Published online May 21, 2009. doi: 10.3748/wjg.15.2314
Revised: April 7, 2009
Accepted: April 14, 2009
Published online: May 21, 2009
Non-classical manifestations of autoimmune hepatitis can delay diagnosis and treatment. Our aims were to describe the clinical phenotypes that can confound the diagnosis, detail scoring systems that can ensure their recognition, and outline advances in treatment that can improve their outcome. Prime source and review articles in English were selected through Medline from 1970-2008 and assimilated into personal libraries spanning 32 years. Acute severe or asymptomatic presentations and atypical histological findings, including centrilobular zone 3 necrosis and concurrent bile duct changes, are compatible with the diagnosis. Cholangiographic abnormalities may be present in children and adults with the disease, and autoimmune hepatitis must be considered in patients without autoantibodies or with antimitochondrial antibodies and no other cholestatic features. Asymptomatic patients frequently become symptomatic; mild disease can progress; and there are no confident indices that justify withholding treatment. Two diagnostic scoring systems with complementary virtues have been developed to evaluate patients with confusing features. Normal liver tests and tissue constitute the optimal end point of treatment, and the first relapse is an indication for long-term azathioprine therapy. Cyclosporine, tacrolimus and mycophenolate mofetil are promising salvage therapies, and budesonide with azathioprine may be a superior frontline treatment. We conclude that the non-classical phenotypes of autoimmune hepatitis can be recognized promptly, diagnosed accurately, and treated effectively.
- Citation: Czaja AJ, Bayraktar Y. Non-classical phenotypes of autoimmune hepatitis and advances in diagnosis and treatment. World J Gastroenterol 2009; 15(19): 2314-2328
- URL: https://www.wjgnet.com/1007-9327/full/v15/i19/2314.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.2314
Autoimmune hepatitis was initially perceived as a self-perpetuating, inflammatory liver disease in young amenorrheic women with hirsutism, acne and cirrhosis[1–3]. The validity of this classical phenotype was subsequently strengthened by technological advances that excluded a viral cause for the condition[4] and by studies that implicated perturbations of the immune system in its pathogenesis[5–8]. The clinical phenotype expanded as the concept of autoimmunity was applied broadly to liver diseases of unknown cause and as the requirement for 6 mo of disease activity was eliminated from its definition[910]. Autoimmune hepatitis still lacks an etiological agent and disease-specific laboratory test, but its designation now applies to patient populations far more diverse and numerous than the original patients with “lupoid hepatitis”[11].
Autoimmune hepatitis must be considered in all individuals with acute and chronic hepatitis of undetermined cause and with graft dysfunction after liver transplantation[11]. Acute[12–15], acute severe[16–19], and asymptomatic[20–22] forms have been described; progression to cirrhosis may be indolent and unsuspected[23–25]; transitions between active and inactive disease may occur spontaneously[2627]; concurrent immune diseases may obscure the diagnosis[2829]; serological markers may be variably expressed and absent at presentation[30–32]; histological findings may include centrilobular zone 3 necrosis[33–37] or concurrent biliary changes[38–42]; and different ethnic groups may have non-classical clinical phenotypes[43–47]. The identification of autoimmune hepatitis in diverse clinical situations is critical since prompt institution of corticosteroid therapy can be life-saving[48–50].
Corticosteroids alone or a lower dose in combination with azathioprine induce clinical, laboratory and histological improvement in 80% of patients within 3 years[5152]. Ten- and twenty-year survivals exceed 80%[24], and hepatic fibrosis is reduced or prevented[53–56]. These therapeutic successes are counterbalanced against adverse outcomes that justify the continued pursuit of new drugs and regimens. Nine percent of treated patients deteriorate despite compliance with corticosteroid schedules (treatment failure)[5758]; 13% develop treatment-related side effects that compel premature withdrawal of medication (drug toxicity)[59]; and 9% improve but not to a degree to justify discontinuation of the medication (incomplete response)[59]. Furthermore, 50%-86% of patients who enter remission relapse after drug withdrawal and require re-treatment[275160–64].
The diagnostic criteria of autoimmune hepatitis have been codified by an international panel[910], and scoring systems can establish the diagnosis in difficult cases[9106566]. The re-definition of treatment goals[67–70] and the revision of current treatment strategies[71–75] promise to improve results. The emergence of powerful immunosuppressive agents, mainly from the transplantation arena, promises to strengthen the treatment repertoire[7677], and the clarification of critical pathogenic pathways make site-specific molecular interventions feasible[767879]. The clinical spectrum of autoimmune hepatitis has expanded, but the diagnostic instruments and therapeutic options have also improved.
The objectives of this review are to describe the non-classical clinical phenotypes of autoimmune hepatitis, detail the diagnostic instruments that can ensure their recognition, and introduce the evolving treatment strategies. Classical syndromes are the cornerstones of clinical practice, but variations from the classical are its realities. The changing spectrum of autoimmune hepatitis and its treatment underscores the importance of the disease and the vigor of the investigative effort that it has generated.
Autoimmune hepatitis is defined as a self-perpetuating inflammation of the liver of unknown cause that is characterized by interface hepatitis on histological examination, hypergammaglobulinemia, and autoantibodies[80]. The diagnosis has great latitude since no features are disease-specific. The phenotypes that satisfy the definition of autoimmune hepatitis but are outside the boundaries of classical disease have acute severe presentations, few or no symptoms, atypical histological findings, absent or variant serological markers, concurrent cholangiographic changes, male gender, and non-Caucasian backgrounds.
The diagnosis of autoimmune hepatitis is no longer restricted by a time requirement for disease activity[910]. An acute severe or fulminant presentation can reflect de novo inflammation[12–15] or the spontaneous exacerbation of a previously unsuspected chronic disease[81]. An acute or abrupt onset occurs in 40% of patients with autoimmune hepatitis[148182], whereas an acute severe presentation is rare[83] (Table 1).
| Non-classical phenotype | Salient features |
| Acute severe disease | Corticosteroids effective in 36%-100%[49] |
| Protracted treatment can be complicated by infection[49] | |
| High mortality if no better within 2 wk of therapy[85] | |
| MELD score ≥ 12 identifies 97% of treatment failures[58] | |
| Asymptomatic mild hepatitis | Common (25%-34%) but unstable state[202122878889] |
| Symptoms develop in 26%-70%[2021] | |
| Progression possible if untreated[20212287] | |
| Improves quickly with therapy[22] | |
| Atypical histological features | Centrilobular necrosis is an early acute form[18333435363792] |
| Transition to interface hepatitis possible[35] | |
| Coincidental biliary changes lack cholestatic profile[41] | |
| Fatty changes may co-exist[5894] | |
| Absent or variant serological markers | Seronegativity possible in 13%[31] |
| Other features and treatment outcome similar[3132100] | |
| Non-standard autoantibodies possible[101102103104] | |
| Conventional autoantibodies may be expressed later[30] | |
| Screen for celiac disease[105106107108] | |
| Concurrent cholangiographic changes | Abnormal cholangiograms in 44% with CUC[116] |
| Poor outcome if biliary changes and CUC[121122123124] | |
| MRC abnormalities in 8% adults without CUC[117] | |
| MRC abnormalities may be associated with fibrosis[119] | |
| Male gender | 0.2-0.5 cases/100 000 per year[127128] |
| Low frequency of concurrent immune diseases[130131132] | |
| No diversity of HLA DRB1*04 alleles[130131132] | |
| Better survival than women[136] | |
| Non-Caucasian | Cholestatic features may be common[45141142143] |
| Male predominance possible[47] | |
| Socioeconomic factors important[137138146147] |
The acute form can be differentiated from the chronic form by laboratory features [higher serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels, total serum bilirubin concentration, and serum γ-glutamyl transpeptidase level] and by histological findings (more frequent centrilobular zone 3 necrosis with plasmacytic infiltration and bile duct injury), but its recognition in individual cases relies mainly on an awareness that acute severe autoimmune hepatitis is possible[84].
Corticosteroid therapy suppresses inflammatory activity in 36%-100% of patients with acute severe presentations[49], whereas delay in treatment can result in a poor outcome[5183] (Table 1). Immediate survival and the need for urgent transplantation depend on the rapidity and nature of the response to corticosteroids[508586]. Failure to improve at least one laboratory abnormality reflective of liver inflammation or function, especially a pre-treatment hyperbilirubinemia, within 2 wk indicates that liver transplantation should be considered[85]. Relentless pursuit of an unachievable benefit from corticosteroid therapy can be complicated by infection and the lost opportunity for a successful transplantation[49].
The Model of End Stage Liver Disease (MELD) can be used to identify individuals with autoimmune hepatitis who are likely to fail corticosteroid therapy and require liver transplantation[58] (Table 1). MELD scores ≥ 12 points at presentation have a sensitivity of 97% and specificity of 68% for treatment failure, and patients with such scores warrant close scrutiny and preparedness for liver transplantation. A MELD score ≥ 12 points at presentation captures all problematic patients, but it does not preclude their salvation through prompt and vigorous corticosteroid treatment.
Autoimmune hepatitis is asymptomatic in 25%-34% of patients at presentation[20–22], and retrospective analyses have estimated that 25%-85% of individuals have mild disease[20–2287–89] (Table 1). These presentations contrast with those described in the classical treatment trials in which selection criteria focused on severe symptomatic and immediately life-threatening disease[519091]. Treatment guidelines have been promulgated for the individuals with severe disease, but they remain arbitrary and inconsistent for those with mild disease[80]. These difficulties reflect in part the lack of a codified definition of mild autoimmune hepatitis and uncertainty about its natural history.
Untreated mild autoimmune hepatitis has a better outcome than severe disease, but it does not have a benign prognosis (Table 1). Cirrhosis develops in 49% of untreated patients within 15 years[87]; liver failure and hepatocellular carcinoma are possible[22]; asymptomatic patients become symptomatic in 26%-70% of instances[2021]; and 10-year mortality exceeds 10%[2122]. Spontaneous resolution is possible, but untreated patients with mild autoimmune hepatitis improve less commonly (12% vs 63%, P = 0.006) and more slowly than treated patients, and they have a lower 10-year survival (67% vs 98%, P = 0.01)[22].
A “safe” subset of patients with non-aggressive autoimmune hepatitis who require no therapy cannot be reliably identified, and the clinical threshold for starting corticosteroid therapy cannot be so high that all patients with mild or asymptomatic disease are excluded (Table 1). Mild autoimmune hepatitis can improve spontaneously, and this prospect may dampen therapeutic zeal, especially if measured against the possibility of serious treatment-related complications[22]. A dictum to do no harm, however, that focuses more concern on the treatment than the disease may be incorrect.
The aggressive potential of mild autoimmune hepatitis at presentation, the inability to predict outcome by clinical parameters, the expected rapidity of the treatment response, and the safety of current treatment regimens favor a proactive management strategy[22]. Until randomized clinical trials are performed comparing treatment against no treatment, the management strategy in patients with mild disease should lean toward conventional therapy. Mild asymptomatic autoimmune hepatitis is a non-classical phenotype, but it should not be regarded or managed as a different disease.
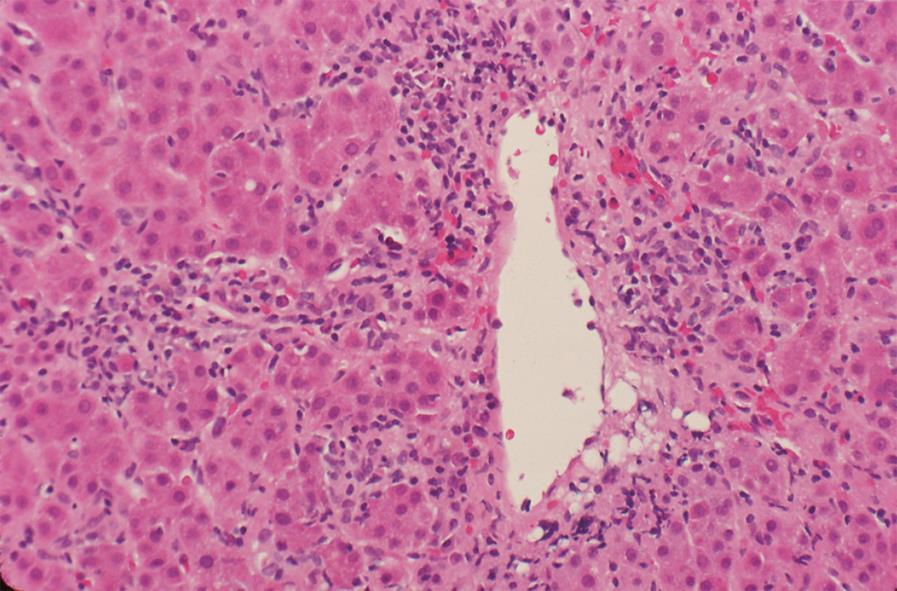
The histological hallmark of autoimmune hepatitis is interface hepatitis, but other histological findings are compatible with the disease[910] (Table 1). Centrilobular zone 3 necrosis (Figure 1) is probably an early form of autoimmune hepatitis that is detected mainly in patients with an acute onset[1833–3792]. Successive liver tissue examinations have disclosed transition of the centrilobular zone 3 pattern of necrosis to that of typical interface hepatitis during the course of the disease[35]. This non-classical finding may suggest an acute viral or toxic injury, but the diagnosis of autoimmune hepatitis should not be discounted.
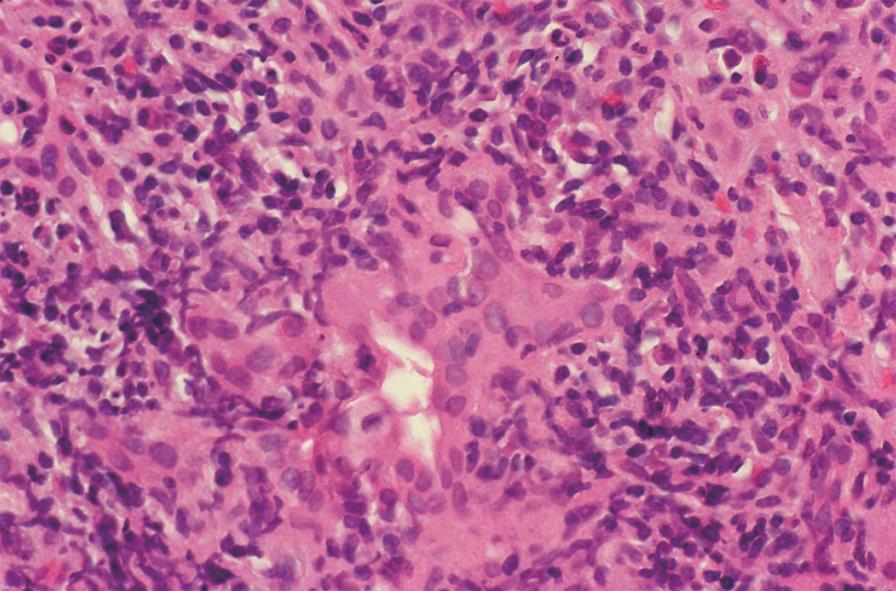
Concurrent biliary changes, including isolated destructive cholangitis (Figure 2), may also be found in patients with otherwise classical autoimmune hepatitis[38–42] (Table 1). These patients do not have a cholestatic clinical or laboratory profile, and successive tissue examinations have not disclosed persistence or progression of the biliary injury[41]. The biliary changes probably reflect collateral damage associated with an exuberant inflammatory process rather than a transition state to a cholestatic disease or variant syndrome. The biliary changes should not alter the diagnosis or the treatment strategy.
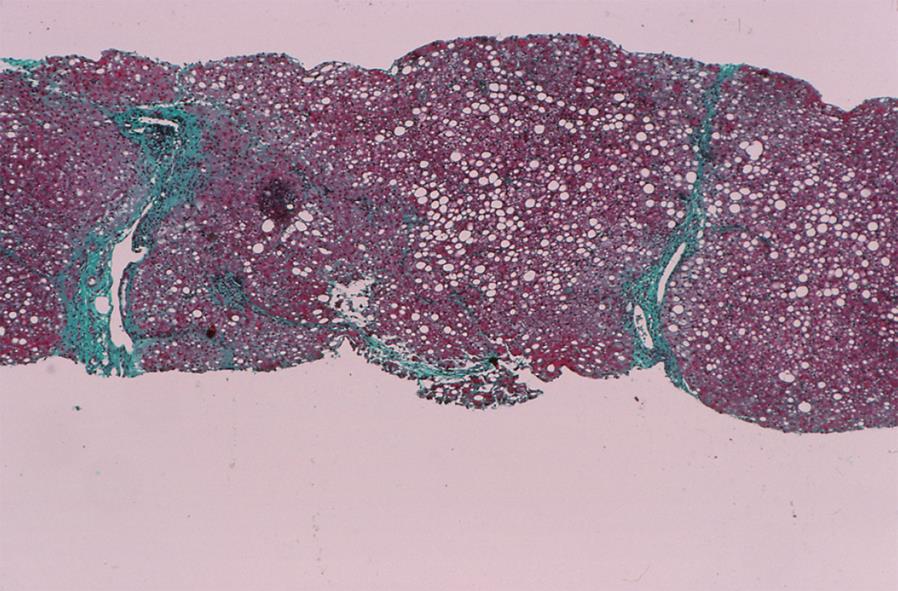
Fatty changes (Figure 3) may also be present at accession or after corticosteroid therapy[589394] (Table 1). Non-alcoholic fatty liver disease (NAFLD) is a common finding in the general population, and it may be associated with autoantibodies and hypergammaglobulinemia[94–97]. Both conditions can co-exist, and corticosteroid therapy can ameliorate the autoimmune hepatitis and intensify the NAFLD[5894]. The presence of coincidental fatty change should not discourage the diagnosis or treatment of autoimmune hepatitis, but it compels an accurate diagnosis. Worsening of the laboratory indices during therapy justifies liver tissue examination and reassessment of the treatment strategy[5894]. Progressive fatty change can be a cause of treatment failure[58].
Antinuclear antibodies (ANA), smooth muscle antibodies (SMA), and antibodies to liver kidney microsome type 1 (anti-LKM1) are the classical serological markers of autoimmune hepatitis[9899]. These antibodies are not pathogenic or disease-specific, and their expression can vary in individual cases and in different geographical regions and ethnic groups. Thirteen percent of white North American adults with classical features of autoimmune hepatitis lack ANA, SMA, and anti-LKM1[31] (Table 1).
Seronegative patients with autoimmune hepatitis have a non-classical phenotype, and they constitute an “autoantibody-negative autoimmune hepatitis”[3132100]. These patients are indistinguishable from those with classical disease, including their HLA profiles, and they also respond to corticosteroid therapy[3132100] (Table 1). Twenty percent may express non-standard autoantibodies, such as antibodies to soluble liver antigen (anti-SLA)[101102] or atypical perinuclear anti-neutrophil cytoplasmic antibodies (atypical pANCA)[103104], and others may express SMA, ANA or both later in their course[30]. Some corticosteroid-responsive patients remain seronegative throughout their disease, and they may await discovery of their signature autoantibody[3132100]. All such patients must be screened for celiac disease by testing for immunoglobulin A antibodies to tissue transglutaminase or endomysium[105–108].
Antimitochondrial antibodies (AMA) can be present in 8%-35% of patients with otherwise classical autoimmune hepatitis, and they define another non-classical serological phenotype[108–112]. These coincidental AMA are not associated with cholestatic features, histological findings of biliary injury, or different response to corticosteroid therapy[110–113]. They may persist for as long as 27 years in the absence of primary biliary cirrhosis (PBC)[111]; they may disappear spontaneously; or they may appear late in the course of the disease without apparent clinical relevance[112]. Severe inflammatory activity may result in modification of the mitochondrial antigens through oxidative stress and facilitate the production of AMA which in turn can disappear when the inflammatory stress subsides[114]. AMA in the absence of cholestatic laboratory or histological features should not dissuade the diagnosis of autoimmune hepatitis or compel a different treatment strategy. The “serological overlap” with PBC does not constitute a hybrid disease or pathological process in transition.
Concurrent cholangiographic changes have been described in children[115] and adults[116117] with autoimmune hepatitis, and these findings constitute another non-classical clinical phenotype (Table 1). The emergence of magnetic resonance cholangiography (MRC) as a safe, effective and non-invasive mechanism by which to assess the biliary system[118] has indicated that cholangiographic changes that resemble primary sclerosing cholangitis (PSC) occur in 8% of adults with autoimmune hepatitis[117]. These changes occur predominately in women who typically lack inflammatory bowel disease, and they are associated with histological features that reflect increased lobular activity rather than biliary injury[117].
The nature and significance of the biliary changes by MRC remain unclear since most adults with these changes respond to corticosteroid therapy[117]. The possibility of a disease process other than typical PSC or an unusual but nonspecific biliary distortion induced by fibrosis cannot be discounted (Table 1). Recent prospective studies have indicated that while adults with autoimmune hepatitis have a high frequency of intrahepatic biliary changes by MRC (24%), the occurrence of PSC is rare (2%)[119]. Furthermore, the frequency of biliary changes by MRC in adults with autoimmune hepatitis is similar to that in patients with cirrhosis of a non-biliary and non-autoimmune nature. Hepatic fibrosis rather than the nature of the liver disease may be the most important parameter independently associated with the biliary changes[119].
Cholangiographic abnormalities by endoscopic or intrahepatic cholangiography are present in 44% of adults with autoimmune hepatitis and inflammatory bowel disease[116120], and patients with these changes are typically refractory to corticosteroid therapy[121–124] (Table 1). This is the non-classical phenotype that has immediate clinical relevance, and its discovery impacts on the diagnosis, treatment, and outcome. Biliary studies should be performed mainly in adult patients with inflammatory bowel disease or recalcitrance to corticosteroid therapy[121125]. Not all biliary changes have independent pathological significance or clinical importance[126].
Autoimmune hepatitis does occur in white northern European men[127128], and its development in this gender constitutes another non-classical phenotype (Table 1). Women with autoimmune hepatitis outnumber men with the disease by more than three-fold[129], and estimates of the incidence of autoimmune hepatitis in northern European men ranges from 0.2-0.5 cases per 100 000 persons per year[127128]. The existence of an important clinical distinction between men and women with autoimmune hepatitis is still unsettled, but clearly the experiences over the decades have not identified a striking difference between the genders.
White North American women with autoimmune hepatitis are distinguished from men with autoimmune hepatitis and the same ethnicity by having higher frequencies of concurrent immune diseases (34% vs 17%, P = 0.05) and HLA DRB1*04 (49% vs 24%, P = 0.007)[130–132] (Table 1). Women with HLA DRB1*04 also have higher frequencies of concurrent immune diseases than women without HLA DRB1*04 (52% vs 22%, P < 0.000001) as do men with HLA DRB1*04 compared to men without HLA DRB1*04 (26% vs 4%, P = 0.002)[129132]. These findings suggest that the clinical phenotype is driven by the genetic predisposition of the host as well as the gender[129133].
Retrospective surveys have suggested gender-based differences in disease behavior and treatment outcome, but results have been discrepant[134–136]. Differences in age at presentation (39 years vs 49 years, P = 0.06) and the frequency of relapse after drug withdrawal (71% vs 55%, P = 0.06) have not reliably distinguished men from women with autoimmune hepatitis. The higher frequency of HLA A1-B8-DRB1*03 in men who relapse (50% vs 23%, P = 0.003) and greater mortality in women than men (P = 0.02) have been contrasting features in some experiences, and these findings require further examination[135136] (Table 1).
The principal clinical concern related to gender is that the diagnosis of autoimmune hepatitis might be overlooked in men. Gender may be a surrogate marker that signifies different antigenic exposures, hormonal effects on immune responsiveness, chromosomal imbalances that favor loss of self-tolerance, and genetic predispositions for immunocyte activation[132]. The diagnosis of autoimmune hepatitis in men should trigger the same treatment strategies and monitoring schedules as in women.
Racial background can affect the clinical phenotype of autoimmune hepatitis, and diagnostic criteria developed mainly in white northern European and North American populations may not apply in different ethnic groups and geographical regions (Table 1). Black North American patients have cirrhosis at presentation more commonly than white North American patients (85% vs 38%)[43137138]. Japanese patients typically have mild, late onset disease[139]. South American patients are younger than white North American counterparts, and they have more severe laboratory abnormalities at presentation[140]. Alaskan natives have a higher frequency of acute icteric disease, asymptomatic illness, and advanced fibrosis at accession than non-native patients[44]. Aboriginal North Americans have disproportionately high frequencies of immune-mediated disorders, cholestatic features, and advanced disease at presentation[141–143]. African, Asian and Arab patients have a higher frequency of biliary changes on histological examination than white northern European patients[45], and patients from Somalia are frequently men with rapidly progressive disease[47] (Table 1).
The variations in clinical phenotype suggest that genetic background and geographical location affect occurrence and behavior of the disease[129133]. Indigenous etiological agents or population-dependent genetic factors may modulate susceptibility to autoimmune hepatitis, determine targets of the immune response, and affect the vigor of the inflammatory reaction[144145]. Socioeconomic status, healthcare access, and quality of care are other factors that must be analyzed when assessing discrepancies in disease occurrence and outcome among different racial groups[137138146147] (Table 1).
The importance of recognizing the diverse manifestations of autoimmune hepatitis in different regions and ethnic groups is illustrated by the appearance and behavior of the disease in Turkey. Autoimmune hepatitis in this region has a character that aligns with the disease of white northern Europeans and North Americans, but it can be difficult to recognize if only the western phenotype is considered.
Autoimmune hepatitis is a relatively rare disorder in Turkey when compared with chronic viral hepatitis, but it is still the most common autoimmune liver disease[148149]. Its high prevalence in women is not unusual, but its 9:1 female-to-male ratio[148149] exceeds the female propensity (3:1 female-to-male ratio) in North America[129]. The age of occurrence in adults (age ranges, 18-59 years; mean age, 42 years) is as broad as elsewhere[150], but there are many patients with signs of hepatitis who are negative for viral markers and the conventional autoantibodies[149]. These patients are typically designated as having “cryptogenic chronic hepatitis”, but they respond well to treatment with corticosteroids and azathioprine. Other liver diseases must be carefully excluded, especially in men and those who lack the conventional autoantibodies, and the diagnosis must be supported by the demonstration of compatible liver enzyme abnormalities, serum immunoglobulin G (IgG) elevation, and histological findings. The presence of periportal lymphoplasmacytic infiltration in liver tissue is an important clue to the diagnosis, and all patients in whom there is a suspicion of autoimmune hepatitis should undergo liver tissue examination.
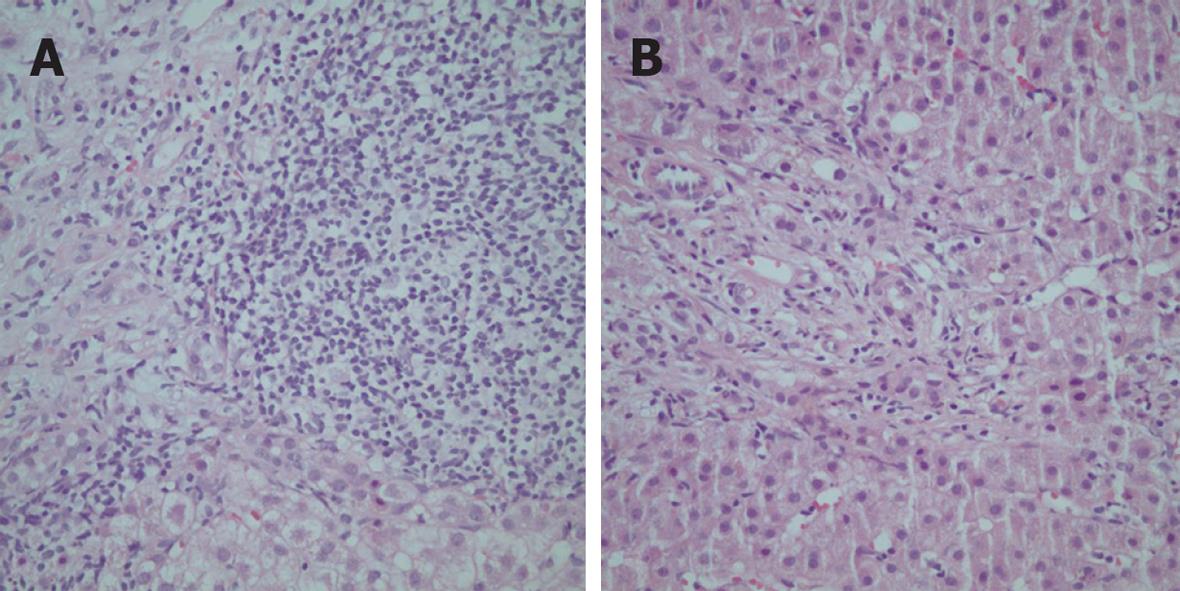
As in other regions, the features of autoimmune hepatitis may be intermixed with those typical of other liver diseases, especially the cholestatic disorders (“overlap syndromes”), and the diagnosis must be secured by expert histological interpretation and cholangiographic studies (Figure 4)[148151152]. In Turkey, as elsewhere, liver tissue examination is the most important tool in directing the diagnosis, and a second examination of the liver tissue after institution of treatment provides a comparison that can support or change the original impression.
Concurrent immune diseases, such as autoimmune thyroiditis[153], celiac disease[154], Sjogren syndrome[155], autoimmune diabetes[155], and various rheumatic conditions[156], can accompany the autoimmune hepatitis of Turkey, but unlike the disease in other regions, the liver disease in Turkey can frequently be linked to different triggers, including indigenous infections [prolonged hepatitis A virus (HAV) infection[157] and brucellosis[158]] and medicinal agents (Echinacea[159], doxycycline[158], estrogen[160], cyproterone acetate[160], and ornidazole[161]). A genetic basis for the liver disease and its immune manifestations has not been well studied in Turkey, but the classical HLA phenotype, A1-B8-DRB1*03, of western countries does not appear to be an important susceptibility factor in this area[162].
Corticosteroid therapy remains the mainstay of treatment in Turkey, but azathioprine, ursodeoxycholic acid and budesonide have been added to the list of available and effective drugs. Combination therapy is the preferred regimen, and budesonide is gaining favor over prednisolone in combination with azathioprine. An example of tailoring the treatment strategy to the population base is the practice of maintaining individuals in remission on low dose prednisolone (4 mg on alternate days) either alone or in combination with azathioprine long-term. By recognizing the regional variations in the clinical phenotype and tailoring therapy to suit the prevalent disease behavior, autoimmune hepatitis in different regions and ethnic groups can be diagnosed promptly and treated successfully in a cost-effective, low risk manner.
New diagnostic instruments have evolved that have the flexibility to accommodate atypical features of autoimmune hepatitis and the sensitivity and specificity to ensure accurate diagnosis of the non-classical phenotypes. A diagnostic scoring system that was promulgated mainly as a research tool in 1993[9] was revised in 1997[10] to exclude cholestatic syndromes. A simplified diagnostic scoring system was added in 2008 to ease clinical application[65], and both systems can now be exploited to strengthen the diagnosis in difficult cases[66].
The revised original diagnostic scoring system developed by the International Autoimmune Hepatitis Group (IAIHG) evaluates 13 clinical components and renders 27 possible grades[10] (Table 2). It is a comprehensive template that grades each component of the disease, including gender, laboratory manifestations of liver inflammation and cholestasis, the conventional autoantibodies, viral markers, epidemiological risk factors such as drug or alcohol exposures, HLA phenotype, concurrent immune diseases, novel autoantibodies, and individual histological features. It also grades the treatment response, and a score can be rendered before and after treatment.
| Variable | Result | Points | Variable | Result | Points |
| Gender | Female | +2 | HLA | DR3 or DR4 | +1 |
| AP:AST (or ALT) ratio | > 3 | -2 | Immune disease | Thyroiditis, colitis, others | +2 |
| < 1.5 | +2 | ||||
| γ-globulin or IgG level above normal | > 2.0 | +3 | Other markers | Anti-SLA, actin, LC1, pANCA | +2 |
| 1.5-2.0 | +2 | ||||
| 1.0-1.5 | +1 | ||||
| < 1.0 | 0 | ||||
| ANA, SMA, or anti-LKM1 titers | > 1:80 | +3 | Histological features | Interface hepatitis | +3 |
| 1:80 | +2 | Plasmacytic | +1 | ||
| 1:40 | +1 | Rosettes | +1 | ||
| < 1:40 | 0 | None of above | -5 | ||
| Biliary changes | -3 | ||||
| Other features | -3 | ||||
| AMA | Positive | -4 | Treatment response | Complete | +2 |
| Relapse | +3 | ||||
| Viral markers | Positive | -3 | |||
| Negative | +3 | ||||
| Drugs | Yes | -4 | Pretreatment aggregate score | ||
| No | +1 | Definite diagnosis | > 15 | ||
| Probable diagnosis | 10-15 | ||||
| Alcohol | < 25 g/d | +2 | Post-treatment aggregate score | ||
| > 60 g/d | -2 | Definite diagnosis | > 17 | ||
| Probable diagnosis | 12-17 | ||||
A pre-treatment score of 10 points or higher or a post-treatment score of 12 points or higher indicates the likelihood of autoimmune hepatitis[10] (Table 2). No single test or finding defeats or ensures the diagnosis of autoimmune hepatitis if other components are sufficiently strong to outweigh it. The Receiver Operating Characteristic (ROC) curve for the revised original scoring system shows that the minimum pre-treatment score of 10 points has a sensitivity of 100% and a specificity of 73% for autoimmune hepatitis. A pre-treatment score of 15 points or higher has a specificity of greater than 90% for autoimmune hepatitis[66].
The principal virtues of the revised original scoring system are that it ensures the systematic assessment of all key features of the disease and it is not compromised by a missing or atypical feature[66]. The revised original diagnostic scoring system is most useful in evaluating patients with few or atypical findings of autoimmune hepatitis, including the variant syndromes, because of its comprehensive nature. It quantifies the strength of the diagnosis, and it is a valuable research tool that ensures comparable study populations within clinical trials.
The simplified diagnostic scoring system eases clinical application by evaluating only 4 clinical components, and it has been validated in diverse ethnic groups and liver diseases[65] (Table 3). The simplified scoring system is based on the presence and level of autoantibody expression by indirect immunofluorescence, serum IgG concentration, compatible or typical histological features, and the absence of viral markers. It does not grade treatment response.
| Variable | Result | Points |
| Autoantibodies | ||
| ANA or SMA | ≥ 1:40 | +1 |
| ANA or SMA | ≥ 1:80 | +2 |
| Antibodies to liver kidney microsome type 1 | ≥ 1:40 | +2 |
| Antibodies to soluble liver antigen | Positive | +2 |
| Immunoglobulin level | ||
| Immunoglobulin G | > UNL | +1 |
| > 1.1 ULN | +2 | |
| Histological findings | ||
| Morphological features | Compatible | +1 |
| Typical | +2 | |
| Viral disease | ||
| Absence of viral hepatitis | No viral markers | +2 |
| Pretreatment aggregate score | ||
| Definite diagnosis | ≥ 7 | |
| Probable diagnosis | 6 |
The ROC curve for the simplified scoring system shows that a minimum score of 6 points has a sensitivity and specificity of 90% for the diagnosis of autoimmune hepatitis[66] (Table 3). Scores of 7 points or higher are nearly 100% specific for the diagnosis of autoimmune hepatitis with only a small decrease in sensitivity. The virtues of the simplified scoring system are the ease of its clinical application, and its combined high sensitivity and specificity for the diagnosis[66]. It is especially useful in excluding autoimmune hepatitis in patients with other distinct conditions who have confusing concurrent immune features. The revised original scoring system has greater sensitivity for the diagnosis, whereas the simplified system has superior specificity and accuracy.
New treatment strategies for autoimmune hepatitis are evolving as current regimens are being used more effectively and new drugs are being exploited in selected situations. The non-classical phenotypes of autoimmune hepatitis are managed in the same fashion as the classical phenotypes, and they benefit similarly from these advances.
The ideal end point of initial corticosteroid therapy has now been defined[67–70]; the treatment adjustments after relapse and incomplete response have been formalized[71–hepatitis have fully resolved73]; and vaccination against hepatitis A (HAV) and hepatitis B (HBV) viruses prior to therapy has been proposed[74]. These improvements constitute advances in the current treatment regimens (Table 4).
| Advance | Nature | Attribute |
| Improved current therapy | Initial therapy until resolution of liver tests and tissue | Prevention of relapse after drug withdrawal[70] |
| Long-term azathioprine therapy after relapse or incomplete response | Prevention of disease progression[7173125] | |
| Pretreatment vaccination for viruses | Protection against morbidity of concurrent viral infection[74] | |
| New drugs | Calcineurin inhibitors (cyclosporine, tacrolimus) | Salvage therapy[150–157] |
| Purine antagonists (6-mercaptopurine, mycophenolate) | Salvage therapy[161–166] | |
| Budesonide (combined with azathioprine) | Effective and safe front line therapy[75] | |
| Potential molecular interventions | Synthetic blocking peptides | Block autoantigen presentation[186187] |
| Cytokine manipulations | Promote anti-inflammatory effects[188] | |
| T cell vaccination | Eliminate cytotoxic liver-infiltrating clone[189] | |
| Oral tolerance (high or low dose regimen) | Reduce immune response (low dose) or induce anergy (high dose)[190191] | |
| Mesenchymal stem cells (human bone marrow-derived) | Replace damaged hepatocytes[200] |
Corticosteroid therapy should be continued until the clinical, laboratory and histological features of autoimmune hepatitis have fully resolved (Table 4)[67–70]. Relapse after drug withdrawal is the most common management problem in autoimmune hepatitis, and this occurrence can be reduced by continuing treatment until liver tests and tissue are normal[5970163]. Patients who sustain remission after treatment withdrawal have better laboratory indices and liver tissue examinations at the time of drug withdrawal than patients who relapse, and treatment until complete resolution of the inflammatory features is the ideal end point of therapy.
Sixty percent of patients who achieve an ideal treatment end point still relapse after drug withdrawal, and 40% of treated patients are unable to achieve full resolution of their disease[70]. The relentless pursuit of an ideal but unachievable treatment end point in these individuals can result in drug-related side effects[5962]. Patients with relapse after drug withdrawal, incomplete response to conventional treatment, and drug intolerances must be managed differently[125].
Repeated relapse and re-treatment is associated with a progressive increase in the cumulative frequencies of cirrhosis, requirement for liver transplantation, and death from hepatic failure[64]. The preferred management strategy after the first relapse is to institute treatment with long-term fixed dose azathioprine (Table 4)[7173]. Prednisone and azathioprine are re-started until clinical and laboratory resolution is achieved. The dose of azathioprine is then increased to 2 mg/kg daily as the dose of prednisone is withdrawn. Azathioprine is then continued indefinitely as a chronic maintenance therapy. Eighty percent of patients are able to sustain remission in this fashion over a 10 year period of observation. Patients who improve during treatment but not to a degree to satisfy remission criteria (incomplete response) can also be managed by this regimen[125].
Vaccination against HAV and HBV is an important adjunct to conventional treatment (Table 4). Susceptibility to infections with HAV (51%) and HBV (86%) is high in patients with autoimmune liver disease, and the incidence of these infections is 1.3-1.4 per 1000 person-years[74]. Vaccination frequencies are only 11% for HBV and 13% for HAV in these patients, and the response to the HBV vaccine is poor or absent in most individuals vaccinated during immunosuppressive therapy[74]. These observations suggest that pre-treatment vaccination for HAV and HBV is under-utilized in autoimmune liver disease and that outcomes can be improved by early vaccination to prevent viral super-infection and mortality.
Treatment options have increased in autoimmune hepatitis as new drugs with targeted immunosuppressive actions have been used empirically[76164] and a third generation corticosteroid has been evaluated by randomized clinical trial[75]. None of these treatments has been incorporated into standard management algorithms, but they constitute an evolving armamentarium that promises to improve outcomes by either interrupting critical pathogenic pathways or eliminating intolerances to the current medications.
The calcineurin inhibitors (cyclosporine[165–169] and tacrolimus[170–172]) have been used as frontline and salvage therapies in children[173–175] and adults[165–172] with autoimmune hepatitis, and these multiple small clinical experiences have supported their efficacy and tolerance (Table 4). Additional clinical trials are necessary to determine their target population, dosing schedule, and safety profile.
The purine antagonists (6-mercaptopurine[176] and mycophenolate mofetil[177–181]) have also been effective in some patients refractory to conventional corticosteroid regimens (Table 4). 6-mercaptopurine has reduced disease activity in patients unresponsive to azathioprine, and should be considered as a salvage therapy[176]. Intolerances to azathioprine based on thiopurine methyltransferase deficiency contraindicate its use, and the drug should not be administered to patients with azathioprine-related side effects[182].
Mycophenolate mofetil is independent of the thiopurine methyltransferase metabolic pathway, and several small experiences have indicated that it can be effective in problematic patients[177–181] (Table 4). Improvement occurs in 39%-84% of patients who tolerate the drug, but the intention to treat is thwarted in 34%-78% of patients because of drug intolerances (nausea, vomiting, pancreatitis, rash, alopecia, deep venous thrombosis, diarrhea and failure to normalize liver tests)[180183184]. Mycophenolate mofetil is another promising alternative drug in the treatment of autoimmune hepatitis, but only a minority of problematic patients may reap its benefits[183–185].
Budesonide is a third generation corticosteroid that has been used empirically as frontline[186–188] and salvage[189] therapy in autoimmune hepatitis (Table 4). Its high first-pass clearance by the liver and its breakdown to inactive metabolites promised to improve efficacy and safety compared to conventional corticosteroid regimens[190]. Its advantage, however, was never fully realized until it was evaluated by randomized clinical trial in 203 treatment-naïve patients with autoimmune hepatitis[75]. Budesonide in combination with azathioprine has been found to be superior to prednisolone and azathioprine in normalizing the serum ALT level (47% vs 18%, P < 0.00001) and reducing the frequency of steroid-related side effects (28% vs 53%, P = 0.0001) after 6 mo of treatment[75]. The frequency of histological improvement and the durability of the results are unknown, but the findings suggest that budesonide may be an alternative, more effective, and safer frontline regimen than a prednisone-based schedule. Budesonide has not been effective as a salvage therapy in patients with severe disease on long-standing corticosteroid treatment[189], and corticosteroid-induced side effects are still possible, especially in patients who have been treated previously with prednisone[189] or who have cirrhosis[189191].
Various other drugs (cyclophosphamide[192], methotrexate[193], rapamycin[194], rituximab[195], intravenous immunoglobulin[196], deflazacort[197], and ursodeoxycholic acid[198]) have been proposed for use in autoimmune hepatitis, and their number reflects the need for better salvage therapies in the treatment of autoimmune hepatitis (Table 4). Prospective and scientifically rigorous collaborative studies are needed to expand the therapeutic repertoire and comprehensive analyses are required to demonstrate that these incremental improvements in outcome are cost-effective[199200].
Site-specific molecular inventions, including antigen-blocking synthetic peptides[201202], cytokine manipulations[203], T cell vaccination[204], and oral tolerance regimens[205206], become feasible when the critical pathogenic mechanisms of the disease are clarified[207–209], and confident animal models of the human disease are developed[210–214] (Table 4). Mesenchymal stem cells from human bone marrow that can differentiate into functional hepatocytes have the potential to rescue individuals from liver failure, reduce reliance on whole organ transplantation, and obviate the complications of whole organ rejection and drug toxicity[215] (Table 4). The treatment options for autoimmune hepatitis are already plentiful and effective, but the drive for further improvement must be continuous and vigorous.
Autoimmune hepatitis can have acute severe or asymptomatic presentations, centrilobular zone 3 necrosis, concurrent bile duct damage or non-alcoholic fatty changes on histological examination, absent or atypical serological markers, cholangiographic abnormalities, and variable clinical phenotypes related to gender or ethnicity. These non-classical manifestations do not alter the management strategy, but they require prompt recognition and confident diagnosis. The revised original diagnostic scoring system of the IAIHG is useful in evaluating patients with few or atypical findings of autoimmune hepatitis because of its comprehensive nature. The simplified diagnostic scoring system is useful in excluding autoimmune hepatitis in patients with other distinct conditions who have confusing concurrent immune features because of its high specificity. Current treatment regimens have been improved by pursuing resolution of liver tests and liver tissue prior to drug withdrawal, instituting long-term azathioprine therapy after the first relapse, and vaccinating against HAV and HBV prior to treatment. Cyclosporine, tacrolimus, 6-mercaptopurine, and mycophenolate mofetil are promising salvage therapies, whereas budesonide in combination with azathioprine may be a superior frontline therapy to prednisone and azathioprine.
| 1. | Bongiovanni AM, Eisenmenger WJ. Adrenal cortical metabolism in chronic liver disease. J Clin Endocrinol Metab. 1951;11:152-172. |
| 2. | Bearn AG, Kunkel HG, Slater RJ. The problem of chronic liver disease in young women. Am J Med. 1956;21:3-15. |
| 4. | Czaja AJ, Carpenter HA, Santrach PJ, Moore SB, Taswell HF, Homburger HA. Evidence against hepatitis viruses as important causes of severe autoimmune hepatitis in the United States. J Hepatol. 1993;18:342-352. |
| 5. | Taft LI, Mackay IR, Cowling DC. Autoclasia: a perpetuacting mechanism in hepatitis. Gastroenterology. 1960;38:563-566. |
| 6. | Cochrane AM, Moussouros A, Thomsom AD, Eddleston AL, Wiiliams R. Antibody-dependent cell-mediated (K cell) cytotoxicity against isolated hepatocytes in chronic active hepatitis. Lancet. 1976;1:441-444. |
| 7. | Hodgson HJ, Wands JR, Isselbacher KJ. Alteration in suppressor cell activity in chronic active hepatitis. Proc Natl Acad Sci USA. 1978;75:1549-1553. |
| 8. | Frazer IH, Mackay IR. T lymphocyte subpopulations defined by two sets of monoclonal antibodies in chronic active hepatitis and systemic lupus erythematosus. Clin Exp Immunol. 1982;50:107-114. |
| 9. | Johnson PJ, McFarlane IG. Meeting report: International Autoimmune Hepatitis Group. Hepatology. 1993;18:998-1005. |
| 10. | Alvarez F, Berg PA, Bianchi FB, Bianchi L, Burroughs AK, Cancado EL, Chapman RW, Cooksley WG, Czaja AJ, Desmet VJ. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999;31:929-938. |
| 11. | Czaja AJ. Diverse manifestations and evolving treatments of autoimmune hepatitis. Minerva Gastroenterol Dietol. 2005;51:313-333. |
| 12. | Crapper RM, Bhathal PS, Mackay IR, Frazer IH. 'Acute' autoimmune hepatitis. Digestion. 1986;34:216-225. |
| 13. | Amontree JS, Stuart TD, Bredfeldt JE. Autoimmune chronic active hepatitis masquerading as acute hepatitis. J Clin Gastroenterol. 1989;11:303-307. |
| 14. | Nikias GA, Batts KP, Czaja AJ. The nature and prognostic implications of autoimmune hepatitis with an acute presentation. J Hepatol. 1994;21:866-871. |
| 15. | Kanda T, Yokosuka O, Hirasawa Y, Imazeki F, Nagao K, Suzuki Y, Saisho H. Acute-onset autoimmune hepatitis resembling acute hepatitis: a case report and review of reported cases. Hepatogastroenterology. 2005;52:1233-1235. |
| 16. | Maggiore G, Porta G, Bernard O, Hadchouel M, Alvarez F, Homberg JC, Alagille D. Autoimmune hepatitis with initial presentation as acute hepatic failure in young children. J Pediatr. 1990;116:280-282. |
| 17. | Herzog D, Rasquin-Weber AM, Debray D, Alvarez F. Subfulminant hepatic failure in autoimmune hepatitis type 1: an unusual form of presentation. J Hepatol. 1997;27:578-582. |
| 18. | Kessler WR, Cummings OW, Eckert G, Chalasani N, Lumeng L, Kwo PY. Fulminant hepatic failure as the initial presentation of acute autoimmune hepatitis. Clin Gastroenterol Hepatol. 2004;2:625-631. |
| 19. | Miyake Y, Iwasaki Y, Terada R, Onishi T, Okamoto R, Sakai N, Sakaguchi K, Shiratori Y. Clinical characteristics of fulminant-type autoimmune hepatitis: an analysis of eleven cases. Aliment Pharmacol Ther. 2006;23:1347-1353. |
| 20. | Kogan J, Safadi R, Ashur Y, Shouval D, Ilan Y. Prognosis of symptomatic versus asymptomatic autoimmune hepatitis: a study of 68 patients. J Clin Gastroenterol. 2002;35:75-81. |
| 21. | Feld JJ, Dinh H, Arenovich T, Marcus VA, Wanless IR, Heathcote EJ. Autoimmune hepatitis: effect of symptoms and cirrhosis on natural history and outcome. Hepatology. 2005;42:53-62. |
| 22. | Czaja AJ. Features and consequences of untreated type 1 autoimmune hepatitis. Liver Int. 2008;42:Epub ahead of print. |
| 23. | Davis GL, Czaja AJ, Ludwig J. Development and prognosis of histologic cirrhosis in corticosteroid-treated hepatitis B surface antigen-negative chronic active hepatitis. Gastroenterology. 1984;87:1222-1227. |
| 24. | Roberts SK, Therneau TM, Czaja AJ. Prognosis of histological cirrhosis in type 1 autoimmune hepatitis. Gastroenterology. 1996;110:848-857. |
| 25. | Czaja AJ, Carpenter HA. Progressive fibrosis during corticosteroid therapy of autoimmune hepatitis. Hepatology. 2004;39:1631-1638. |
| 26. | Schalm SW, Korman MG, Summerskill WH, Czaja AJ, Baggenstoss AH. Severe chronic active liver disease. Prognostic significance of initial morphologic patterns. Am J Dig Dis. 1977;22:973-980. |
| 27. | Czaja AJ, Ludwig J, Baggenstoss AH, Wolf A. Corticosteroid-treated chronic active hepatitis in remission: uncertain prognosis of chronic persistent hepatitis. N Engl J Med. 1981;304:5-9. |
| 28. | Bittencourt PL, Farias AQ, Porta G, Cançado EL, Miura I, Pugliese R, Kalil J, Goldberg AC, Carrilho FJ. Frequency of concurrent autoimmune disorders in patients with autoimmune hepatitis: effect of age, gender, and genetic background. J Clin Gastroenterol. 2008;42:300-305. |
| 29. | Czaja AJ. Clinical features, differential diagnosis and treatment of autoimmune hepatitis in the elderly. Drugs Aging. 2008;25:219-239. |
| 30. | Czaja AJ. Behavior and significance of autoantibodies in type 1 autoimmune hepatitis. J Hepatol. 1999;30:394-401. |
| 31. | Czaja AJ, Carpenter HA, Santrach PJ, Moore SB, Homburger HA. The nature and prognosis of severe cryptogenic chronic active hepatitis. Gastroenterology. 1993;104:1755-1761. |
| 32. | Gassert DJ, Garcia H, Tanaka K, Reinus JF. Corticosteroid-responsive cryptogenic chronic hepatitis: evidence for seronegative autoimmune hepatitis. Dig Dis Sci. 2007;52:2433-2437. |
| 33. | Pratt DS, Fawaz KA, Rabson A, Dellelis R, Kaplan MM. A novel histological lesion in glucocorticoid-responsive chronic hepatitis. Gastroenterology. 1997;113:664-668. |
| 34. | Singh R, Nair S, Farr G, Mason A, Perrillo R. Acute autoimmune hepatitis presenting with centrizonal liver disease: case report and review of the literature. Am J Gastroenterol. 2002;97:2670-2673. |
| 35. | Okano N, Yamamoto K, Sakaguchi K, Miyake Y, Shimada N, Hakoda T, Terada R, Baba S, Suzuki T, Tsuji T. Clinicopathological features of acute-onset autoimmune hepatitis. Hepatol Res. 2003;25:263-270. |
| 36. | Hofer H, Oesterreicher C, Wrba F, Ferenci P, Penner E. Centrilobular necrosis in autoimmune hepatitis: a histological feature associated with acute clinical presentation. J Clin Pathol. 2006;59:246-249. |
| 37. | Zen Y, Notsumata K, Tanaka N, Nakanuma Y. Hepatic centrilobular zonal necrosis with positive antinuclear antibody: a unique subtype or early disease of autoimmune hepatitis? Hum Pathol. 2007;38:1669-1675. |
| 38. | Ludwig J, Czaja AJ, Dickson ER, LaRusso NF, Wiesner RH. Manifestations of nonsuppurative cholangitis in chronic hepatobiliary diseases: morphologic spectrum, clinical correlations and terminology. Liver. 1984;4:105-116. |
| 39. | Ben-Ari Z, Dhillon AP, Sherlock S. Autoimmune cholangiopathy: part of the spectrum of autoimmune chronic active hepatitis. Hepatology. 1993;18:10-15. |
| 40. | Czaja AJ, Carpenter HA, Santrach PJ, Moore SB. Autoimmune cholangitis within the spectrum of autoimmune liver disease. Hepatology. 2000;31:1231-1238. |
| 41. | Czaja AJ, Carpenter HA. Autoimmune hepatitis with incidental histologic features of bile duct injury. Hepatology. 2001;34:659-665. |
| 42. | Czaja AJ, Muratori P, Muratori L, Carpenter HA, Bianchi FB. Diagnostic and therapeutic implications of bile duct injury in autoimmune hepatitis. Liver Int. 2004;24:322-329. |
| 43. | Lim KN, Casanova RL, Boyer TD, Bruno CJ. Autoimmune hepatitis in African Americans: presenting features and response to therapy. Am J Gastroenterol. 2001;96:3390-3394. |
| 44. | Hurlburt KJ, McMahon BJ, Deubner H, Hsu-Trawinski B, Williams JL, Kowdley KV. Prevalence of autoimmune liver disease in Alaska Natives. Am J Gastroenterol. 2002;97:2402-2407. |
| 45. | Zolfino T, Heneghan MA, Norris S, Harrison PM, Portmann BC, McFarlane IG. Characteristics of autoimmune hepatitis in patients who are not of European Caucasoid ethnic origin. Gut. 2002;50:713-717. |
| 46. | Gohar S, Desai D, Joshi A, Bhaduri A, Deshpande R, Balkrishna C, Chawla M, Rodrigues C, Joshi VR. Autoimmune hepatitis: a study of 50 patients. Indian J Gastroenterol. 2003;22:140-142. |
| 47. | D'Souza R, Sinnott P, Glynn MJ, Sabin CA, Foster GR. An unusual form of autoimmune hepatitis in young Somalian men. Liver Int. 2005;25:325-330. |
| 48. | Viruet EJ, Torres EA. Steroid therapy in fulminant hepatic failure secondary to autoimmune hepatitis. P R Health Sci J. 1998;17:297-300. |
| 49. | Ichai P, Duclos-Vallée JC, Guettier C, Hamida SB, Antonini T, Delvart V, Saliba F, Azoulay D, Castaing D, Samuel D. Usefulness of corticosteroids for the treatment of severe and fulminant forms of autoimmune hepatitis. Liver Transpl. 2007;13:996-1003. |
| 50. | Czaja AJ. Corticosteroids or not in severe acute or fulminant autoimmune hepatitis: therapeutic brinksmanship and the point beyond salvation. Liver Transpl. 2007;13:953-955. |
| 51. | Soloway RD, Summerskill WH, Baggenstoss AH, Geall MG, Gitnićk GL, Elveback IR, Schoenfield LJ. Clinical, biochemical, and histological remission of severe chronic active liver disease: a controlled study of treatments and early prognosis. Gastroenterology. 1972;63:820-833. |
| 52. | Summerskill WH, Korman MG, Ammon HV, Baggenstoss AH. Prednisone for chronic active liver disease: dose titration, standard dose, and combination with azathioprine compared. Gut. 1975;16:876-883. |
| 53. | Dufour JF, DeLellis R, Kaplan MM. Reversibility of hepatic fibrosis in autoimmune hepatitis. Ann Intern Med. 1997;127:981-985. |
| 54. | Cotler SJ, Jakate S, Jensen DM. Resolution of cirrhosis in autoimmune hepatitis with corticosteroid therapy. J Clin Gastroenterol. 2001;32:428-430. |
| 55. | Czaja AJ, Carpenter HA. Decreased fibrosis during corticosteroid therapy of autoimmune hepatitis. J Hepatol. 2004;40:646-652. |
| 56. | Mohamadnejad M, Malekzadeh R, Nasseri-Moghaddam S, Hagh-Azali S, Rakhshani N, Tavangar SM, Sedaghat M, Alimohamadi SM. Impact of immunosuppressive treatment on liver fibrosis in autoimmune hepatitis. Dig Dis Sci. 2005;50:547-551. |
| 57. | Schalm SW, Ammon HV, Summerskill WH. Failure of customary treatment in chronic active liver disease: causes and management. Ann Clin Res. 1976;8:221-227. |
| 58. | Montano-Loza AJ, Carpenter HA, Czaja AJ. Features associated with treatment failure in type 1 autoimmune hepatitis and predictive value of the model of end-stage liver disease. Hepatology. 2007;46:1138-1145. |
| 59. | Czaja AJ, Davis GL, Ludwig J, Taswell HF. Complete resolution of inflammatory activity following corticosteroid treatment of HBsAg-negative chronic active hepatitis. Hepatology. 1984;4:622-627. |
| 60. | Czaja AJ, Ammon HV, Summerskill WH. Clinical features and prognosis of severe chronic active liver disease (CALD) after corticosteroid-induced remission. Gastroenterology. 1980;78:518-523. |
| 61. | Hegarty JE, Nouri Aria KT, Portmann B, Eddleston AL, Williams R. Relapse following treatment withdrawal in patients with autoimmune chronic active hepatitis. Hepatology. 1983;3:685-689. |
| 62. | Czaja AJ, Beaver SJ, Shiels MT. Sustained remission after corticosteroid therapy of severe hepatitis B surface antigen-negative chronic active hepatitis. Gastroenterology. 1987;92:215-219. |
| 63. | Czaja AJ, Menon KV, Carpenter HA. Sustained remission after corticosteroid therapy for type 1 autoimmune hepatitis: a retrospective analysis. Hepatology. 2002;35:890-897. |
| 64. | Montano-Loza AJ, Carpenter HA, Czaja AJ. Consequences of treatment withdrawal in type 1 autoimmune hepatitis. Liver Int. 2007;27:507-515. |
| 65. | Hennes EM, Zeniya M, Czaja AJ, Parés A, Dalekos GN, Krawitt EL, Bittencourt PL, Porta G, Boberg KM, Hofer H. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008;48:169-176. |
| 66. | Czaja AJ. Performance parameters of the diagnostic scoring systems for autoimmune hepatitis. Hepatology. 2008;48:1540-1548. |
| 67. | Kanzler S, Gerken G, Löhr H, Galle PR, Meyer zum Büschenfelde KH, Lohse AW. Duration of immunosuppressive therapy in autoimmune hepatitis. J Hepatol. 2001;34:354-355. |
| 68. | Verma S, Gunuwan B, Mendler M, Govindrajan S, Redeker A. Factors predicting relapse and poor outcome in type I autoimmune hepatitis: role of cirrhosis development, patterns of transaminases during remission and plasma cell activity in the liver biopsy. Am J Gastroenterol. 2004;99:1510-1516. |
| 69. | Miyake Y, Iwasaki Y, Terada R, Takagi S, Okamaoto R, Ikeda H, Sakai N, Makino Y, Kobashi H, Takaguchi K. Persistent normalization of serum alanine aminotransferase levels improves the prognosis of type 1 autoimmune hepatitis. J Hepatol. 2005;43:951-957. |
| 70. | Montano-Loza AJ, Carpenter HA, Czaja AJ. Improving the end point of corticosteroid therapy in type 1 autoimmune hepatitis to reduce the frequency of relapse. Am J Gastroenterol. 2007;102:1005-1012. |
| 71. | Stellon AJ, Keating JJ, Johnson PJ, McFarlane IG, Williams R. Maintenance of remission in autoimmune chronic active hepatitis with azathioprine after corticosteroid withdrawal. Hepatology. 1988;8:781-784. |
| 72. | Czaja AJ. Low-dose corticosteroid therapy after multiple relapses of severe HBsAg-negative chronic active hepatitis. Hepatology. 1990;11:1044-1049. |
| 73. | Johnson PJ, McFarlane IG, Williams R. Azathioprine for long-term maintenance of remission in autoimmune hepatitis. N Engl J Med. 1995;333:958-963. |
| 74. | Wörns MA, Teufel A, Kanzler S, Shrestha A, Victor A, Otto G, Lohse AW, Galle PR, Höhler T. Incidence of HAV and HBV infections and vaccination rates in patients with autoimmune liver diseases. Am J Gastroenterol. 2008;103:138-146. |
| 75. | Manns MP, Bahr MJ, Woynarowski M, Kreisel W, Oren R, Günther R, Hultcrantz R, Proels M, Rust C, Spengler U. Budesonide 3 mg TID is superior to prednisolone in combination with azathioprine in the treatment of autoimmune hepatitis (abstract). J Hepatol. 2008;48 suppl 2:S369-S370. |
| 76. | Vierling JM, Flores PA. Evolving new therapies of autoimmune hepatitis. Clin Liver Dis. 2002;6:825-850, ix. |
| 77. | Montano Loza AJ, Czaja AJ. Current therapy for autoimmune hepatitis. Nat Clin Pract Gastroenterol Hepatol. 2007;4:202-214. |
| 78. | Czaja AJ. Autoimmune hepatitis. Part A: pathogenesis. Expert Rev Gastroenterol Hepatol. 2007;1:113-128. |
| 79. | Czaja AJ. Evolving concepts in the diagnosis, pathogenesis and treatment of autoimmune hepatitis. Minerva Gastroenterol Dietol. 2007;53:43-78. |
| 80. | Czaja AJ, Freese DK. Diagnosis and treatment of autoimmune hepatitis. Hepatology. 2002;36:479-497. |
| 81. | Burgart LJ, Batts KP, Ludwig J, Nikias GA, Czaja AJ. Recent-onset autoimmune hepatitis. Biopsy findings and clinical correlations. Am J Surg Pathol. 1995;19:699-708. |
| 82. | Czaja AJ, Davis GL, Ludwig J, Baggenstoss AH, Taswell HF. Autoimmune features as determinants of prognosis in steroid-treated chronic active hepatitis of uncertain etiology. Gastroenterology. 1983;85:713-717. |
| 83. | Davis GL, Czaja AJ, Baggenstoss AH, Taswell HF. Prognostic and therapeutic implications of extreme serum aminotransferase elevation in chronic active hepatitis. Mayo Clin Proc. 1982;57:303-309. |
| 84. | Iwai M, Jo M, Ishii M, Mori T, Harada Y. Comparison of clinical features and liver histology in acute and chronic autoimmune hepatitis. Hepatol Res. 2008;38:784-789. |
| 85. | Czaja AJ, Rakela J, Ludwig J. Features reflective of early prognosis in corticosteroid-treated severe autoimmune chronic active hepatitis. Gastroenterology. 1988;95:448-453. |
| 86. | Tan P, Marotta P, Ghent C, Adams P. Early treatment response predicts the need for liver transplantation in autoimmune hepatitis. Liver Int. 2005;25:728-733. |
| 87. | De Groote J, Fevery J, Lepoutre L. Long-term follow-up of chronic active hepatitis of moderate severity. Gut. 1978;19:510-513. |
| 88. | Koretz RL, Lewin KJ, Higgins J, Fagen ND, Gitnick GL. Chronic active hepatitis. Who meets treatment criteria? Dig Dis Sci. 1980;25:695-699. |
| 89. | Hodges JR, Millward-Sadler GH, Wright R. Chronic active hepatitis: the spectrum of disease. Lancet. 1982;1:550-552. |
| 90. | Cook GC, Mulligan R, Sherlock S. Controlled prospective trial of corticosteroid therapy in active chronic hepatitis. Q J Med. 1971;40:159-185. |
| 91. | Murray-Lyon IM, Stern RB, Williams R. Controlled trial of prednisone and azathioprine in active chronic hepatitis. Lancet. 1973;1:735-737. |
| 92. | Misdraji J, Thiim M, Graeme-Cook FM. Autoimmune hepatitis with centrilobular necrosis. Am J Surg Pathol. 2004;28:471-478. |
| 93. | Czaja AJ, Carpenter HA. Sensitivity, specificity, and predictability of biopsy interpretations in chronic hepatitis. Gastroenterology. 1993;105:1824-1832. |
| 94. | Adams LA, Lindor KD, Angulo P. The prevalence of autoantibodies and autoimmune hepatitis in patients with nonalcoholic Fatty liver disease. Am J Gastroenterol. 2004;99:1316-1320. |
| 95. | Tajiri K, Takenawa H, Yamaoka K, Yamane M, Marumo F, Sato C. Nonalcoholic steatohepatitis masquerading as autoimmune hepatitis. J Clin Gastroenterol. 1997;25:538-540. |
| 96. | Loria P, Lonardo A, Leonardi F, Fontana C, Carulli L, Verrone AM, Borsatti A, Bertolotti M, Cassani F, Bagni A. Non-organ-specific autoantibodies in nonalcoholic fatty liver disease: prevalence and correlates. Dig Dis Sci. 2003;48:2173-2181. |
| 97. | Czaja AJ, Carpenter HA. Optimizing diagnosis from the medical liver biopsy. Clin Gastroenterol Hepatol. 2007;5:898-907. |
| 98. | Czaja AJ. Autoantibodies in autoimmune liver disease. Adv Clin Chem. 2005;40:127-164. |
| 99. | Czaja AJ. Autoimmune hepatitis. Part B: diagnosis. Expert Rev Gastroenterol Hepatol. 2007;1:129-143. |
| 100. | Czaja AJ, Hay JE, Rakela J. Clinical features and prognostic implications of severe corticosteroid-treated cryptogenic chronic active hepatitis. Mayo Clin Proc. 1990;65:23-30. |
| 101. | Baeres M, Herkel J, Czaja AJ, Wies I, Kanzler S, Cancado EL, Porta G, Nishioka M, Simon T, Daehnrich C. Establishment of standardised SLA/LP immunoassays: specificity for autoimmune hepatitis, worldwide occurrence, and clinical characteristics. Gut. 2002;51:259-264. |
| 102. | Czaja AJ, Shums Z, Norman GL. Frequency and significance of antibodies to soluble liver antigen/liver pancreas in variant autoimmune hepatitis. Autoimmunity. 2002;35:475-483. |
| 103. | Targan SR, Landers C, Vidrich A, Czaja AJ. High-titer antineutrophil cytoplasmic antibodies in type-1 autoimmune hepatitis. Gastroenterology. 1995;108:1159-1166. |
| 104. | Zauli D, Ghetti S, Grassi A, Descovich C, Cassani F, Ballardini G, Muratori L, Bianchi FB. Anti-neutrophil cytoplasmic antibodies in type 1 and 2 autoimmune hepatitis. Hepatology. 1997;25:1105-1107. |
| 105. | Volta U, De Franceschi L, Molinaro N, Cassani F, Muratori L, Lenzi M, Bianchi FB, Czaja AJ. Frequency and significance of anti-gliadin and anti-endomysial antibodies in autoimmune hepatitis. Dig Dis Sci. 1998;43:2190-2195. |
| 106. | Kaukinen K, Halme L, Collin P, Färkkilä M, Mäki M, Vehmanen P, Partanen J, Höckerstedt K. Celiac disease in patients with severe liver disease: gluten-free diet may reverse hepatic failure. Gastroenterology. 2002;122:881-888. |
| 107. | Abdo A, Meddings J, Swain M. Liver abnormalities in celiac disease. Clin Gastroenterol Hepatol. 2004;2:107-112. |
| 108. | Kenny RP, Czaja AJ, Ludwig J, Dickson ER. Frequency and significance of antimitochondrial antibodies in severe chronic active hepatitis. Dig Dis Sci. 1986;31:705-711. |
| 109. | Muratori P, Muratori L, Gershwin ME, Czaja AJ, Pappas G, MacCariello S, Granito A, Cassani F, Loria P, Lenzi M. 'True' antimitochondrial antibody-negative primary biliary cirrhosis, low sensitivity of the routine assays, or both? Clin Exp Immunol. 2004;135:154-158. |
| 110. | Nezu S, Tanaka A, Yasui H, Imamura M, Nakajima H, Ishida H, Takahashi S. Presence of antimitochondrial autoantibodies in patients with autoimmune hepatitis. J Gastroenterol Hepatol. 2006;21:1448-1454. |
| 111. | O'Brien C, Joshi S, Feld JJ, Guindi M, Dienes HP, Heathcote EJ. Long-term follow-up of antimitochondrial antibody-positive autoimmune hepatitis. Hepatology. 2008;48:550-556. |
| 112. | Montano-Loza AJ, Carpenter HA, Czaja AJ. Frequency, behavior, and prognostic implications of antimitochondrial antibodies in type 1 autoimmune hepatitis. J Clin Gastroenterol. 2008;42:1047-1053. |
| 113. | Mishima S, Omagari K, Ohba K, Kadokawa Y, Masuda J, Mishima R, Kinoshita H, Hayashida K, Isomoto H, Shikuwa S. Clinical implications of antimitochondrial antibodies in type 1 autoimmune hepatitis: a longitudinal study. Hepatogastroenterology. 2008;55:221-227. |
| 114. | Leung PS, Rossaro L, Davis PA, Park O, Tanaka A, Kikuchi K, Miyakawa H, Norman GL, Lee W, Gershwin ME. Antimitochondrial antibodies in acute liver failure: implications for primary biliary cirrhosis. Hepatology. 2007;46:1436-1442. |
| 115. | Gregorio GV, Portmann B, Karani J, Harrison P, Donaldson PT, Vergani D, Mieli-Vergani G. Autoimmune hepatitis/sclerosing cholangitis overlap syndrome in childhood: a 16-year prospective study. Hepatology. 2001;33:544-553. |
| 116. | Perdigoto R, Carpenter HA, Czaja AJ. Frequency and significance of chronic ulcerative colitis in severe corticosteroid-treated autoimmune hepatitis. J Hepatol. 1992;14:325-331. |
| 117. | Abdalian R, Dhar P, Jhaveri K, Haider M, Guindi M, Heathcote EJ. Prevalence of sclerosing cholangitis in adults with autoimmune hepatitis: evaluating the role of routine magnetic resonance imaging. Hepatology. 2008;47:949-957. |
| 118. | Sahni VA, Mortele KJ. Magnetic resonance cholangiopancreatography: current use and future applications. Clin Gastroenterol Hepatol. 2008;6:967-977. |
| 119. | Lewin M, Vilgrain V, Ozenne V, Lemoine M, Wendum D, Paradis V, Ziol M, Beaugrand M, Poupon R, Valla D. MRI abnormalities of bile ducts in adults with autoimmune hepatitis: myth or reality? Results of a controlled prospective study (abstract). Hepatology (Suppl). 2008;48:421A. |
| 120. | Gohlke F, Lohse AW, Dienes HP, Löhr H, Märker-Hermann E, Gerken G, Meyer zum Büschenfelde KH. Evidence for an overlap syndrome of autoimmune hepatitis and primary sclerosing cholangitis. J Hepatol. 1996;24:699-705. |
| 121. | Czaja AJ. Frequency and nature of the variant syndromes of autoimmune liver disease. Hepatology. 1998;28:360-365. |
| 122. | McNair AN, Moloney M, Portmann BC, Williams R, McFarlane IG. Autoimmune hepatitis overlapping with primary sclerosing cholangitis in five cases. Am J Gastroenterol. 1998;93:777-784. |
| 123. | Floreani A, Rizzotto ER, Ferrara F, Carderi I, Caroli D, Blasone L, Baldo V. Clinical course and outcome of autoimmune hepatitis/primary sclerosing cholangitis overlap syndrome. Am J Gastroenterol. 2005;100:1516-1522. |
| 124. | Al-Chalabi T, Portmann BC, Bernal W, McFarlane IG, Heneghan MA. Autoimmune hepatitis overlap syndromes: an evaluation of treatment response, long-term outcome and survival. Aliment Pharmacol Ther. 2008;28:209-220. |
| 125. | Czaja AJ. Treatment strategies in autoimmune hepatitis. Clin Liver Dis. 2002;6:799-824. |
| 126. | Carpenter HA, Czaja AJ. The role of histologic evaluation in the diagnosis and management of autoimmune hepatitis and its variants. Clin Liver Dis. 2002;6:685-705. |
| 127. | Boberg KM, Aadland E, Jahnsen J, Raknerud N, Stiris M, Bell H. Incidence and prevalence of primary biliary cirrhosis, primary sclerosing cholangitis, and autoimmune hepatitis in a Norwegian population. Scand J Gastroenterol. 1998;33:99-103. |
| 128. | Werner M, Prytz H, Ohlsson B, Almer S, Björnsson E, Bergquist A, Wallerstedt S, Sandberg-Gertzén H, Hultcrantz R, Sangfelt P. Epidemiology and the initial presentation of autoimmune hepatitis in Sweden: a nationwide study. Scand J Gastroenterol. 2008;43:1232-1240. |
| 129. | Czaja AJ, dos Santos RM, Porto A, Santrach PJ, Moore SB. Immune phenotype of chronic liver disease. Dig Dis Sci. 1998;43:2149-2155. |
| 130. | Czaja AJ, Carpenter HA, Santrach PJ, Moore SB. Significance of HLA DR4 in type 1 autoimmune hepatitis. Gastroenterology. 1993;105:1502-1507. |
| 131. | Czaja AJ, Strettell MD, Thomson LJ, Santrach PJ, Moore SB, Donaldson PT, Williams R. Associations between alleles of the major histocompatibility complex and type 1 autoimmune hepatitis. Hepatology. 1997;25:317-323. |
| 132. | Czaja AJ, Donaldson PT. Gender effects and synergisms with histocompatibility leukocyte antigens in type 1 autoimmune hepatitis. Am J Gastroenterol. 2002;97:2051-2057. |
| 133. | Czaja AJ, Carpenter HA, Santrach PJ, Moore SB. Genetic predispositions for the immunological features of chronic active hepatitis. Hepatology. 1993;18:816-822. |
| 134. | Tong MJ, Hsieh J, Blatt LM. Demographics, treatment and a predictive model of survival in a population of chronic auto- immune hepatitis patients (abstract). Hepatology. 1998;28:549A. |
| 135. | Heneghan MA, Kosar Y, McDougall NI, Portmann B, McFarlane IG, Harrison PM. Characteristics and outcome of a large male cohort with autoimmune hepatitis: Male sex as a favorable prognostic indicator: Treat-ment response and outcome (abstract). Gastroenterology. 2000;118:A1009. |
| 136. | Al-Chalabi T, Underhill JA, Portmann BC, McFarlane IG, Heneghan MA. Impact of gender on the long-term outcome and survival of patients with autoimmune hepatitis. J Hepatol. 2008;48:140-147. |
| 137. | Verma S, Torbenson M, Thuluvath PJ. The impact of ethnicity on the natural history of autoimmune hepatitis. Hepatology. 2007;46:1828-1835. |
| 138. | Nguyen GC, Thuluvath PJ. Racial disparity in liver disease: Biological, cultural, or socioeconomic factors. Hepatology. 2008;47:1058-1066. |
| 139. | Nakamura K, Yoneda M, Yokohama S, Tamori K, Sato Y, Aso K, Aoshima M, Hasegawa T, Makino I. Efficacy of ursodeoxycholic acid in Japanese patients with type 1 autoimmune hepatitis. J Gastroenterol Hepatol. 1998;13:490-495. |
| 140. | Czaja AJ, Souto EO, Bittencourt PL, Cancado EL, Porta G, Goldberg AC, Donaldson PT. Clinical distinctions and pathogenic implications of type 1 autoimmune hepatitis in Brazil and the United States. J Hepatol. 2002;37:302-308. |
| 141. | Chung HV, Riley M, Ho JK, Leung B, Jevon GP, Arbour LT, Barker C, Schreiber R, Yoshida EM. Retrospective review of pediatric and adult autoimmune hepatitis in two quaternary care centres in British Columbia: increased prevalence seen in British Columbia's First Nations community. Can J Gastroenterol. 2007;21:565-568. |
| 142. | Minuk GY, Liu S, Kaita K, Wong S, Renner E, Rempel J, Uhanova J. Autoimmune hepatitis in a North American Aboriginal/First Nations population. Can J Gastroenterol. 2008;22:829-834. |
| 143. | Scott JD, Garland N. Chronic liver disease in Aboriginal North Americans. World J Gastroenterol. 2008;14:4607-4615. |
| 144. | Donaldson PT, Czaja AJ. Genetic effects on susceptibility, clinical expression, and treatment outcome of type 1 autoimmune hepatitis. Clin Liver Dis. 2002;6:707-725. |
| 145. | Czaja AJ. Genetic factors affecting the occurrence, clinical phenotype, and outcome of autoimmune hepatitis. Clin Gastroenterol Hepatol. 2008;6:379-388. |
| 146. | Siegel AB, McBride RB, El-Serag HB, Hershman DL, Brown RS Jr, Renz JF, Emond J, Neugut AI. Racial disparities in utilization of liver transplantation for hepatocellular carcinoma in the United States, 1998-2002. Am J Gastroenterol. 2008;103:120-127. |
| 147. | Flores YN, Yee HF Jr, Leng M, Escarce JJ, Bastani R, Salmerón J, Morales LS. Risk factors for chronic liver disease in Blacks, Mexican Americans, and Whites in the United States: results from NHANES IV, 1999-2004. Am J Gastroenterol. 2008;103:2231-2238. |
| 148. | Günsar F, Akarca US, Ersöz G, Karasu Z, Yüce G, Batur Y. Clinical and biochemical features and therapy responses in primary biliary cirrhosis and primary biliary cirrhosis-autoimmune hepatitis overlap syndrome. Hepatogastroenterology. 2002;49:1195-1200. |
| 149. | Kaymakoglu S, Cakaloglu Y, Demir K, Türkoglu S, Badur S, Gürel S, Beşişik F, Cevikbaş U, Okten A. Is severe cryptogenic chronic hepatitis similar to autoimmune hepatitis? J Hepatol. 1998;28:78-83. |
| 150. | Balaban YH, Batman F, Us D, Hascelik G, Bayraktar D. Serum hepatocyte growth factor in autoimmune and hepatitis B-associated liver diseases. Indian J Gastroenterol. 2007;26:192-193. |
| 151. | Usta Y, Gurakan F, Akcoren Z, Ozen S. An overlap syndrome involving autoimmune hepatitis and systemic lupus erythematosus in childhood. World J Gastroenterol. 2007;13:2764-2767. |
| 152. | Kayacetin E, Köklü S, Temuçin T. Overlap syndrome of primary biliary cirrhosis and autoimmune hepatitis with unusual initial presentation as fulminant hepatic failure. Dig Liver Dis. 2004;36:419-422. |
| 153. | Cindoruk M, Yetkin I, Karakan T, Kandilci U. The prevalence of autoimmune hepatitis in Hashimoto's thyroiditis in a Turkish population. Acta Gastroenterol Belg. 2002;65:143-145. |
| 154. | Arslan N, Büyükgebiz B, Oztürk Y, Ozer E. The prevalence of liver function abnormalities in pediatric celiac disease patients and its relation with intestinal biopsy findings. Acta Gastroenterol Belg. 2005;68:424-427. |
| 155. | Kocaman O, Aygun C, Gurbuz Y, Cefle A, Konduk T, Senturk O, Celebi A, Hulagu S. Burst of autoimmunity with the emergence of primary Sjogren syndrome, cholestatic autoimmune hepatitis and latent autoimmune diabetes of adults (LADA). South Med J. 2006;99:1014-1015. |
| 156. | Soy M, Guldiken S, Arikan E, Altun BU, Tugrul A. Frequency of rheumatic diseases in patients with autoimmune thyroid disease. Rheumatol Int. 2007;27:575-577. |
| 157. | Tabak F, Ozdemir F, Tabak O, Erer B, Tahan V, Ozaras R. Autoimmune hepatitis induced by the prolonged hepatitis A virus infection. Ann Hepatol. 2008;7:177-179. |
| 158. | Selimoglu MA, Ertekin V. Autoimmune hepatitis triggered by Brucella infection or doxycycline or both. Int J Clin Pract. 2003;57:639-641. |
| 159. | Kocaman O, Hulagu S, Senturk O. Echinacea-induced severe acute hepatitis with features of cholestatic autoimmune hepatitis. Eur J Intern Med. 2008;19:148. |
| 160. | Kacar S, Akdogan M, Koşar Y, Parlak E, Sasmaz N, Oguz P, Aydog G. Estrogen and cyproterone acetate combination-induced autoimmune hepatitis. J Clin Gastroenterol. 2002;35:98-100. |
| 161. | Koşar Y, Saşmaz N, Oguz P, Kacar S, Erden E, Parlak E, Akdogan M. Ornidazole-induced autoimmune hepatitis. Eur J Gastroenterol Hepatol. 2001;13:737-739. |
| 162. | Kosar Y, Kacar S, Sasmaz N, Oguz P, Turhan N, Parlak E, Heneghan MA, McFarlane IG. Type 1 autoimmune hepatitis in Turkish patients: absence of association with HLA B8. J Clin Gastroenterol. 2002;35:185-190. |
| 163. | Czaja AJ, Carpenter HA. Histological features associated with relapse after corticosteroid withdrawal in type 1 autoimmune hepatitis. Liver Int. 2003;23:116-123. |
| 164. | Heneghan MA, McFarlane IG. Current and novel immunosuppressive therapy for autoimmune hepatitis. Hepatology. 2002;35:7-13. |
| 165. | Mistilis SP, Vickers CR, Darroch MH, McCarthy SW. Cyclosporin, a new treatment for autoimmune chronic active hepatitis. Med J Aust. 1985;143:463-465. |
| 166. | Hyams JS, Ballow M, Leichtner AM. Cyclosporine treatment of autoimmune chronic active hepatitis. Gastroenterology. 1987;93:890-893. |
| 167. | Jackson LD, Song E. Cyclosporin in the treatment of corticosteroid resistant autoimmune chronic active hepatitis. Gut. 1995;36:459-461. |
| 168. | Fernandes NF, Redeker AG, Vierling JM, Villamil FG, Fong TL. Cyclosporine therapy in patients with steroid resistant autoimmune hepatitis. Am J Gastroenterol. 1999;94:241-248. |
| 169. | Malekzadeh R, Nasseri-Moghaddam S, Kaviani MJ, Taheri H, Kamalian N, Sotoudeh M. Cyclosporin A is a promising alternative to corticosteroids in autoimmune hepatitis. Dig Dis Sci. 2001;46:1321-1327. |
| 170. | Van Thiel DH, Wright H, Carroll P, Abu-Elmagd K, Rodriguez-Rilo H, McMichael J, Irish W, Starzl TE. Tacrolimus: a potential new treatment for autoimmune chronic active hepatitis: results of an open-label preliminary trial. Am J Gastroenterol. 1995;90:771-776. |
| 171. | Aqel BA, Machicao V, Rosser B, Satyanarayana R, Harnois DM, Dickson RC. Efficacy of tacrolimus in the treatment of steroid refractory autoimmune hepatitis. J Clin Gastroenterol. 2004;38:805-809. |
| 172. | Larsen FS, Vainer B, Eefsen M, Bjerring PN, Adel Hansen B. Low-dose tacrolimus ameliorates liver inflammation and fibrosis in steroid refractory autoimmune hepatitis. World J Gastroenterol. 2007;13:3232-3236. |
| 173. | Alvarez F, Ciocca M, Cañero-Velasco C, Ramonet M, de Davila MT, Cuarterolo M, Gonzalez T, Jara-Vega P, Camarena C, Brochu P. Short-term cyclosporine induces a remission of autoimmune hepatitis in children. J Hepatol. 1999;30:222-227. |
| 174. | Debray D, Maggiore G, Girardet JP, Mallet E, Bernard O. Efficacy of cyclosporin A in children with type 2 autoimmune hepatitis. J Pediatr. 1999;135:111-114. |
| 175. | Cuarterolo M, Ciocca M, Velasco CC, Ramonet M, González T, López S, Garsd A, Alvarez F. Follow-up of children with autoimmune hepatitis treated with cyclosporine. J Pediatr Gastroenterol Nutr. 2006;43:635-639. |
| 176. | Pratt DS, Flavin DP, Kaplan MM. The successful treatment of autoimmune hepatitis with 6-mercaptopurine after failure with azathioprine. Gastroenterology. 1996;110:271-274. |
| 177. | Richardson PD, James PD, Ryder SD. Mycophenolate mofetil for maintenance of remission in autoimmune hepatitis in patients resistant to or intolerant of azathioprine. J Hepatol. 2000;33:371-375. |
| 178. | Devlin SM, Swain MG, Urbanski SJ, Burak KW. Mycophenolate mofetil for the treatment of autoimmune hepatitis in patients refractory to standard therapy. Can J Gastroenterol. 2004;18:321-326. |
| 179. | Chatur N, Ramji A, Bain VG, Ma MM, Marotta PJ, Ghent CN, Lilly LB, Heathcote EJ, Deschenes M, Lee SS. Transplant immunosuppressive agents in non-transplant chronic autoimmune hepatitis: the Canadian association for the study of liver (CASL) experience with mycophenolate mofetil and tacrolimus. Liver Int. 2005;25:723-727. |
| 180. | Czaja AJ, Carpenter HA. Empiric therapy of autoimmune hepatitis with mycophenolate mofetil: comparison with conventional treatment for refractory disease. J Clin Gastroenterol. 2005;39:819-825. |
| 181. | Inductivo-Yu I, Adams A, Gish RG, Wakil A, Bzowej NH, Frederick RT, Bonacini M. Mycophenolate mofetil in autoimmune hepatitis patients not responsive or intolerant to standard immunosuppressive therapy. Clin Gastroenterol Hepatol. 2007;5:799-802. |
| 182. | Czaja AJ. Safety issues in the management of autoimmune hepatitis. Expert Opin Drug Saf. 2008;7:319-333. |
| 183. | Hlivko JT, Shiffman ML, Stravitz RT, Luketic VA, Sanyal AJ, Fuchs M, Sterling RK. A single center review of the use of mycophenolate mofetil in the treatment of autoimmune hepatitis. Clin Gastroenterol Hepatol. 2008;6:1036-1040. |
| 184. | Hennes EM, Oo YH, Schramm C, Denzer U, Buggisch P, Wiegard C, Kanzler S, Schuchmann M, Boecher W, Galle PR. Mycophenolate mofetil as second line therapy in autoimmune hepatitis? Am J Gastroenterol. 2008;103:3063-3070. |
| 185. | Oo YH, Neuberger J. Use of mycophenolate in the treatment of autoimmune hepatitis. Liver Int. 2005;25:687-691. |
| 186. | Danielsson A, Prytz H. Oral budesonide for treatment of autoimmune chronic active hepatitis. Aliment Pharmacol Ther. 1994;8:585-590. |
| 187. | Wiegand J, Schüler A, Kanzler S, Lohse A, Beuers U, Kreisel W, Spengler U, Koletzko S, Jansen PL, Hochhaus G. Budesonide in previously untreated autoimmune hepatitis. Liver Int. 2005;25:927-934. |
| 188. | Zandieh I, Krygier D, Wong V, Howard J, Worobetz L, Minuk G, Witt-Sullivan H, Yoshida EM. The use of budesonide in the treatment of autoimmune hepatitis in Canada. Can J Gastroenterol. 2008;22:388-392. |
| 189. | Czaja AJ, Lindor KD. Failure of budesonide in a pilot study of treatment-dependent autoimmune hepatitis. Gastroenterology. 2000;119:1312-1316. |
| 190. | Clissold SP, Heel RC. Budesonide. A preliminary review of its pharmacodynamic properties and therapeutic efficacy in asthma and rhinitis. Drugs. 1984;28:485-518. |
| 191. | Geier A, Gartung C, Dietrich CG, Wasmuth HE, Reinartz P, Matern S. Side effects of budesonide in liver cirrhosis due to chronic autoimmune hepatitis: influence of hepatic metabolism versus portosystemic shunts on a patient complicated with HCC. World J Gastroenterol. 2003;9:2681-2685. |
| 192. | Kanzler S, Gerken G, Dienes HP, Meyer zum Büschenfelde KH, Lohse AW. Cyclophosphamide as alternative immunosuppressive therapy for autoimmune hepatitis--report of three cases. Z Gastroenterol. 1997;35:571-578. |
| 193. | Burak KW, Urbanski SJ, Swain MG. Successful treatment of refractory type 1 autoimmune hepatitis with methotrexate. J Hepatol. 1998;29:990-993. |
| 194. | Kerkar N, Dugan C, Rumbo C, Morotti RA, Gondolesi G, Shneider BL, Emre S. Rapamycin successfully treats post-transplant autoimmune hepatitis. Am J Transplant. 2005;5:1085-1089. |
| 195. | Santos ES, Arosemena LR, Raez LE, O'Brien C, Regev A. Successful treatment of autoimmune hepatitis and idiopathic thrombocytopenic purpura with the monoclonal antibody, rituximab: case report and review of literature. Liver Int. 2006;26:625-629. |
| 196. | Carmassi F, Morale M, Puccetti R, Pistelli F, Palla R, Bevilacqua G, Viacava P, Antonelli A, Mariani G. Efficacy of intravenous immunoglobulin therapy in a case of autoimmune-mediated chronic active hepatitis. Clin Exp Rheumatol. 1992;10:13-17. |
| 197. | Rebollo Bernárdez J, Cifuentes Mimoso C, Piñar Moreno A, Caunedo Alvarez A, Salas Herrero E, Jiménez-Sáenz M, Herrerías Gutiérrez J. Deflazacort for long-term maintenance of remission in type I autoimmune hepatitis. Rev Esp Enferm Dig. 1999;91:630-638. |
| 198. | Czaja AJ, Carpenter HA, Lindor KD. Ursodeoxycholic acid as adjunctive therapy for problematic type 1 autoimmune hepatitis: a randomized placebo-controlled treatment trial. Hepatology. 1999;30:1381-1386. |
| 199. | Czaja AJ, Bianchi FB, Carpenter HA, Krawitt EL, Lohse AW, Manns MP, McFarlane IG, Mieli-Vergani G, Toda G, Vergani D. Treatment challenges and investigational opportunities in autoimmune hepatitis. Hepatology. 2005;41:207-215. |
| 200. | Heneghan MA, Al-Chalabi T, McFarlane IG. Cost-effectiveness of pharmacotherapy for autoimmune hepatitis. Expert Opin Pharmacother. 2006;7:145-156. |
| 201. | Fridkis-Hareli M, Rosloniec EF, Fugger L, Strominger JL. Synthetic amino acid copolymers that bind to HLA-DR proteins and inhibit type II collagen-reactive T cell clones. Proc Natl Acad Sci USA. 1998;95:12528-12531. |
| 202. | Fridkis-Hareli M, Rosloniec EF, Fugger L, Strominger JL. Synthetic peptides that inhibit binding of the collagen type II 261-273 epitope to rheumatoid arthritis-associated HLA-DR1 and -DR4 molecules and collagen-specific T-cell responses. Hum Immunol. 2000;61:640-650. |
| 203. | Boetticher NC, Peine CJ, Kwo P, Abrams GA, Patel T, Aqel B, Boardman L, Gores GJ, Harmsen WS, McClain CJ. A randomized, double-blinded, placebo-controlled multicenter trial of etanercept in the treatment of alcoholic hepatitis. Gastroenterology. 2008;135:1953-1960. |
| 204. | Lohse AW, Dienes HP, Meyer zum Büschenfelde KH. Suppression of murine experimental autoimmune hepatitis by T-cell vaccination or immunosuppression. Hepatology. 1998;27:1536-1543. |
| 205. | Wardrop RM 3rd, Whitacre CC. Oral tolerance in the treatment of inflammatory autoimmune diseases. Inflamm Res. 1999;48:106-119. |
| 206. | Nagler A, Pines M, Abadi U, Pappo O, Zeira M, Rabbani E, Engelhardt D, Ohana M, Chowdhury NR, Chowdhury JR. Oral tolerization ameliorates liver disorders associated with chronic graft versus host disease in mice. Hepatology. 2000;31:641-648. |
| 207. | Czaja AJ. Understanding the pathogenesis of autoimmune hepatitis. Am J Gastroenterol. 2001;96:1224-1231. |
| 208. | Manns MP, Vogel A. Autoimmune hepatitis, from mechanisms to therapy. Hepatology. 2006;43:S132-S144. |
| 209. | Lapierre P, Béland K, Alvarez F. Pathogenesis of autoimmune hepatitis: from break of tolerance to immune-mediated hepatocyte apoptosis. Transl Res. 2007;149:107-113. |
| 210. | Jaeckel E. Animal models of autoimmune hepatitis. Semin Liver Dis. 2002;22:325-338. |
| 211. | Christen U, Holdener M, Hintermann E. Animal models for autoimmune hepatitis. Autoimmun Rev. 2007;6:306-311. |
| 212. | Bowen DG. Of mice and molecular mimicry: modeling autoimmune hepatitis. Hepatology. 2008;48:1013-1015. |
| 213. | Lapierre P, Djilali-Saiah I, Vitozzi S, Alvarez F. A murine model of type 2 autoimmune hepatitis: Xenoimmunization with human antigens. Hepatology. 2004;39:1066-1074. |
| 214. | Holdener M, Hintermann E, Bayer M, Rhode A, Rodrigo E, Hintereder G, Johnson EF, Gonzalez FJ, Pfeilschifter J, Manns MP. Breaking tolerance to the natural human liver autoantigen cytochrome P450 2D6 by virus infection. J Exp Med. 2008;205:1409-1422. |
| 215. | Kuo TK, Hung SP, Chuang CH, Chen CT, Shih YR, Fang SC, Yang VW, Lee OK. Stem cell therapy for liver disease: parameters governing the success of using bone marrow mesenchymal stem cells. Gastroenterology. 2008;134:2111-2121, 2121.e1-2121.e3. |