Published online Jun 14, 2009. doi: 10.3748/wjg.15.2714
Revised: May 5, 2009
Accepted: May 12, 2009
Published online: June 14, 2009
AIM: To investigate the influence of CO2-insufflation pressure on adhesion, invasion and metastatic potential of colon cancer cells based on adhesion molecules expression.
METHODS: With an in vitro artificial pneumoperitoneum model, SW1116 human colon carcinoma cells were exposed to CO2-insufflation in 5 different pressure groups: 6 mmHg, 9 mmHg, 12 mmHg, 15 mmHg and control group, respectively for 1 h. Expression of E-cadherin, ICAM-1, CD44 and E-selectin was measured at 0, 12, 24, 48 and 72 h after CO2-insufflation using flow cytometry. The adhesion and invasion capacity of SW1116 cells before and after exposure to CO2-insufflation was detected by cell adhesion/invasion assay in vitro. Each group of cells was injected intraperitoneally into 16 BALB/C mice. The number of visible abdominal cavity tumor nodules, visceral metastases and survival of the mice were recorded in each group.
RESULTS: The expression of E-cadherin, ICAM-1, CD44 and E-selectin in SW1116 cells were changed significantly following exposure to CO2 insufflation at different pressures (P < 0.05). The expression of E-cadherin, CD44 and ICAM-1 decreased with increasing CO2-insufflation pressure. The adhesive/invasive cells also decreased gradually with increasing pressure as determined by the adhesion/invasion assay. In animal experiments, the number of abdominal cavity tumor nodules in the 15 mmHg group was also significantly lower than that in the 6 mmHg group (29.7 ± 9.91 vs 41.7 ± 14.90, P = 0.046). However, the survival in each group was not statistically different.
CONCLUSION: CO2-insufflation induced a temporary change in the adhesion and invasion capacity of cancer cells in vitro. Higher CO2-insufflation pressure inhibited adhesion, invasion and metastatic potential in vitro and in vivo, which was associated with reduced expression of adhesion molecules.
- Citation: Ma JJ, Feng B, Zhang Y, Li JW, Lu AG, Wang ML, Peng YF, Hu WG, Yue F, Zheng MH. Higher CO2-insufflation pressure inhibits the expression of adhesion molecules and the invasion potential of colon cancer cells. World J Gastroenterol 2009; 15(22): 2714-2722
- URL: https://www.wjgnet.com/1007-9327/full/v15/i22/2714.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.2714
The laparoscopic approach is generally considered to be less invasive and less immunosuppressive than conventional open surgery[1]. However, port site metastases and abdominal wall recurrences although rare nowadays, remain a problem with laparoscopic surgery for cancer[2]. Recently, several studies demonstrated that the five-year survival rates were similar after laparoscopically assisted colectomy and open colectomy for cancer patients, suggesting that the laparoscopic approach is an acceptable alternative to open surgery for colorectal cancer[3–5]. However, whether CO2 insufflation, which is widely used in laparoscopic surgery, increases the metastatic potential of tumor cells is still the focus of related studies[6–8].
Adhesion molecules play an important role in cell-cell, cell-extracellular matrix (ECM) interactions at tumor metastasis[910]. ICAM-1, E-cadherin, and CD44 are representative molecules involved in the interaction not only between the adhesion of inflammatory cells but also between tumor cells and the endothelium. Previous studies have shown that reduced E-cadherin expression correlates with increased invasiveness in colorectal carcinoma cell lines[11]. The decrease or deletion of E-cadherin was reported to induce liver metastasis of colorectal tumors[1213], and the increased expression of CD44 also induces tumor metastasis[14]. Recently it has been reported that CO2 insufflation can effect the expression of adhesion molecules, such as E-cadherin, CD44 and ICAM-1, which are related to tumor metastasis[15–17]. However, there are few studies on the influence of CO2-insufflation pressure on adhesion molecules and how CO2 insufflation further impacts on metastatic potential following altered expression of these adhesion molecules.
In this study, we investigated whether expression of adhesion molecules are changed after CO2 insufflation in vitro. We further investigated the influence of CO2 pressure on the expression of these adhesion molecules, and how CO2-insufflation influenced the adhesion and invasion potential of colon cancer cells in vitro and in vivo.
The human colorectal adenocarcinoma cell line SW1116 (human, Caucasian, colon, adenocarcinoma, grade III, CCL-233™) was cultured in RPMI 1640 medium (Hangzhou Genom Co. Cat. No. GNM31800.25) supplemented with 10% fetal calf serum (Life Technologies). The human colorectal adenocarcinoma cell line Lovo (human, colon, adenocarcinoma, grade IV, CCL-229™) used in the invasive assay was cultured in F12K medium (Hangzhou Genom Co. Cat. No. GNM21700.25) supplemented with 10% fetal calf serum. The monolayer cell culture was maintained in culture flasks under standard culture conditions at 37°C in air with 5% CO2.
For flow cytometry, the following monoclonal antibodies (Mab) were used: anti-ICAM-1-PE-Cy5 (Clone HA58) and anti-E-selectin-PE (Clone 68-5H11). They were purchased from BD Biosciences (San Diego, CA, USA). Anti-CD44-FITC (Clone J.173) and unconjugated anti-E-cadherin (Clone 67A4) were purchased from Beckman Coulter™. Secondary goat anti-mouse IgG-PE, isotype control mouse IgG-PE-Cy5, and mouse IgG-PE were purchased from BD Biosciences. Mouse IgG FITC, and mouse IgG PE were purchased from Beckman Coulter™.
Four to eight week-old male BALB/C mice (Chinese Academy of Sciences, Shanghai, China) were kept under standard laboratory conditions and fed a standard laboratory diet with access to water added libitum before and after injection.
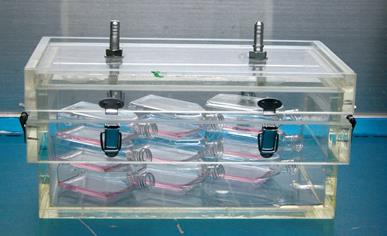
An in vitro pneumoperitoneum was established by connecting an electronic CO2 insufflator (Stryker® Endoscopy) to a 5 L volume airtight Perspex box with two ventilating pits (Figure 1). Medical grade carbon dioxide at a concentration of 99.5% was used. To ensure exhaustion of air in the box, 10 L CO2 were insufflated into the Perspex box before the tumor cells were placed into the box. The cells were divided into 5 groups with different insufflation pressures (6 mmHg, 9 mmHg, 12 mmHg, 15 mmHg, and room air as a control). The tumor cells and the boxes were transferred into an incubator at 37°C and the in vitro pneumoperitoneum model was retained for 1 h at different insufflation pressures during a continuous CO2 flow of 2.5 L/min.
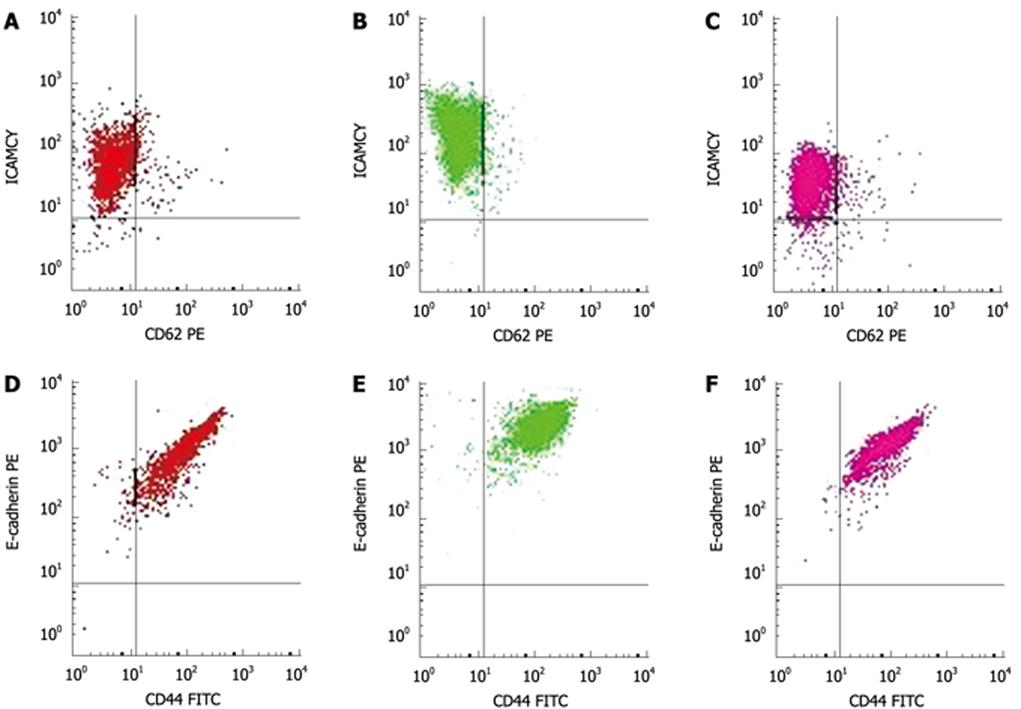
At 0, 12, 24, 48, and 72 h after exposure, the cells were detached from the tissue culture flasks using ice-cold phosphate-buffered saline (PBS) containing 0.1% sodium azide and resuspended at 5 × 105 cells/mL. One milliliter of the cell suspension was incubated with the monoclonal antibodies (1:100). The cells (5 × 105/group) were incubated with anti-ICAM-1-PE-Cy5, 10 &mgr;L, anti-E-selectin-PE, 10 &mgr;L, anti-CD44-FITC, 20 &mgr;L and unconjugated anti-E-cadherin, 10 &mgr;L for 30 min at room temperature. At the end of the incubation period, the cells were washed with PBS. After treatment with unconjugated anti-E-cadherin the cells were incubated with a secondary goat anti-mouse IgG-PE (3 &mgr;L/5 × 105 cells) for 30 min at room temperature. After washing with PBS, the cells were fixed with 0.5% formalin, and analyzed for surface receptor expression by flow cytometry (BD Calibur). The expression of 4 different cell adhesion molecules was measured at 5 time points (0, 12, 24, 48 and 72 h) after CO2 insufflation. Expression of these adhesion molecules is given as mean fluorescence intensity of the cell population.
A cell adhesion assay (Cat. No. ECM 554, Chemicon International, USA) was used, in which the wells were coated with human fibronectin, one of the component proteins of the extracellular matrix (ECM). At 0 h and 72 h after exposure to CO2 insufflation (at 6 mmHg, 9 mmHg, 12 mmHg, 15 mmHg and room air for 1 h, respectively), the cells were detached using 0.25% trypsin and washed with PBS. The wells were rehydrated with 200 &mgr;L of PBS per well for at least 15 min at room temperature before being plated at a concentration of 1 × 105 cells per well. After the plate was incubated at 37°C in air with 5% CO2 for 1 h, each well was washed gently 3 times with PBS. The cells which had adhered to the wells were then dyed with crystal violet and incubated for 5 min at room temperature. After the plate was gently washed 3 times with PBS, 100 &mgr;L of Solubilization Buffer (a 50/50 mixture of 0.1 mol/L NaH2PO4, pH 4.5 and 50% ethanol) was added to each well. The plate was shaken at room temperature gently to solubilize the cell-bound stain completely. Finally, a microplate reader was used to determine the absorbance at 570 nm as a measure of tumor cell adhesion on the ECM layer.
The SW1116 cells were starved by incubation in serum-free RPMI 1640 for 18-24 h prior to assay. At 0 h and 72 h after CO2 insufflation (6 mmHg, 9 mmHg, 12 mmHg, 15 mmHg and room air for 1 h, respectively), the cells were harvested by trypsinization, and resuspended in RPMI 1640 at 1 × 106 cells/mL. Twenty-four-well QCM™ Cell The Invasion Assay kit (Cat. No. ECM 101, Chemicon International, USA) was used. After rehydration of the ECM layer by adding 300 &mgr;L of pre-warmed serum-free medium to the interior of the inserts for 30 min at room temperature, 250 &mgr;L of the medium was removed from the inserts without disturbing the membrane. The prepared cell suspension (250 &mgr;L) as described previously was then added into each insert, and RPMI 1640 with 5% BSA was added to the lower chamber. After incubation for 24 h at 37°C in the incubator with 5% CO2, the medium and suspended cells were removed and the invasion chamber was placed into a clean well containing 225 &mgr;L of the cell detachment solution. The cells were then incubated at 37°C for 30 min, and both lyses buffer and dye solution were added to each well containing the cell detachment solution with cells which had invaded the ECM coated membrane. Finally, the mixture was detected by a fluorescence plate reader using a 480/520 nm filter set. The fluorescence intensity was measured as an index of the quantity of invasive cells. Lovo cells underwent the same procedure in the invasive assay.
To analyze the potential for abdominal cavity dissemination in each group of cells (exposed to CO2 insufflation at 6 mmHg, 9 mmHg, 12 mmHg, 15 mmHg and room air), each group of cells was injected intraperitoneally into 16 BALB/C mice (1 × 106 cells/mouse). Fourteen days later, 10 mice in each group were sacrificed by cervical dislocation, and the number of visible abdominal cavity tumor nodules, reflecting the metastatic potential, was counted. The rate of viscera (liver, kidney, spleen, peritoneum or mesentery) metastasis was also recorded in each group. The remaining mice in each group were monitored for their survival.
For studies on the expression of adhesion molecules, each experiment was performed in triplicate. Cell adhesion and invasion assays were repeated five times. Data are expressed as mean ± SD. The repeated-measures ANOVA test, one-way ANOVA test and paired t test were used to compare the control mean with the various treatment means: adhesion molecules, cell adhesion and invasion assay in vitro, and abdominal cavity tumor metastasis in vivo. The rates of organ (liver, kidney, spleen, peritoneum or mesentery) metastasis were analyzed using Fisher’s exact test. Statistical significance was set at the 5% level.
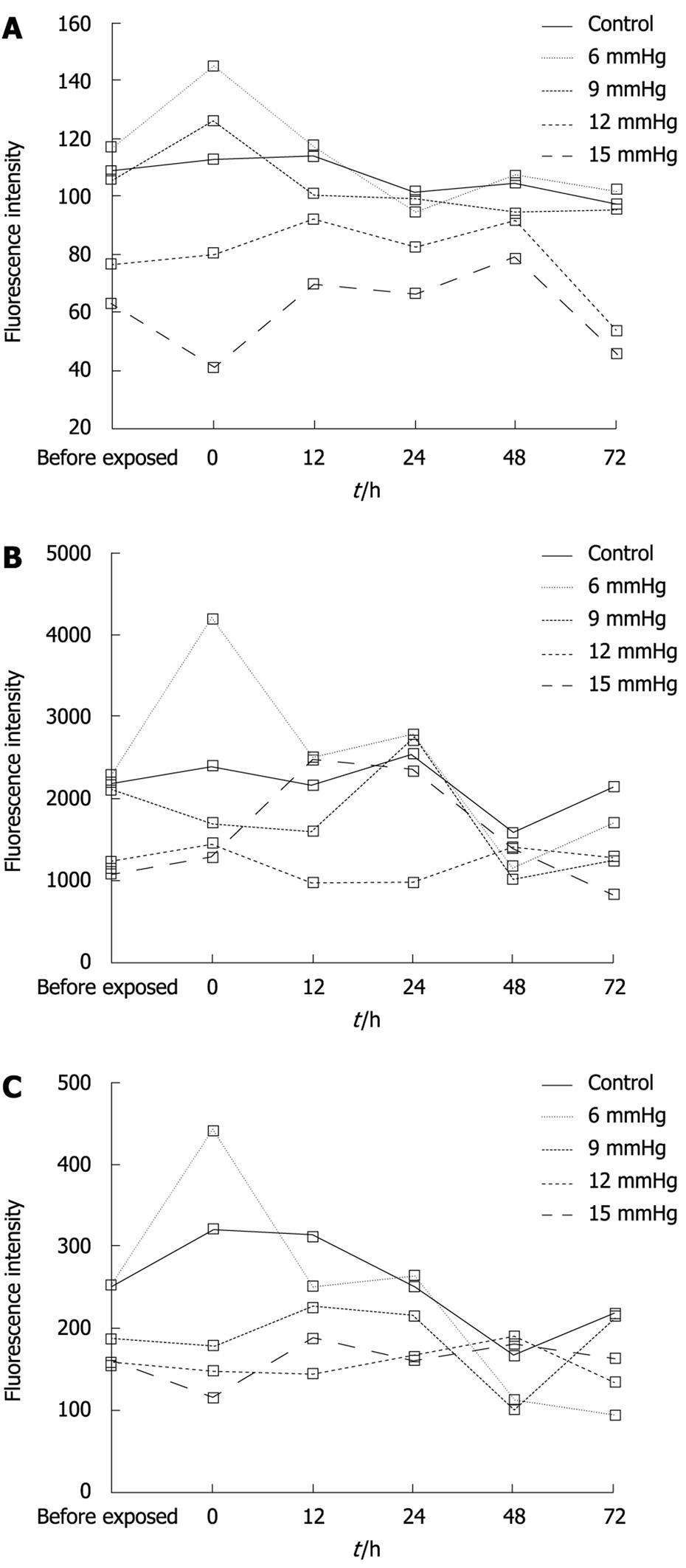
Immediately after the 6 mmHg CO2 insufflation, ICAM-1 (Figure 2A-C) and CD44 expression significantly increased compared to room air controls (F = 106.38, 297.73; P < 0.01), while the expression of ICAM-1 decreased at 48 h and 72 h after the 6 mmHg CO2 insufflation (F = 17 222.3, 385.61; P < 0.01) (Figure 2A-C). Immediately after the 9 mmHg CO2 insufflation, E-selectin expression significantly increased compared to the controls (F = 147.75, P = 0.01), and CD44 expression increased significantly compared to controls (F = 39.20, P = 0.025); 24 h after 9 mmHg CO2 insufflation, E-cadherin expression increased significantly compared to controls (F = 26.79, P = 0.035) (Figure 2D-F), while a significant reduction in E-selectin expression was observed (F = 33.43, P = 0.029). At 48 h after 9 mmHg CO2 insufflation, a significant reduction in ICAM-1 expression was demonstrated (F = 282.94, P < 0.01). At 48 h after 12 mmHg CO2 insufflation, CD44 expression increased significantly (F = 93.70, P = 0.011), while 72 h after 12 mmHg CO2 insufflation, the expression of CD44 decreased significantly compared to the controls (F = 679.38, P < 0.01). Immediately after the 15 mmHg CO2 insufflation, CD44 expression decreased significantly compared to the controls (F = 70.01, P = 0.014). At 12 h and 24 h after 15 mmHg CO2 insufflation, an increased expression of E-cadherin was demonstrated (F = 53.53, 68.14, P = 0.018, 0.014). No significant differences in the expression of these adhesion molecules at other time points and pressures were observed.
The expression of CD44, E-cadherin and ICAM-1 decreased with increasing CO2-insufflation pressure. At the 0 h time point, the differences in CD44 expression between the 6 mmHg and the 9 mmHg group, the 9 mmHg and the 12 mmHg group, the 12 mmHg and the 15 mmHg group were significant (t = 4.4291, 4.5725, 7.3587, P < 0.01) (Figure 3A). At the same time point, E-cadherin expression in the 9 mmHg group was less than that in the 6 mmHg group (t = 7.1839, P < 0.01), while in the 12 mmHg and the 15 mmHg group, E-cadherin expression was significantly less than that in the 9 mmHg group (t = 4.5148, 4.4582, P < 0.01) (Figure 3B). ICAM-1 expression in the 9 mmHg, 12 mmHg and 15 mmHg groups was less than that in the 6 mmHg group, respectively (t = 3.3359, 2.3189, 2.7546, P < 0.01, P = 0.046, 0.022), and the expression at 15 mmHg was significantly less than that at 12 mmHg (t = 2.2912, P = 0.048) (Figure 3C).
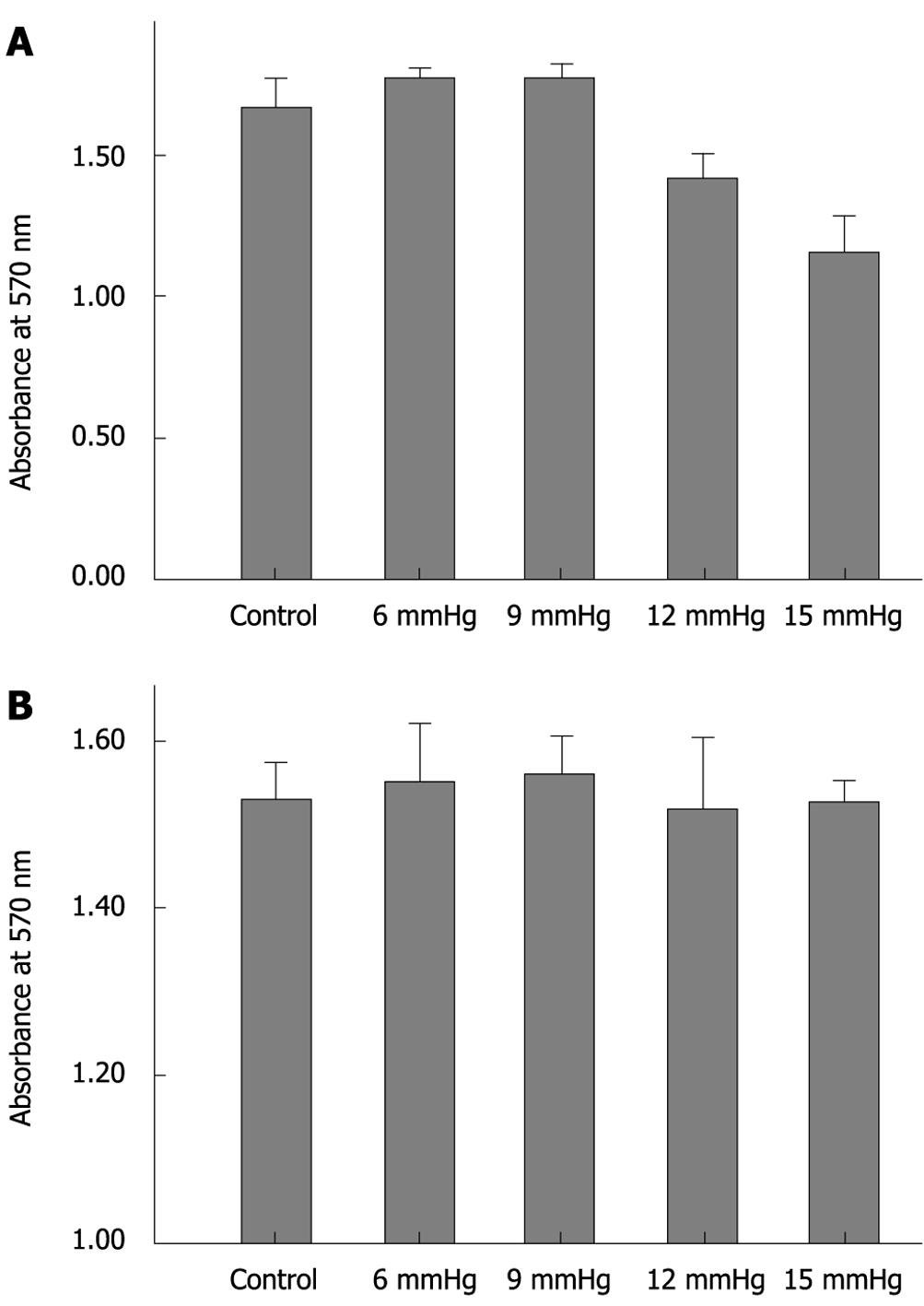
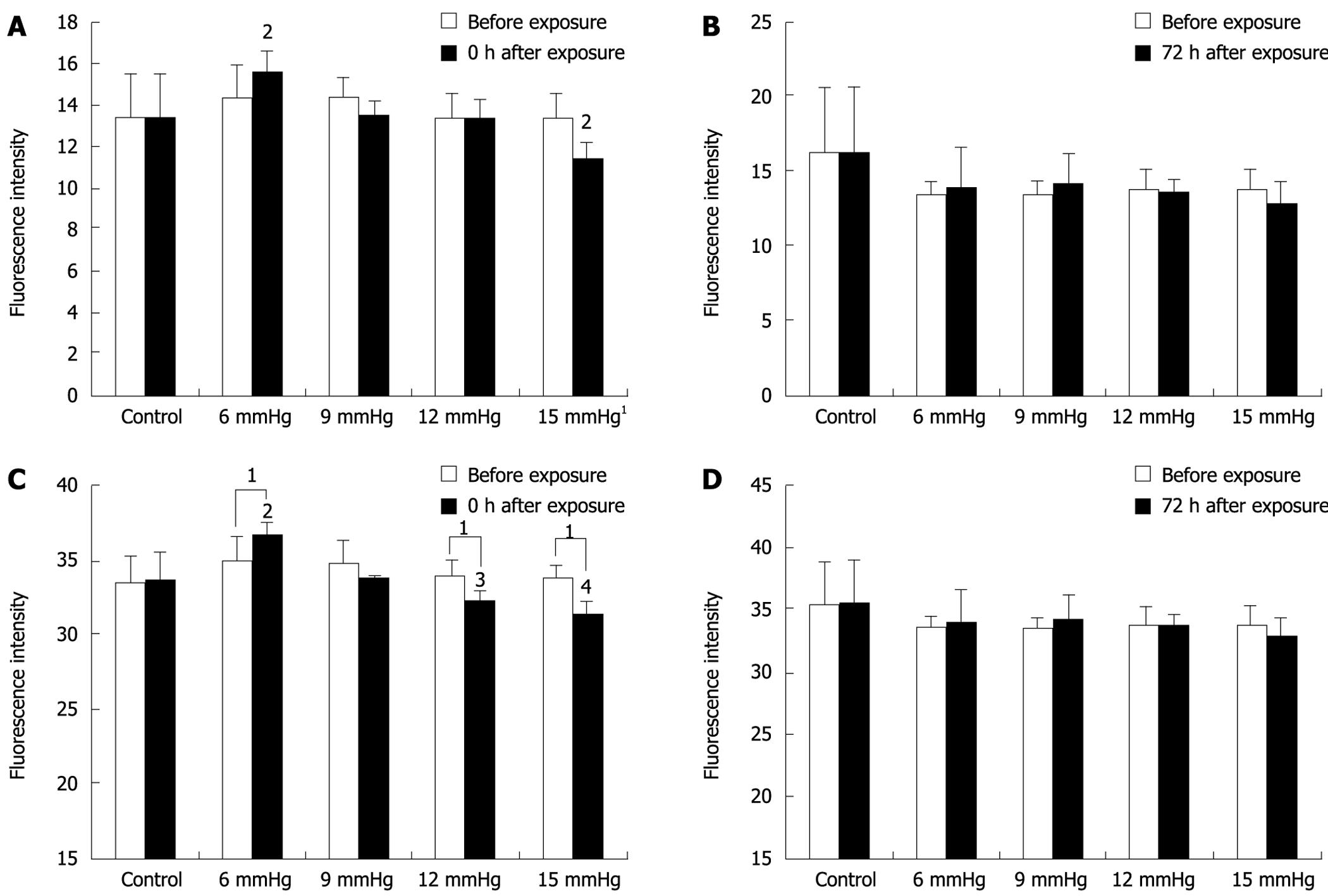
Immediately after exposure to increasing CO2-insufflation pressure, cell adhesion decreased gradually. Cell adhesion in the 15 mmHg group was significantly less than that at 12 mmHg, 9 mmHg, 6 mmHg and the control (all P < 0.01), and cell adhesion in the 12 mmHg group was also less than that in the 9 mmHg, 6 mmHg and control groups (all P < 0.01). The cells exposed to 6 mmHg had more adhesion capacity than those exposed to 9 mmHg or the control group, however, the differences were not significant (P = 0.886, P = 0.058) (Figure 4A). At 72 h after CO2 insufflation, the differences between the various insufflation pressure groups were not significant (Figure 4B).
Immediately after exposure to 15 mmHg CO2 insufflation, the invasion of SW1116 cells decreased significantly compared to the cells before exposure (11.36 ± 0.861 vs 13.43 ± 1.113; P = 0.019), while immediately after exposure to 6 mmHg insufflation, invasive cells increased compared to before exposure (15.58 ± 1.015 vs 14.42 ± 1.491; P = 0.056), however, the difference was not significant (Figure 5A). At 72 h after CO2 insufflation, cell invasion was similar to that for cells before exposure (Figure 5B).
The differences between various insufflation pressures were also compared. At the 0 h time point, the cells exposed to 15 mmHg were significantly less invasive than those exposed to the other insufflation pressures (vs 6 mmHg, P < 0.01; vs 9 mmHg, P = 0.011; vs 12 mmHg, P = 0.016; and vs room air, P = 0.014), and the cells exposed to 6 mmHg were more invasive than cells exposed to the other insufflation pressures (vs 9 mmHg, P = 0.013; vs 12 mmHg, P < 0.01; vs 15 mmHg P < 0.01; and vs room air, P = 0.011). However, these differences were not significant (Figure 5A). At 72 h after CO2 insufflation, the differences between the various insufflation pressure groups were not significant (Figure 5B).
The human colon cancer cell line Lovo was also tested in the invasive assay, and a similar trend was observed (Figure 5C and D).
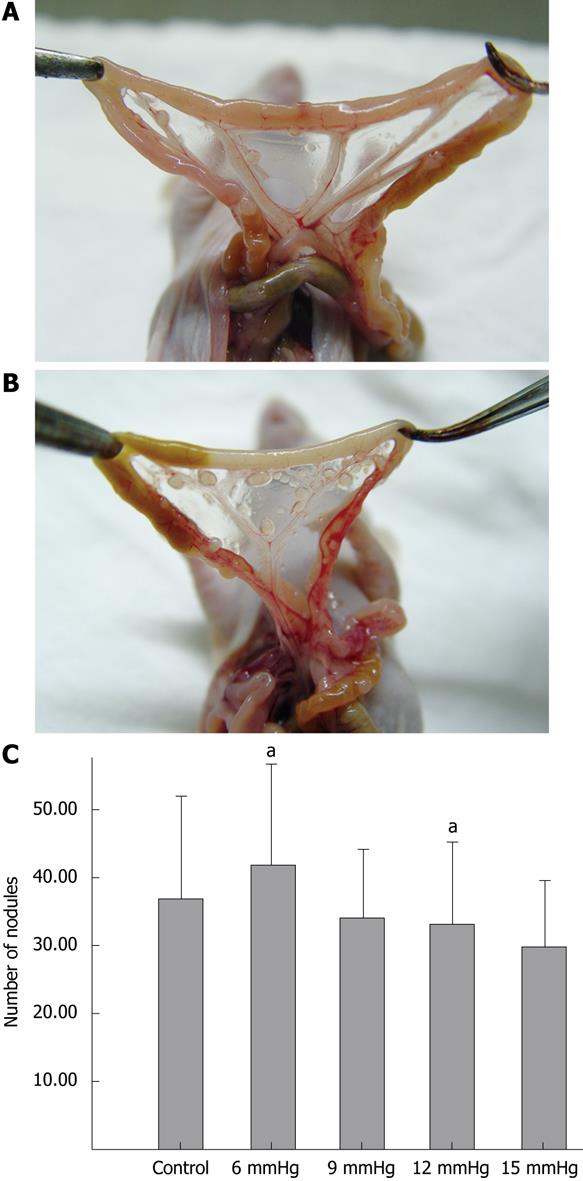
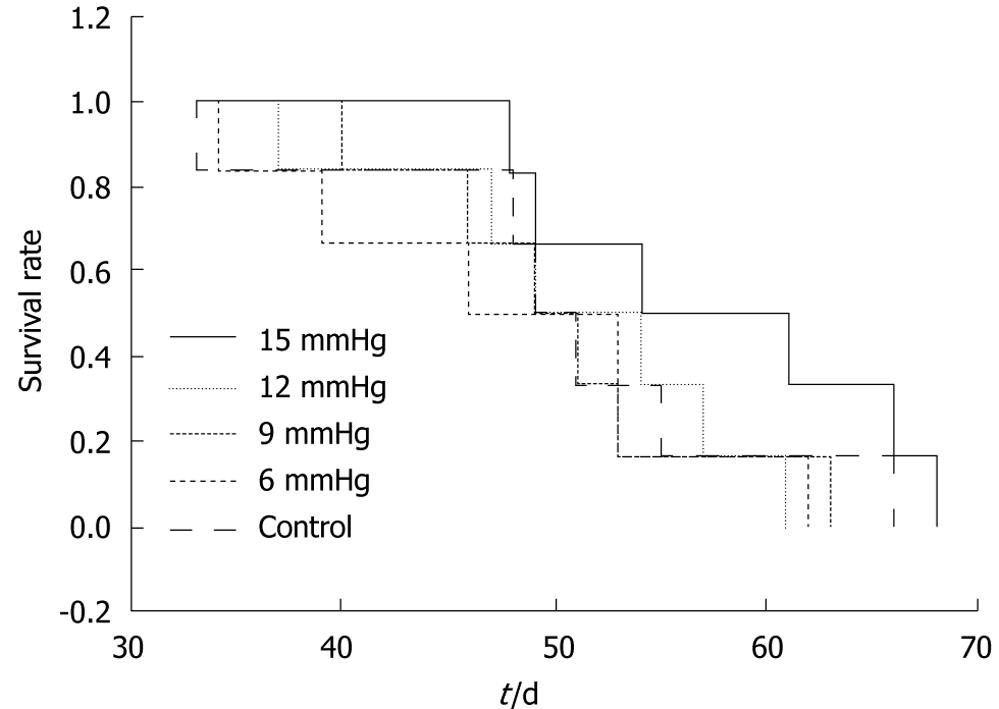
The ratios of the number of mice which developed intra-abdominal tumors/the number of mice which received inoculation of SW1116 cells in the various groups were as follows: (1) control group, 10/10; (2) 6 mmHg group, 9/10; (3) 9 mmHg group, 9/10; (4) 12 mmHg group, 9/10; (5) 15 mmHg group, 10/10. The differences were not significant. The mean number of intra-abdominal tumor nodules in the various groups was as follows: (1) control group = 36.8 ± 15.32, (2) 6 mmHg group = 41.7 ± 14.90, (3) 9 mmHg group = 33.9 ± 10.29, (4) 12 mmHg group = 33.2 ± 11.72, (5) 15 mmHg = 29.7 ± 9.91. The difference between the 6 mmHg group and the 15 mmHg group was significant (P = 0.046) (Figure 6A-C), but the differences among the other groups were not. The results of visceral (liver, kidney, spleen, peritoneum or mesentery) metastases (the number of mice with certain visceral metastasis/the number of mice with abdominal cavity metastasis after inoculation of SW1116 cells) are shown in Table 1. No significant differences in survival rate between the various groups were observed (P = 0.426). The overall survival curve is shown in Figure 7.
| Liver (n1/n2) | Spleen (n1/n2) | Mesentery (n1/n2) | Kidney (n1/n2) | Peritoneum (n1/n2) | |
| Control | 10/10 | 4/10 | 10/10 | 5/10 | 4/10 |
| 6 mmHg | 9/9 | 0/9 | 9/9 | 0/9 | 2/9 |
| 9 mmHg | 9/9 | 1/9 | 9/9 | 2/9 | 1/9 |
| 12 mmHg | 8/9 | 1/9 | 9/9 | 4/9 | 0/9 |
| 15 mmHg | 10/10 | 1/10 | 10/10 | 4/10 | 1/10 |
| P | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 |
The use of minimal access techniques in the surgical management of colorectal cancer should be safe and feasible in terms of the oncologic effect on the tumor, as compared with conventional surgery. Recent clinical trials in Europe and America have demonstrated that the recurrence and 5-year survival of laparoscopic colorectal cancer surgery are identical to those obtained by open surgery[34]. Similar results have also been reported from Hong Kong[18]. However, the occurrence of port-site metastasis and peritoneal metastasis reported in early studies of laparoscopic surgery for cancer led to many studies investigating the biologic effects of positive pressure pneumoperitoneum on tumor growth and the development of trocar recurrences. Several experimental studies suggested that laparoscopic surgery can promote tumor growth[619]. Some animal studies have also shown that CO2, especially at high insufflation pressures increased the incidence of distant metastases[2021]. However, other researchers failed to confirm these experimental observations in similar animal models[22]. Thus, although the problem of port-site deposits in patients undergoing laparoscopic surgery for cancer has largely been resolved by improved oncological operative techniques, from a biological standpoint, the adverse effect of a sustained positive pressure CO2 pneumoperitoneum is still debatable, and it is prudent to investigate the mechanism of tumor metastasis induced by CO2 pneumoperitoneum.
Some adhesion molecules in cancer cells have been reported to play an important role in tumor metastasis. There is evidence that decreased expression of E-cadherin can initiate tumor invasion and metastasis because E-cadherin prevents detachment of tumor cells from the primary tumor[112324]. ICAM-1 mediates the adhesion of circulating cells to the intravascular endothelium. ICAM-1 may also play a role in tumor cell adhesion to the mesothelium[25]. Changes in the expression of CD44 are reported to be related to increased tumor metastasis. The expression of E-selectin in primary colorectal cancer is significantly less than in secondary deposits from this tumor[26]. A number of recent studies have addressed the effects of the laparoscopic environment on these adhesion molecules in cancer cells. Kim et al[16] demonstrated a significant alteration in E-cadherin, ICAM-1 and CD44 expression in colon cancer cells after exposure to CO2. Tahara et al[27] reported that CO2 insufflation increased the expression of P-cadherin mRNA in the mouse peritoneum. However, neither of these two groups investigated how these CO2-induced changes affected the invasive and metastatic potential of colon cancer cells.
In our study, we found that the expression of these adhesion molecules in the SW1116 cell line increased significantly 0-48 h after the cells were exposed to continuous CO2-insufflation for 1 h (P < 0.05), and returned to the control level by 72 h. We found that these changes are temporary. At certain time points, ICAM-1, E-selectin, and CD44 were found to be even lower than the control levels (P < 0.05). In addition, contrary to the results reported by Kim et al[16], the expression of E-cadherin significantly increased, rather than decreased after exposure to CO2-insufflation, although the effect was temporary. The important findings of the present study are that the changes in these adhesion molecules are bi-directional. Collectively increased expression of ICAM-1, E-selectin, and CD44 and decreased expression of E-cadherin signify increasing potential for tumor metastasis. However, the results of the present study also demonstrated the temporary and bi-directional nature of the changes in the expression of these adhesion molecules, indicating that CO2-insufflation during laparoscopic surgery does not appear to increase the metastatic potential of solid cancers. Our results document that with increasing CO2-insufflation pressure, the expression of E-cadherin, CD44 and ICAM-1 decreased, especially at the start of CO2-insufflation exposure (P < 0.05, Figure 3A-C). We believe that higher pressures of CO2-insufflation could inhibit the expression of adhesion molecules.
We also investigated the effect of CO2-insufflation on the adhesion and invasion potential of colon cancer cells in vitro and the effect on the metastatic capacity of the tumor cells in vivo. The cell invasion assays showed that CO2-insufflation also changed the invasion potential of tumor cells (both the SW1116 and the Lovo cell line) temporarily but with increasing CO2-insufflation pressures, invasion potential decreased. Similar results were obtained with the adhesion assays.
The in vivo animal studies with rising CO2-insufflation pressure, showed a decrease in the number of tumor nodules when the data between the 15 mmHg and 6 mmHg groups were compared. This observation suggests that high CO2-insufflation pressures could inhibit the adhesion, invasion and metastatic potential of colon cancer cells. Ziprin et al[28] reported that laparoscopic enhancement of tumor cell binding to the peritoneum is inhibited by anti-ICAM-1 monoclonal antibody. High CO2-insufflation pressures could inhibit the adhesion, invasion or metastatic capacity of tumor cells in vitro and in vivo by inhibiting expression of the adhesion molecules ICAM-1 and CD44.
In the present study, the adhesion potential of the tumor cells from the CO2-insufflation groups did not increase when compared to the control group. In addition, the numbers of tumor nodules in the four CO2-insufflation groups were similar to the control group. Likewise, survival rates were not significantly different indicating that CO2-insufflation did not increase the adhesion potential of colon cancer cells in vitro or the metastatic capacity in vivo. However, reaction of the host seems also to be highly important. Various host defence mechanisms in the organism are involved in tumouricidal killing processes. Some studies have reported that the mononuclear phagocytotic system, which largely consists of hepatic tissue macrophages (Kupffer cells), plays an important tumouricidal role. Previously, laparoscopic insufflation with CO2 had been demonstrated to impair the activity of the mononuclear phagocyte system and was responsible for potential hepatic disadvantages[29]. In this study, only tumor cells treated with CO2-insufflation in vitro, and the in vivo metastatic capacity of colon cancer cells treated with different CO2-insufflation in vitro were the focus of this investigation. The reaction of the host after high pressure pneumoperitoneum, and the effect that the pneumoperitoneum may have on intraperitoneal cells was not considered. Further investigations are required.
In conclusion, this study has shown that the expression of adhesion molecules can be affected temporarily and bi-directionally by continuous CO2-insufflation, which also induced a temporary change in the adhesion and invasion capacity of these cells in vitro. CO2-insufflation at high pressures plays an important role in inhibiting the expression of adhesion molecules. In addition, high insufflation pressures inhibited the adhesion and invasion potential of colon cancer cells in vitro and inhibited metastatic potential in vivo, which was associated with inhibited expression of adhesion molecules. The results of our study do not provide any evidence that CO2-insufflation increases the metastatic potential of colon cancer cells.
In conclusion, CO2-insufflation induced a temporary change in the adhesion and invasion capacity of cancer cells in vitro. High pressures of CO2-insufflation inhibited the adhesion, invasion and metastatic potential in vitro and in vivo, which was associated with reduced expression of adhesion molecules.
Laparoscopic CO2-insufflation has been reported to correlate with growth and metastasis of colorectal cancer. However, few studies have addressed the influence of CO2-insufflation pressure on the biological behaviour of colorectal cancer. The aim of this study is to investigate the influence of CO2-insufflation pressure on adhesion, invasion and metastatic potential of colon cancer cells based on adhesion molecules expression.
Recently, it has been reported that the CO2 insufflation can effect the expression of some adhesion molecules, such as E-cadherin, CD44 and ICAM-1, which are related to the tumor metastasis. But few studies concerning the influence of the CO2-insufflation pressure on the adhesion molecules and how CO2 insufflation further impacts on the metastatic potential following altered expression of these adhesion molecules.
In this study, the authors found that CO2-insufflation induced a temporary change in the adhesion and invasion capacity of cancer cells in vitro. High pressures of CO2-insufflation inhibited the adhesion, invasion and metastatic potential in vitro and in vivo, which was associated with reduced expression of adhesion molecules.
The results of their study do not provide any evidence that CO2-insufflation increases the metastatic potential of colon cancer cells. CO2-insufflation may be used with oncological safety in laparoscopic surgery for colorectal cancer.
The manuscript by Jun-Jun Ma and co-authors is well presented, and deals with a significant clinical issue when tumor tissue is resected. Indeed reoccurrences of tumor nodes stand central as a major post-operative complication. Furthermore, the authors applied an original approach to study this problem in vitro and in situ. This paper contains important information which might have significant translational medical implications.
| 1. | Vittimberga FJ Jr, Foley DP, Meyers WC, Callery MP. Laparoscopic surgery and the systemic immune response. Ann Surg. 1998;227:326-334. |
| 2. | Alexander RJ, Jaques BC, Mitchell KG. Laparoscopically assisted colectomy and wound recurrence. Lancet. 1993;341:249-250. |
| 3. | Clinical Outcomes of Surgical Therapy Study Group. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004;350:2050-2059. |
| 4. | Buunen M, Veldkamp R, Hop WC, Kuhry E, Jeekel J, Haglind E, Pahlman L, Cuesta MA, Msika S, Morino M. Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol. 2009;10:44-52. |
| 5. | Zheng MH, Feng B, Lu AG, Li JW, Wang ML, Mao ZH, Hu YY, Dong F, Hu WG, Li DH. Laparoscopic versus open right hemicolectomy with curative intent for colon carcinoma. World J Gastroenterol. 2005;11:323-326. |
| 6. | Lecuru F, Agostini A, Camatte S, Robin F, Aggerbeck M, Jaes JP, Vilde F, Taurelle R. Impact of pneumoperitoneum on tumor growth. Surg Endosc. 2002;16:1170-1174. |
| 7. | Ishida H, Idezuki Y, Yokoyama M, Nakada H, Odaka A, Murata N, Fujioka M, Hashimoto D. Liver metastasis following pneumoperitoneum with different gases in a mouse model. Surg Endosc. 2001;15:189-192. |
| 8. | Gutt CN, Kim ZG, Hollander D, Bruttel T, Lorenz M. CO2 environment influences the growth of cultured human cancer cells dependent on insufflation pressure. Surg Endosc. 2001;15:314-318. |
| 9. | Haier J, Nasralla M, Nicolson GL. Cell surface molecules and their prognostic values in assessing colorectal carcinomas. Ann Surg. 2000;231:11-24. |
| 10. | Jiang WG. Cell adhesion molecules in the formation of liver metastasis. J Hepatobiliary Pancreat Surg. 1998;5:375-382. |
| 11. | Kinsella AR, Lepts GC, Hill CL, Jones M. Reduced E-cadherin expression correlates with increased invasiveness in colorectal carcinoma cell lines. Clin Exp Metastasis. 1994;12:335-342. |
| 12. | Shimoyama Y, Nagafuchi A, Fujita S, Gotoh M, Takeichi M, Tsukita S, Hirohashi S. Cadherin dysfunction in a human cancer cell line: possible involvement of loss of alpha-catenin expression in reduced cell-cell adhesiveness. Cancer Res. 1992;52:5770-5774. |
| 13. | Mohri Y. Prognostic significance of E-cadherin expression in human colorectal cancer tissue. Surg Today. 1997;27:606-612. |
| 14. | Naor D, Sionov RV, Ish-Shalom D. CD44: structure, function, and association with the malignant process. Adv Cancer Res. 1997;71:241-319. |
| 15. | Paraskeva PA, Ridgway PF, Olsen S, Isacke C, Peck DH, Darzi AW. A surgically induced hypoxic environment causes changes in the metastatic behaviour of tumours in vitro. Clin Exp Metastasis. 2006;23:149-157. |
| 16. | Kim ZG, Mehl C, Lorenz M, Gutt CN. Impact of laparoscopic CO2-insufflation on tumor-associated molecules in cultured colorectal cancer cells. Surg Endosc. 2002;16:1182-1186. |
| 17. | Cai KL, Wang GB, Xiong LJ. Effects of carbon dioxide and nitrogen on adhesive growth and expressions of E-cadherin and VEGF of human colon cancer cell CCL-228. World J Gastroenterol. 2003;9:1594-1597. |
| 18. | Leung KL, Kwok SP, Lam SC, Lee JF, Yiu RY, Ng SS, Lai PB, Lau WY. Laparoscopic resection of rectosigmoid carcinoma: prospective randomised trial. Lancet. 2004;363:1187-1192. |
| 19. | Gutt CN, Kim ZG, Schmandra T, Paolucci V, Lorenz M. Carbon dioxide pneumoperitoneum is associated with increased liver metastases in a rat model. Surgery. 2000;127:566-570. |
| 20. | Ishida H, Murata N, Idezuki Y. Increased insufflation pressure enhances the development of liver metastasis in a mouse laparoscopy model. World J Surg. 2001;25:1537-1541. |
| 21. | Ishida H, Hashimoto D, Nakada H, Takeuchi I, Hoshino T, Murata N, Idezuki Y, Hosono M. Increased insufflation pressure enhances the development of liver metastasis in a mouse laparoscopy model: possible mechanisms. Surg Endosc. 2002;16:331-335. |
| 22. | Kuntz C, Kienle P, Schmeding M, Benner A, Autschbach F, Schwalbach P. Comparison of laparoscopic versus conventional technique in colonic and liver resection in a tumor-bearing small animal model. Surg Endosc. 2002;16:1175-1181. |
| 23. | Shiozaki H, Oka H, Inoue M, Tamura S, Monden M. E-cadherin mediated adhesion system in cancer cells. Cancer. 1996;77:1605-1613. |
| 24. | Jiang WG. E-cadherin and its associated protein catenins, cancer invasion and metastasis. Br J Surg. 1996;83:437-446. |
| 25. | Nomura M, Sugiura Y, Tatsumi Y, Miyamoto K. Adhesive interaction of highly malignant hepatoma AH66F cells with mesothelial cells. Biol Pharm Bull. 1999;22:738-740. |
| 26. | Ye C, Kiriyama K, Mistuoka C, Kannagi R, Ito K, Watanabe T, Kondo K, Akiyama S, Takagi H. Expression of E-selectin on endothelial cells of small veins in human colorectal cancer. Int J Cancer. 1995;61:455-460. |
| 27. | Tahara K, Fujii K, Yamaguchi K, Suematsu T, Shiraishi N, Kitano S. Increased expression of P-cadherin mRNA in the mouse peritoneum after carbon dioxide insufflation. Surg Endosc. 2001;15:946-949. |
| 28. | Ziprin P, Ridgway PF, Peck DH, Darzi AW. Laparoscopic enhancement of tumour cell binding to the peritoneum is inhibited by anti-intercellular adhesion molecule-1 monoclonal antibody. Surg Endosc. 2003;17:1812-1817. |
| 29. | Gutt CN, Gessmann T, Schemmer P, Mehrabi A, Schmandra T, Kim ZG. The impact of carbon dioxide and helium insufflation on experimental liver metastases, macrophages, and cell adhesion molecules. Surg Endosc. 2003;17:1628-1631. |