the prognosis for pancreatic cancer is very poor. Novel treatments such as adenovirus mediated gene therapy are needed to improve this.
Adenoviral vector have been widely applied to treat solid tumors. Although the results in animal models were promising the results in subsequent clinical trials were disappointing due to limited transduction of the tumors. Poor transduction of cancer cells was mainly caused by their low expression of coxsackie and adenovirus receptor (CAR), the receptor that mediates adenoviral entry in to the cell.
Retargeting of adenoviral vectors can be used to circumvent CAR mediated entry and can improve the transduction of human cancer cells. In this study, the authors targeted adenoviral vectors to the EphA2 receptor that is highly expressed on pancreatic cancer cells in vitro and in vivo. To further improve specific targeting to cancer cells they removed the regions in the adenoviral capsid that mediate transduction of the liver. In vitro, this strategy indeed proved very specific. The results in a nude mouse model were however disappointing most likely due increased uptake of the retargeted vector by macrophages, a route that is enhanced in these mice due to a compensatory mechanism for loss of other immune responses.
The authors show that adenovirus can be targeted to the EphA2 receptor in vitro. However to allow application in vivo further ablation of endogenous adenobinding sites seem needed.
EphA2 or ephrine A2 receptor is a receptor involved in embryogenesis and is upregulated in several solid tumors. Adenovirus has a icosahedral symmetry with 12 fibers spiking out at all corners. The adenofibers are trimeric proteins. At the end they form a knob with a peptide stretch exposed to the surface, the HI-loop.
The goal of this study was to generate an adenoviral vector specifically targeting the EphA2 receptor, which is highly expressed on pancreatic cancer cells in vivo by first using an in vitro model. The methodology incorporated into this particular study was sound and that their findings were novel.
| 1. | Boeck S, Hinke A, Wilkowski R, Heinemann V. Importance of performance status for treatment outcome in advanced pancreatic cancer. World J Gastroenterol. 2007;13:224-227. |
| 2. | Ma WW, Hidalgo M. Exploiting novel molecular targets in gastrointestinal cancers. World J Gastroenterol. 2007;13:5845-5856. |
| 3. | Tanaka T, Kuroki M, Hamada H, Kato K, Kinugasa T, Shibaguchi H, Zhao J, Kuroki M. Cancer-targeting gene therapy using tropism-modified adenovirus. Anticancer Res. 2007;27:3679-3684. |
| 4. | Mulvihill S, Warren R, Venook A, Adler A, Randlev B, Heise C, Kirn D. Safety and feasibility of injection with an E1B-55 kDa gene-deleted, replication-selective adenovirus (ONYX-015) into primary carcinomas of the pancreas: a phase I trial. Gene Ther. 2001;8:308-315. |
| 5. | Hecht JR, Bedford R, Abbruzzese JL, Lahoti S, Reid TR, Soetikno RM, Kirn DH, Freeman SM. A phase I/II trial of intratumoral endoscopic ultrasound injection of ONYX-015 with intravenous gemcitabine in unresectable pancreatic carcinoma. Clin Cancer Res. 2003;9:555-561. |
| 6. | Rittner K, Schreiber V, Erbs P, Lusky M. Targeting of adenovirus vectors carrying a tumor cell-specific peptide: in vitro and in vivo studies. Cancer Gene Ther. 2007;14:509-518. |
| 7. | Wesseling JG, Bosma PJ, Krasnykh V, Kashentseva EA, Blackwell JL, Reynolds PN, Li H, Parameshwar M, Vickers SM, Jaffee EM. Improved gene transfer efficiency to primary and established human pancreatic carcinoma target cells via epidermal growth factor receptor and integrin-targeted adenoviral vectors. Gene Ther. 2001;8:969-976. |
| 8. | Mudali SV, Fu B, Lakkur SS, Luo M, Embuscado EE, Iacobuzio-Donahue CA. Patterns of EphA2 protein expression in primary and metastatic pancreatic carcinoma and correlation with genetic status. Clin Exp Metastasis. 2006;23:357-365. |
| 9. | Abraham S, Knapp DW, Cheng L, Snyder PW, Mittal SK, Bangari DS, Kinch M, Wu L, Dhariwal J, Mohammed SI. Expression of EphA2 and Ephrin A-1 in carcinoma of the urinary bladder. Clin Cancer Res. 2006;12:353-360. |
| 10. | Miyazaki T, Kato H, Fukuchi M, Nakajima M, Kuwano H. EphA2 overexpression correlates with poor prognosis in esophageal squamous cell carcinoma. Int J Cancer. 2003;103:657-663. |
| 11. | Zelinski DP, Zantek ND, Stewart JC, Irizarry AR, Kinch MS. EphA2 overexpression causes tumorigenesis of mammary epithelial cells. Cancer Res. 2001;61:2301-2306. |
| 12. | Koolpe M, Dail M, Pasquale EB. An ephrin mimetic peptide that selectively targets the EphA2 receptor. J Biol Chem. 2002;277:46974-46979. |
| 13. | Roelvink PW, Mi Lee G, Einfeld DA, Kovesdi I, Wickham TJ. Identification of a conserved receptor-binding site on the fiber proteins of CAR-recognizing adenoviridae. Science. 1999;286:1568-1571. |
| 14. | Einfeld DA, Schroeder R, Roelvink PW, Lizonova A, King CR, Kovesdi I, Wickham TJ. Reducing the native tropism of adenovirus vectors requires removal of both CAR and integrin interactions. J Virol. 2001;75:11284-11291. |
| 15. | Leissner P, Legrand V, Schlesinger Y, Hadji DA, van Raaij M, Cusack S, Pavirani A, Mehtali M. Influence of adenoviral fiber mutations on viral encapsidation, infectivity and in vivo tropism. Gene Ther. 2001;8:49-57. |
| 16. | Alemany R, Curiel DT. CAR-binding ablation does not change biodistribution and toxicity of adenoviral vectors. Gene Ther. 2001;8:1347-1353. |
| 17. | Mizuguchi H, Koizumi N, Hosono T, Ishii-Watabe A, Uchida E, Utoguchi N, Watanabe Y, Hayakawa T. CAR- or alphav integrin-binding ablated adenovirus vectors, but not fiber-modified vectors containing RGD peptide, do not change the systemic gene transfer properties in mice. Gene Ther. 2002;9:769-776. |
| 18. | Martin K, Brie A, Saulnier P, Perricaudet M, Yeh P, Vigne E. Simultaneous CAR- and alpha V integrin-binding ablation fails to reduce Ad5 liver tropism. Mol Ther. 2003;8:485-494. |
| 19. | Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973;52:2745-2756. |
| 20. | Koizumi N, Mizuguchi H, Sakurai F, Yamaguchi T, Watanabe Y, Hayakawa T. Reduction of natural adenovirus tropism to mouse liver by fiber-shaft exchange in combination with both CAR- and alphav integrin-binding ablation. J Virol. 2003;77:13062-13072. |
| 21. | Von Seggern DJ, Kehler J, Endo RI, Nemerow GR. Complementation of a fibre mutant adenovirus by packaging cell lines stably expressing the adenovirus type 5 fibre protein. J Gen Virol. 1998;79:1461-1468. |
| 22. | Ma L, Bluyssen HA, De Raeymaeker M, Laurysens V, van der Beek N, Pavliska H, van Zonneveld AJ, Tomme P, van Es HH. Rapid determination of adenoviral vector titers by quantitative real-time PCR. J Virol Methods. 2001;93:181-188. |
| 23. | van Beusechem VW, Mastenbroek DC, van den Doel PB, Lamfers ML, Grill J, Würdinger T, Haisma HJ, Pinedo HM, Gerritsen WR. Conditionally replicative adenovirus expressing a targeting adapter molecule exhibits enhanced oncolytic potency on CAR-deficient tumors. Gene Ther. 2003;10:1982-1991. |
| 24. | Boom R, Sol CJ, Salimans MM, Jansen CL, Wertheim-van Dillen PM, van der Noordaa J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol. 1990;28:495-503. |
| 25. | Akiyama M, Thorne S, Kirn D, Roelvink PW, Einfeld DA, King CR, Wickham TJ. Ablating CAR and integrin binding in adenovirus vectors reduces nontarget organ transduction and permits sustained bloodstream persistence following intraperitoneal administration. Mol Ther. 2004;9:218-230. |
| 26. | Sandovici M, Deelman LE, Smit-van Oosten A, van Goor H, Rots MG, de Zeeuw D, Henning RH. Enhanced transduction of fibroblasts in transplanted kidney with an adenovirus having an RGD motif in the HI loop. Kidney Int. 2006;69:45-52. |
| 27. | Dmitriev I, Krasnykh V, Miller CR, Wang M, Kashentseva E, Mikheeva G, Belousova N, Curiel DT. An adenovirus vector with genetically modified fibers demonstrates expanded tropism via utilization of a coxsackievirus and adenovirus receptor-independent cell entry mechanism. J Virol. 1998;72:9706-9713. |
| 28. | Murugesan SR, Akiyama M, Einfeld DA, Wickham TJ, King CR. Experimental treatment of ovarian cancers by adenovirus vectors combining receptor targeting and selective expression of tumor necrosis factor. Int J Oncol. 2007;31:813-822. |
| 29. | Kritz AB, Nicol CG, Dishart KL, Nelson R, Holbeck S, Von Seggern DJ, Work LM, McVey JH, Nicklin SA, Baker AH. Adenovirus 5 fibers mutated at the putative HSPG-binding site show restricted retargeting with targeting peptides in the HI loop. Mol Ther. 2007;15:741-749. |
| 30. | Koizumi N, Kawabata K, Sakurai F, Watanabe Y, Hayakawa T, Mizuguchi H. Modified adenoviral vectors ablated for coxsackievirus-adenovirus receptor, alphav integrin, and heparan sulfate binding reduce in vivo tissue transduction and toxicity. Hum Gene Ther. 2006;17:264-279. |
| 31. | Shayakhmetov DM, Gaggar A, Ni S, Li ZY, Lieber A. Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity. J Virol. 2005;79:7478-7491. |
| 32. | Xu Z, Tian J, Smith JS, Byrnes AP. Clearance of adenovirus by Kupffer cells is mediated by scavenger receptors, natural antibodies, and complement. J Virol. 2008;82:11705-11713. |
| 33. | Lieber A, He CY, Meuse L, Schowalter D, Kirillova I, Winther B, Kay MA. The role of Kupffer cell activation and viral gene expression in early liver toxicity after infusion of recombinant adenovirus vectors. J Virol. 1997;71:8798-8807. |
| 34. | Holub M, Fornůsek L, Vĕtvicka V, Chalupná J. Enhanced phagocytic activity of blood leukocytes in athymic nude mice. J Leukoc Biol. 1984;35:605-615. |
| 35. | Vĕtvicka V, Fornusek L, Holub M, Zídková J, Kopecek J. Macrophages of athymic nude mice: Fc receptors, C receptors, phagocytic and pinocytic activities. Eur J Cell Biol. 1984;35:35-40. |
| 36. | Tao N, Gao GP, Parr M, Johnston J, Baradet T, Wilson JM, Barsoum J, Fawell SE. Sequestration of adenoviral vector by Kupffer cells leads to a nonlinear dose response of transduction in liver. Mol Ther. 2001;3:28-35. |
| 37. | Manickan E, Smith JS, Tian J, Eggerman TL, Lozier JN, Muller J, Byrnes AP. Rapid Kupffer cell death after intravenous injection of adenovirus vectors. Mol Ther. 2006;13:108-117. |
| 38. | Havenga MJ, Lemckert AA, Ophorst OJ, van Meijer M, Germeraad WT, Grimbergen J, van Den Doel MA, Vogels R, van Deutekom J, Janson AA. Exploiting the natural diversity in adenovirus tropism for therapy and prevention of disease. J Virol. 2002;76:4612-4620. |
| 39. | Yamamoto M, Davydova J, Wang M, Siegal GP, Krasnykh V, Vickers SM, Curiel DT. Infectivity enhanced, cyclooxygenase-2 promoter-based conditionally replicative adenovirus for pancreatic cancer. Gastroenterology. 2003;125:1203-1218. |
| 40. | Vivès RR, Lortat-Jacob H, Fender P. Heparan sulphate proteoglycans and viral vectors : ally or foe? Curr Gene Ther. 2006;6:35-44. |
| 41. | Conejo JR, Kleeff J, Koliopanos A, Matsuda K, Zhu ZW, Goecke H, Bicheng N, Zimmermann A, Korc M, Friess H. Syndecan-1 expression is up-regulated in pancreatic but not in other gastrointestinal cancers. Int J Cancer. 2000;88:12-20. |
| 42. | Kleeff J, Ishiwata T, Kumbasar A, Friess H, Büchler MW, Lander AD, Korc M. The cell-surface heparan sulfate proteoglycan glypican-1 regulates growth factor action in pancreatic carcinoma cells and is overexpressed in human pancreatic cancer. J Clin Invest. 1998;102:1662-1673. |
| 43. | Liu J, Shriver Z, Pope RM, Thorp SC, Duncan MB, Copeland RJ, Raska CS, Yoshida K, Eisenberg RJ, Cohen G. Characterization of a heparan sulfate octasaccharide that binds to herpes simplex virus type 1 glycoprotein D. J Biol Chem. 2002;277:33456-33467. |
| 44. | Bayo-Puxan N, Cascallo M, Gros A, Huch M, Fillat C, Alemany R. Role of the putative heparan sulfate glycosaminoglycan-binding site of the adenovirus type 5 fiber shaft on liver detargeting and knob-mediated retargeting. J Gen Virol. 2006;87:2487-2495. |