Published online Jun 7, 2009. doi: 10.3748/wjg.15.2638
Revised: April 15, 2009
Accepted: April 22, 2009
Published online: June 7, 2009
AIM: To evaluate the feasibility and efficacy of percutaneous radiofrequency ablation (RFA) of the feeding artery of hepatocellular carcinoma (HCC) in reducing the blood-flow-induced heat-sink effect of RFA.
METHODS: A total of 154 HCC patients with 177 pathologically confirmed hypervascular lesions participated in the study and were randomly assigned into two groups. Seventy-one patients with 75 HCCs (average tumor size, 4.3 ± 1.1 cm) were included in group A, in which the feeding artery of HCC was identified by color Doppler flow imaging, and were ablated with multiple small overlapping RFA foci [percutaneous ablation of feeding artery (PAA)] before routine RFA treatment of the tumor. Eighty-three patients with 102 HCC (average tumor size, 4.1 ± 1.0 cm) were included in group B, in which the tumors were treated routinely with RFA. Contrast-enhanced computed tomography was used as post-RFA imaging, when patients were followed-up for 1, 3 and 6 mo.
RESULTS: In group A, feeding arteries were blocked in 66 (88%) HCC lesions, and the size of arteries decreased in nine (12%). The average number of punctures per HCC was 2.76 ± 1.12 in group A, and 3.36 ± 1.60 in group B (P = 0.01). The tumor necrosis rate at 1 mo post-RFA was 90.67% (68/75 lesions) in group A and 90.20% (92/102 lesions) in group B. HCC recurrence rate at 6 mo post-RFA was 17.33% (13/75) in group A and 31.37% (32/102) in group B (P = 0.04).
CONCLUSION: PAA blocked effectively the feeding artery of HCC. Combination of PAA and RFA significantly decreased post-RFA recurrence and provided an alternative treatment for hypervascular HCC.
- Citation: Hou YB, Chen MH, Yan K, Wu JY, Yang W. Adjuvant percutaneous radiofrequency ablation of feeding artery of hepatocellular carcinoma before treatment. World J Gastroenterol 2009; 15(21): 2638-2643
- URL: https://www.wjgnet.com/1007-9327/full/v15/i21/2638.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.2638
Hepatocellular carcinoma (HCC) is the fifth most common cancer in the world (564 000 cases per year) and the third most frequent cause of cancer-related death[1]. Surgical resection is considered to be potentially curative therapy. However, only about 20% of HCC patients are eligible for resection[12]; the remainder are ineligible because of multifocal tumors, advanced tumors, tumor location precluding complete resection, or poor hepatic functional reserve. Therefore, a variety of imaging-guided tumor ablation therapies such as ethanol injection, microwave coagulation, percutaneous radiofrequency ablation (RFA) and laser ablation are often considered as alternative options[3–6]. Among them, RFA has been used increasingly as a safe technique for treating hepatic tumors[7–9]. However, for hypervascular HCC, RFA appears less effective because of a blood-flow-induced heat sink effect, which might cause incomplete ablation or recurrence[10]. Transcatheter arterial chemoembolization (TACE) can reduce blood supply of HCC by occlusion of tumor arteries, and the efficacy of combination TACE and RFA has been confirmed[1112]. Treatment difficulty remains for those patients who cannot tolerate or are ineligible for TACE because of liver cirrhosis or difficulty in manipulation of vessels with abnormal curvature that have resulted from surgical resection and liver transplantation.
We hypothesized that, if percutaneous ablation of the feeding artery (PAA) of HCC could block or reduce the blood flow of HCC, the ablation volume of coagulation necrosis of subsequent RFA of the tumor would be increased. To the best of our knowledge, the application of PAA in the treatment of hypervascular HCC has not been reported in a large number of patients. In the present study, we evaluated the feasibility and adjuvant value of PAA performed before routine RFA treatment (PAA-RFA) of hypervascular HCC.
From January 2003 to June 2007, patients with HCC who met the entry criteria and agreed to participate were included in the study. The inclusion criteria for the patients were: pathologically confirmed HCC lesions, ineligibility for surgical resection or TACE, tumor size > 3 cm, no significant tumor direct invasion of adjacent organs, tumor not invading the main bile duct or being obviously exophytic, tumor’s feeding artery visible on color Doppler flow imaging (CDFI), prothrombin time ratio > 50%, or platelet count > 60 × 109/L. Exclusion criteria were: tumor thrombus in main or lobar portal vein system, extrahepatic metastasis, or Child-Pugh class C liver cirrhosis.
Each patient who participated in the study was assigned a random number of 1 or 2. The patients with number 1 were allocated to group A, which was treated with PAA-RFA, and those with number 2 were allocated to group B, which was treated with routine RFA[13].
This study was performed with the approval of the ethics committee and informed consent was obtained from each patient after the nature of the procedure had been fully explained.
The RFA system used in this study was a 460-kHz generator, 150 W output power (Model 1500; RITA Medical Systems, Mountain View, CA, USA). The expandable electrodes consisted of an outer 14-gauge, 15-cm long outer insulated needle, and nine prongs that were deployed and retracted by a movable hub, with deployment diameter ranging from 3 to 5cm. Twenty minutes were required to produce a 5-cm ablation sphere during RFA. Track ablation was performed when withdrawing the RFA electrode in all patients, to avoid implantation metastasis and hemorrhage.
Real-time ultrasound (US) systems (Aloka 5500 and Aloka α-10, Tokyo, Japan) were used for scanning with 3.5-5.0-MHz convex probes with needle guide devices. Computed tomography (CT) was performed with a Plus 4 scanner (Siemens, Germany) with 5 mm collimation and a table speed of 7.5 mm/s. A total of 100 mL of non-ionic contrast material (300 mg iodine/mL, Omnipaque; Amersham, Shanghai, China) was administered at a rate of 3 mL/s with a power injector (OP 100; Medrad, Pittsburgh, PA, USA). Images were acquired before contrast material injection and 25 and 60 s after the administration of intravenous contrast material, during the hepatic arterial and portal venous phases, respectively.
All RFA was performed by two radiologists (M.H.C. and K.Y.) who had more than 10 years’ experience of US-guided interventional procedures. Before RFA, the patients were examined by ultrasound and contrast CT or magnetic resonance imaging, and the size, shape and border of the tumor were determined, mainly based on US scans.
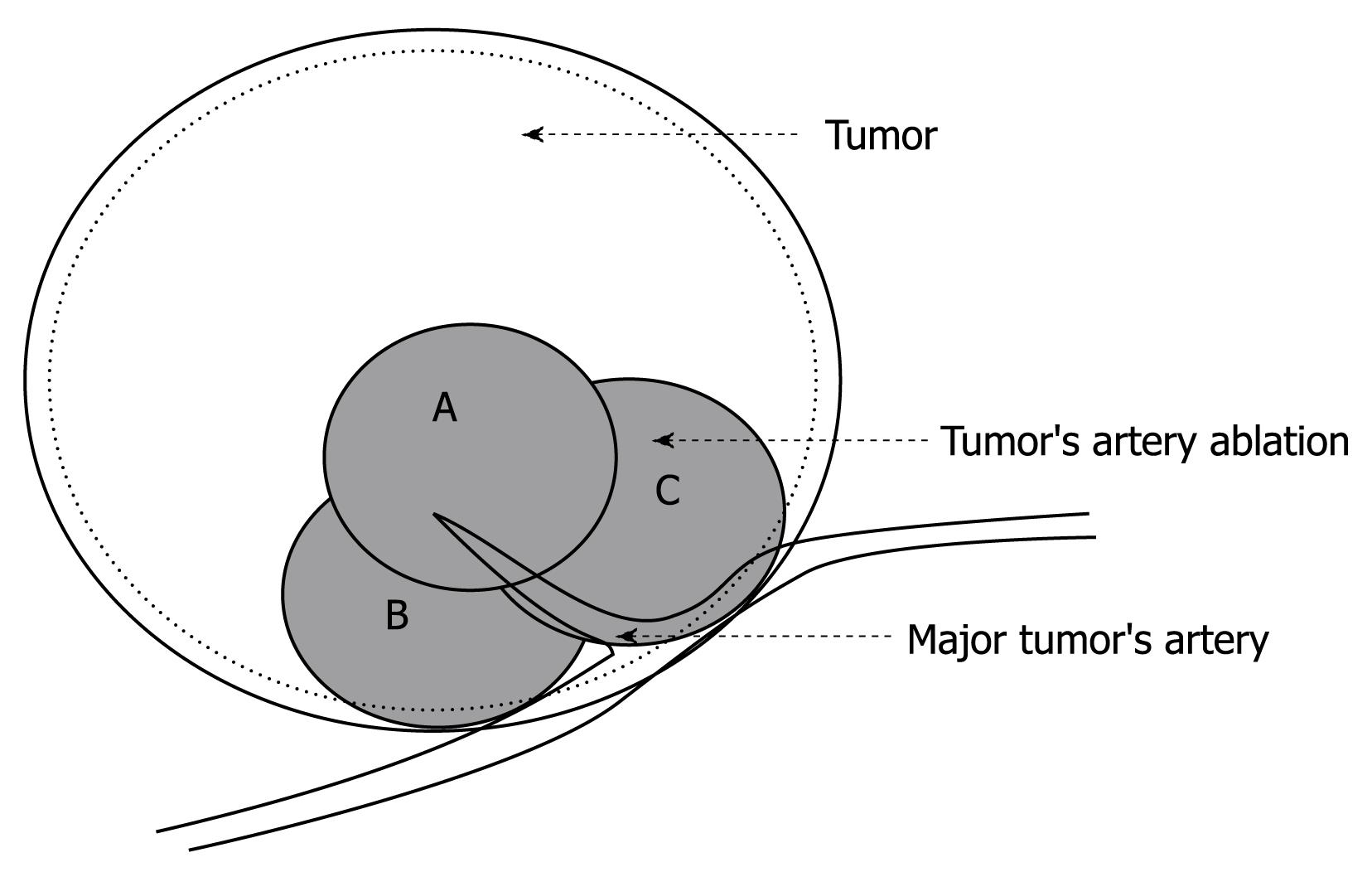
CDFI was used to identify the major feeding artery and guide the RFA needle to puncture the area where the artery entered the tumor. This area was ablated with 2-3 overlapping, high-energy ablation foci (2-3 cm each in diameter) in different direction or depths (Figure 1). The flow rate and vessel size of the feeding artery was measured before and immediately after PAA to evaluate the blood supply. After PAA, routine RFA treatment of the tumor was performed immediately, and the ablated area covered 0.5-1 cm beyond the tumor margin. As a result of the hypervascular character of the lesions, special attention was paid to using a slow withdrawal process of the electrode needle to avoid bleeding.
Patients in group B underwent routine RFA treatment using multiple overlapping ablation spheres to cover the tumor and the safe margin beyond the tumor of 0.5-1 cm[13]. During RFA, moderate intravenous sedation was induced with 2.5-5.0 mg midazolam (Roche, Basel, Switzerland) and 50-100 &mgr;g fentanyl (Fentaini; Renfu, Yichang, China). Local infiltration anesthesia was induced by 5-15 mL 1% lidocaine (Liduokayin; Yimin, Beijing, China). If patients with tumors adjacent to the diaphragm and hepatic hilum experienced local and right shoulder pain when the ablation was extended, intravenous infusion of propofol (Diprivan, 1-2 mg/kg; Zeneca, Macclesfield, UK) was given for temporary anesthesia enhancement.
The patients were conscious when the RFA electrode was placed, and vital signs and oxygen saturation were monitored continuously during the procedure. After RFA, the patients underwent close medical observation and were rescanned within 1-2 h after the procedure to detect any bleeding in the liver or the peritoneal cavity. All patients stayed in the hospital overnight.
Any adverse events were evaluated and recorded. Major complications were defined as those that, if left untreated, might have threatened the patient’s life, led to substantial morbidity and disability, or resulted in hospital admission or a substantially lengthened hospital stay. All other complications were considered minor.
Treatment response was assessed by contrast-enhanced spiral CT at 1 mo after RFA and complete response was considered to be achieved if the CT scans revealed: (1) the ablation zone was beyond the original tumor borders; (2) the margin of the ablation zone was clear and smooth; and (3) no arterial enhancement or abnormal wash-out was detected within or around the tumor. Subsequently, the patients were followed-up with serum alpha-fetoprotein (AFP) measurement, abdominal US, and contrast-enhanced CT every 2-3 mo in the first year, and then every 4-6 mo thereafter. All patients were followed-up for at least 6 mo. Recurrence was defined as enhancement within or at the periphery of the ablated area in the follow-up CT scan. Recurrent HCC was treated with another session of RFA. All CT scans were reviewed by two radiologists with more > 10 years experience, who were unaware of patient clinical data or treatment assignment.
Differences in complete necrosis rate at 1 mo and recurrence rate at 6 mo were analyzed by χ2 and t tests where appropriate. The recurrence rate was determined by log-rank tests, and multivariate hazard ratio was calculated using the Cox proportional hazard model. The Kaplan-Meier estimate of the cumulative recurrence rates over time was also carried out. All P values were two-sided, and P ≤ 0.05 was considered statistically significant. All analyses were performed with SAS software, version 6.12.
A total of 154 patients participated in the study from January 2003 to June 2007. No patient withdrew from the trial during 6 mo follow-up. Seventy-one subjects were assigned in group A, whose average tumor size was 4.3 ± 1.1 cm. Eighty-three participants were in group B, whose average tumor size was 4.1 ± 1.0 cm. The characteristics of the two groups are shown in Table 1. There was no significant difference between the two groups in patients’ clinical profile except for the gender ratio.
| Group A (n = 71) | Group B (n = 83) | P value | |
| Age (yr) | 59.3 ± 12.0 | 61.3 ± 12.0 | 0.28 |
| HCC size (cm) | 4.3 ± 1.1 | 4.1 ± 1.0 | 0.21 |
| Male | 63 (88.73) | 61 (73.49) | 0.02 |
| Cirrhosis | 53 (74.65) | 72 (86.75) | 0.06 |
| Previous surgery | 18 (25.35) | 14 (16.87) | 0.20 |
| Liver disease history | |||
| Hepatitis B | 54 (76.05) | 72 (86.75) | 0.06 |
| Hepatitis C | 6 (8.45) | 1 (1.20) | |
| Alcohol-related hepatitis | 2 (2.82) | 0 (0) | |
| No | 9 (12.68) | 10 (12.05) | |
| Child-Pugh class | |||
| A | 58 (81.69) | 61 (73.49) | 0.23 |
| B | 13 (18.31) | 22 (26.51) | |
| TNM staging | |||
| T1 | 32 (45.07) | 37 (44.58) | 0.27 |
| T2 | 19 (26.76) | 23 (27.71) | |
| T3 | 16 (22.54) | 18 (21.69) | |
| T4 | 4 (5.63) | 5 (6.02) |
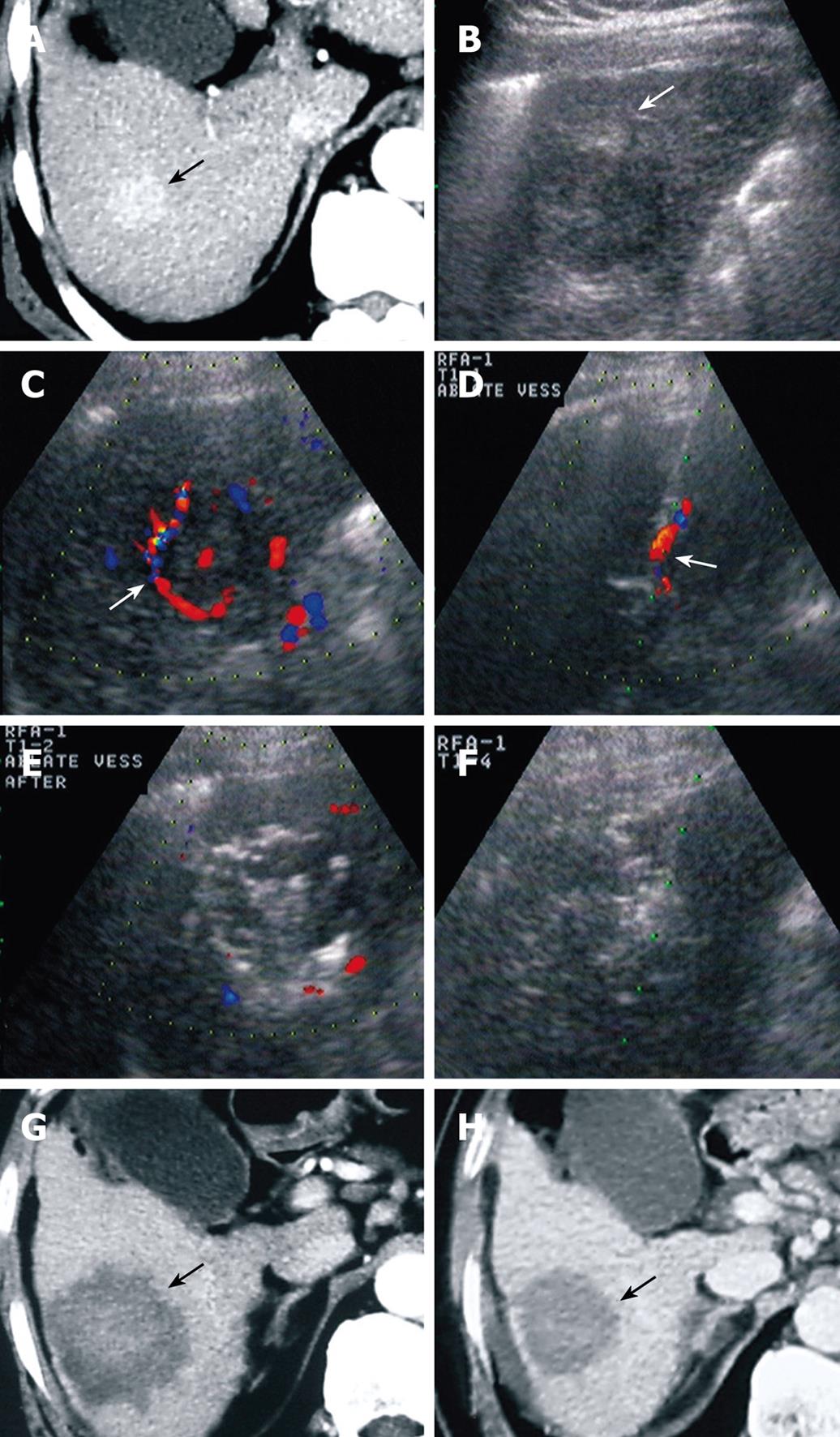
For patients in group A, the feeding artery was blocked in 66/75 (88%) HCC lesions, and decreased in size in 9/75 (12%) lesions. The average number of punctures per HCC was 2.76 ± 1.12. Complete necrosis rate at 1 mo after RFA was 90.67% (68/75), and recurrence rate at 6 mo was 17.33% (13/75, lesions) (Figure 2). For patients in group B, the average number of punctures per HCC was 3.36 ± 1.60. Complete necrosis rate at 1 mo after RFA was 90.20% (92/102), and recurrence rate at 6 mo was 31.37% (32/102).
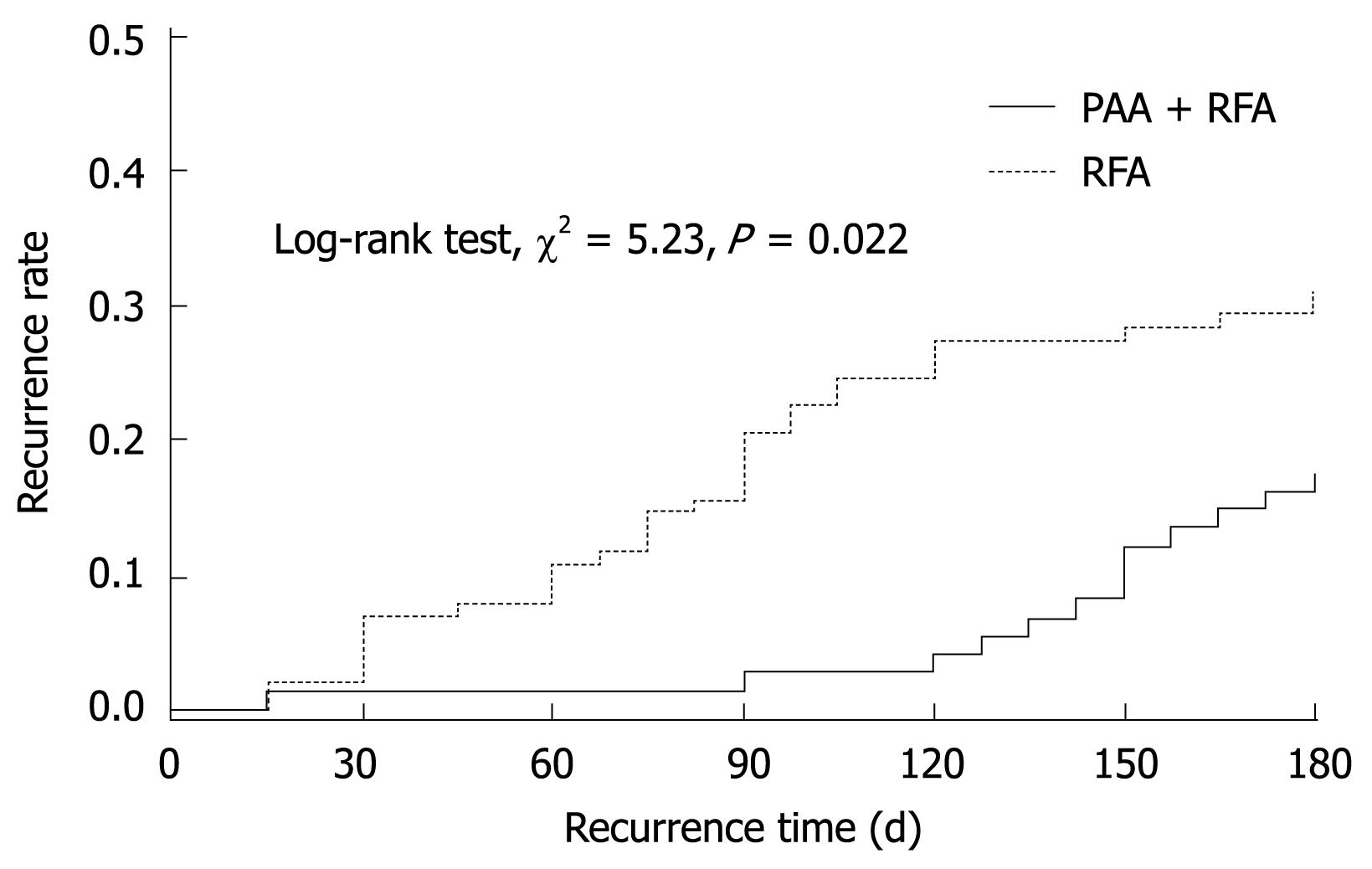
No significant difference was found in the necrosis rate at 1 mo post-RFA. However, there was a significant different between the two groups in the average number of punctures per HCC and recurrence rate at 6 mo after RFA. The recurrence time between the two groups was also significantly different by the log-rank test (χ2 = 5.23, P = 0.02; Figure 3). When adjusted for gender, age and cirrhosis, it was still significantly different (χ2 = 4.58, P = 0.03).
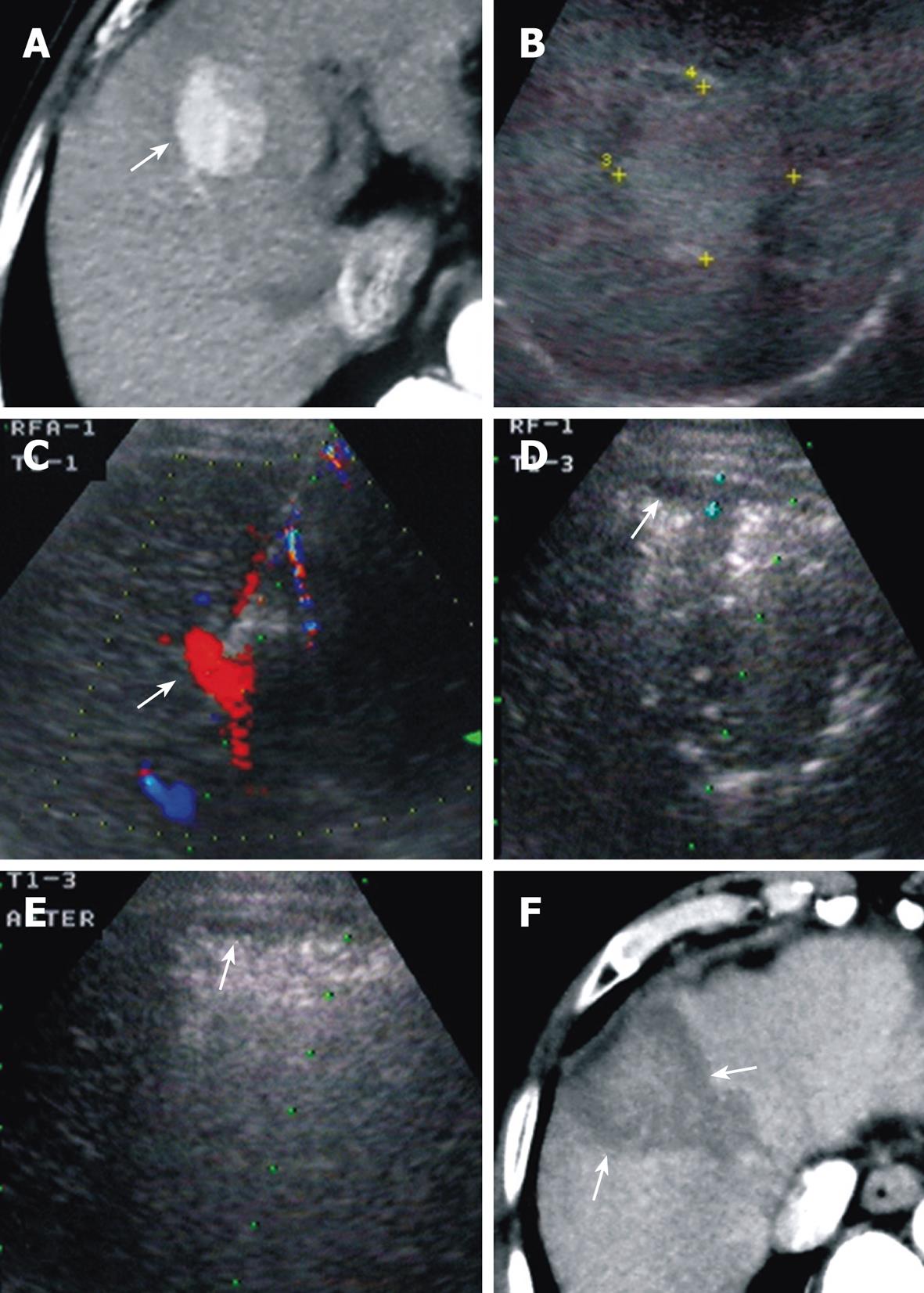
In group A, RFA-related major complications were seen in five patients (7.04%), including three cases of pleural fluid collection, one of bowel wall edema, and one of intrahepatic biliary duct dilation with jaundice, which were all relieved by conservative therapy. A small amount of subcapsular hemorrhage around the puncture site, approximately 0.5-1 cm thick, during RFA was seen with US in 13 patients (18.31%) (Figure 4). It was not considered as a major complication because homeostasis was achieved after injection of hemocoagulase, without additional intervention and no change in blood pressure. In group B, major complications were detected in nine cases (10.84%), including one of pneumothorax with pleural fluid collection, six of pleural effusion, one of abdominal wall abscess, and one of intraperitoneal hemorrhage in a tumor > 5 cm and protruding liver lateral surface. All complications were controlled with conservative treatment. There was no significant difference between the incidence of complications between the two groups.
The outcomes of RFA for HCC correlate closely with the location and blood supply of the tumor. Goldberg et al[10] have demonstrated that blood-flow-induced thermal loss in the tumor and liver tissue is the main reason for the decreased ablation effect of thermotherapy. The high-velocity blood flow of the tumor vessels created a heat sink effect that compromised the ablation effect, which led to residual and recurrent HCC. For the treatment of hypervascular HCC, many studies have focused on reducing flow perfusion to improve the thermal effect of RFA. Curley et al[14] have used the Pringle method[15] intraoperatively to reduce liver blood flow, by temporarily stopping portal vein and hepatic artery flow, and improving ablation outcomes. Goldberg et al[16] have used vascular agents, such as halothane, vasopressin and adrenaline, to adjust liver blood volume in order to increase ablation area.
TACE is one of the major interventional methods for HCC treatment, and when performed before RFA, it can increase therapeutic efficacy as a result of the decreased heat sink effect[17–19]. Kitamoto et al[20] have compared the therapeutic effects of RFA alone and in combination with TACE in 21 patients with 26 HCCs smaller than 3.0 cm. The size of the ablated necrotic area in the TACE/RFA group was significantly larger than that with RFA alone. However, repeated TACE treatment worsened liver function and quality of life[21], and then prolonged the interval between TACE and RFA. Therefore, for those who were ineligible or could not tolerate TACE treatment, other minimally invasive methods of reducing tumor blood supply before RFA were needed.
The study used PAA to ablate the area where the feeding artery entered the tumor, with small overlapping, high-energy ablation foci. This procedure was conducted through one puncture point using three ablations in different directions or depths. After PAA, the tumor’s feeding artery was blocked, thus reducing the blood-flow-induced heat loss, and achieving a similar result to that with TACE before RFA. PAA avoided damage to the surrounding liver parenchyma and liver function compared with TACE, and was well-tolerated by patients.
Recurrence after RFA remains an unsolved problem for large HCC. Harrison et al[22] reported percutaneous RFA in 46 HCC patients within 3 years, and only 14 (28%) of them showed no liver tumor tissue by imaging and AFP follow-up. Ruzzenete et al[23] have reviewed RFA of 104 HCCs in 88 patients with an average tumor size of 3.9 ± 1.3 cm. The necrotic rate for tumors < 3 cm, 3-5 cm and > 5 cm was 100%, 87.7% and 57.1%, respectively. In an average 19.2-mo follow-up period, 17 (19.3%) patients showed local recurrence. In our study, all the HCCs were > 3 cm in both groups A. For HCC treated with PAA and RFA in group A, the local recurrence rate at 6 mo was 17.33% (13/75), which was significantly lower than that in group B (31.37%, 32/102), which was treated with RFA alone (P = 0.0382). Although the proportion of cirrhosis was higher in group B than in group A, which might have influenced HCC recurrence, after adjusting for cirrhosis, the results still showed a significant difference between the two groups for recurrence time. Thus, PAA that was performed before routine RFA improved the treatment efficacy in hypervascular HCC. With the new strategy of PAA combined with RFA, the number of percutaneous punctures per HCC was reduced in group A to an average of 2.76 ± 1.12, which was significantly less than that in group B, 3.36 ± 1.60 (P = 0.001), thus injury to patients was reduced. Kitamoto et al[20] reported that the average duration between TACE and RFA was 18.2 d. In our study, RFA could be performed immediately after blocking of the major feeding artery with PAA, which could reduce hospital stay.
In group A, 13 (17.33%) patients had a small amount of bleeding during PAA, most of which was detected at the first puncture, where the RFA needle punctured the area where the feeding artery entered the tumor. We supposed that the bleeding might have been caused by damage of the feeding vessels and incomplete ablation. Additional focal ablation in the area was helpful in stopping bleeding.
One limitation of our study was the short duration of follow-up. However, our main goal was to demonstrate the benefit of PAA/RFA in treating hypervascular HCC, and our data confirmed this. PAA blocked the feeding artery of the tumor, and then blood-flow-induced heat loss was reduced during RFA treatment. We think that the short-term benefits of PAA/RFA compared with RFA alone for hypervascular HCC provide some insight for the future wider application of this treatment.
In conclusion, for hypervascular HCC patients who were unsuitable for surgical resection or TACE, PAA was an alternative for blocking the feeding artery of the tumor, and reducing heat loss during subsequent RFA. The combination of PAA and RFA could significantly decrease post-RFA recurrence and provide a safe and effective treatment for hypervascular HCC.
Hepatocellular carcinoma (HCC) is the most common primary malignant liver neoplasm worldwide. Although surgical resection is the gold standard for treatment of HCC, only a limited number of patients are surgical candidates because of their lack of hepatic reserve that results from coexisting advanced cirrhosis, widespread intrahepatic involvement, and concomitant diseases. Therefore, a variety of imaging-guided tumor ablation therapies such as ethanol injection, microwave coagulation, percutaneous radiofrequency ablation (RFA) and laser ablation are often considered as alternative options. Among them, RFA has been used increasingly as a safe technique for treating hepatic tumors.
For hypervascular HCC, RFA appears less effective because of the blood-flow-induced heat sink effect, which might cause incomplete ablation or recurrence. Previous experiments have shown that mechanical and pharmacological strategies that are aimed at lowering hepatic perfusion can increase the size of thermally induced lesions.
Repeated transcatheter arterial chemoembolization (TACE) worsened liver function and quality of life, and then prolonged the interval between TACE and RFA. In order to overcome these disadvantages, the study used PAA to ablate the area where the feeding artery entered the tumor, with small overlapping, high-energy ablating foci. This procedure was conducted through one puncture point using three ablations in different directions or depths. After PAA, the tumor’s feeding artery was blocked, thus reducing the blood-flow-induced heat loss, and achieving a similar result as that with TACE before RFA. PAA avoided damage to the surrounding liver parenchyma and liver function compared with TACE, and was well-tolerated by patients.
The study results suggested that, for hypervascular HCC patients who were unsuitable for surgical resection or TACE, PAA was an alternative for effectively blocking the feeding artery of the tumor, and reducing heat loss during subsequent RFA. The combination of PAA and RFA may significantly decrease post-RFA recurrence and provide a safe and effective treatment for hypervascular HCC.
PAA: Color Doppler flow imaging was used to identify the major feeding artery and guide the RFA needle to puncture the area where the feeding artery entered the tumor. This area was ablated with 2-3 overlapping high-energy ablation foci (2-3 cm each in diameter) in different directions or depths.
This is a good original study in which authors performed a new approach to block the major feeding artery and reduce heat loss during subsequent RFA. The results are interesting and suggest that the combination of PAA and RFA significantly decreases post-RFA recurrence and provides a safe and effective treatment for hypervascular HCC.
| 1. | Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153-156. |
| 2. | Stuart KE, Anand AJ, Jenkins RL. Hepatocellular carcinoma in the United States. Prognostic features, treatment outcome, and survival. Cancer. 1996;77:2217-2222. |
| 3. | Luo BM, Wen YL, Yang HY, Zhi H, Xiao XY, Ou B, Pan JS, Ma JH. Percutaneous ethanol injection, radiofrequency and their combination in treatment of hepatocellular carcinoma. World J Gastroenterol. 2005;11:6277-6280. |
| 4. | Shibata T, Murakami T, Ogata N. Percutaneous microwave coagulation therapy for patients with primary and metastatic hepatic tumors during interruption of hepatic blood flow. Cancer. 2000;88:302-311. |
| 5. | Pearson AS, Izzo F, Fleming RY, Ellis LM, Delrio P, Roh MS, Granchi J, Curley SA. Intraoperative radiofrequency ablation or cryoablation for hepatic malignancies. Am J Surg. 1999;178:592-599. |
| 6. | Pacella CM, Bizzarri G, Cecconi P, Caspani B, Magnolfi F, Bianchini A, Anelli V, Pacella S, Rossi Z. Hepatocellular carcinoma: long-term results of combined treatment with laser thermal ablation and transcatheter arterial chemoembolization. Radiology. 2001;219:669-678. |
| 7. | Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Ierace T, Solbiati L, Gazelle GS. Hepatocellular carcinoma: radio-frequency ablation of medium and large lesions. Radiology. 2000;214:761-768. |
| 8. | Giorgio A, Tarantino L, de Stefano G, Scala V, Liorre G, Scarano F, Perrotta A, Farella N, Aloisio V, Mariniello N. Percutaneous sonographically guided saline-enhanced radiofrequency ablation of hepatocellular carcinoma. AJR Am J Roentgenol. 2003;181:479-484. |
| 9. | Solmi L, Nigro G, Roda E. Therapeutic effectiveness of echo-guided percutaneous radiofrequency ablation therapy with a LeVeen needle electrode in hepatocellular carcinoma. World J Gastroenterol. 2006;12:1098-1104. |
| 10. | Goldberg SN, Hahn PF, Tanabe KK, Mueller PR, Schima W, Athanasoulis CA, Compton CC, Solbiati L, Gazelle GS. Percutaneous radiofrequency tissue ablation: does perfusion-mediated tissue cooling limit coagulation necrosis? J Vasc Interv Radiol. 1998;9:101-111. |
| 11. | Rossi S, Garbagnati F, Lencioni R, Allgaier HP, Marchiano A, Fornari F, Quaretti P, Tolla GD, Ambrosi C, Mazzaferro V. Percutaneous radio-frequency thermal ablation of nonresectable hepatocellular carcinoma after occlusion of tumor blood supply. Radiology. 2000;217:119-126. |
| 12. | Yamasaki T, Kurokawa F, Shirahashi H, Kusano N, Hironaka K, Okita K. Percutaneous radiofrequency ablation therapy for patients with hepatocellular carcinoma during occlusion of hepatic blood flow. Comparison with standard percutaneous radiofrequency ablation therapy. Cancer. 2002;95:2353-2360. |
| 13. | Chen MH, Yang W, Yan K, Zou MW, Solbiati L, Liu JB, Dai Y. Large liver tumors: protocol for radiofrequency ablation and its clinical application in 110 patients--mathematic model, overlapping mode, and electrode placement process. Radiology. 2004;232:260-271. |
| 14. | Curley SA, Izzo F, Delrio P, Ellis LM, Granchi J, Vallone P, Fiore F, Pignata S, Daniele B, Cremona F. Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: results in 123 patients. Ann Surg. 1999;230:1-8. |
| 15. | Delva E, Camus Y, Nordlinger B, Hannoun L, Parc R, Deriaz H, Lienhart A, Huguet C. Vascular occlusions for liver resections. Operative management and tolerance to hepatic ischemia: 142 cases. Ann Surg. 1989;209:211-218. |
| 16. | Goldberg SN, Hahn PF, Halpern EF, Fogle RM, Gazelle GS. Radio-frequency tissue ablation: effect of pharmacologic modulation of blood flow on coagulation diameter. Radiology. 1998;209:761-767. |
| 17. | de Baere T, Bessoud B, Dromain C, Ducreux M, Boige V, Lassau N, Smayra T, Girish BV, Roche A, Elias D. Percutaneous radiofrequency ablation of hepatic tumors during temporary venous occlusion. AJR Am J Roentgenol. 2002;178:53-59. |
| 18. | Patterson EJ, Scudamore CH, Owen DA, Nagy AG, Buczkowski AK. Radiofrequency ablation of porcine liver in vivo: effects of blood flow and treatment time on lesion size. Ann Surg. 1998;227:559-565. |
| 19. | Chinn SB, Lee FT Jr, Kennedy GD, Chinn C, Johnson CD, Winter TC 3rd, Warner TF, Mahvi DM. Effect of vascular occlusion on radiofrequency ablation of the liver: results in a porcine model. AJR Am J Roentgenol. 2001;176:789-795. |
| 20. | Kitamoto M, Imagawa M, Yamada H, Watanabe C, Sumioka M, Satoh O, Shimamoto M, Kodama M, Kimura S, Kishimoto K. Radiofrequency ablation in the treatment of small hepatocellular carcinomas: comparison of the radiofrequency effect with and without chemoembolization. AJR Am J Roentgenol. 2003;181:997-1003. |
| 21. | Nishizaki T, Takenaka K, Yoshida K, Ikeda T, Sugimachi K. Influence of lipiodolization on a cirrhotic liver. J Surg Oncol. 1995;58:263-268. |
| 22. | Harrison LE, Koneru B, Baramipour P, Fisher A, Barone A, Wilson D, Dela Torre A, Cho KC, Contractor D, Korogodsky M. Locoregional recurrences are frequent after radiofrequency ablation for hepatocellular carcinoma. J Am Coll Surg. 2003;197:759-764. |
| 23. | Ruzzenente A, Manzoni GD, Molfetta M, Pachera S, Genco B, Donataccio M, Guglielmi A. Rapid progression of hepatocellular carcinoma after Radiofrequency Ablation. World J Gastroenterol. 2004;10:1137-1140. |