Published online Apr 7, 2009. doi: 10.3748/wjg.15.1587
Revised: March 9, 2009
Accepted: March 16, 2009
Published online: April 7, 2009
AIM: To evaluate the ability of endoscopic ultrasound (EUS) elastography to distinguish benign from malignant pancreatic masses and lymph nodes.
METHODS: A multicenter study was conducted and included 222 patients who underwent EUS examination with assessment of a pancreatic mass (n = 121) or lymph node (n = 101). The classification as benign or malignant, based on the real time elastography pattern, was compared with the classification based on the B-mode EUS images and with the final diagnosis obtained by EUS-guided fine needle aspiration (EUS-FNA) and/or by surgical pathology. An interobserver study was performed.
RESULTS: The sensitivity and specificity of EUS elastography to differentiate benign from malignant pancreatic lesions are 92.3% and 80.0%, respectively, compared to 92.3% and 68.9%, respectively, for the conventional B-mode images. The sensitivity and specificity of EUS elastography to differentiate benign from malignant lymph nodes was 91.8% and 82.5%, respectively, compared to 78.6% and 50.0%, respectively, for the B-mode images. The kappa coefficient was 0.785 for the pancreatic masses and 0.657 for the lymph nodes.
CONCLUSION: EUS elastography is superior compared to conventional B-mode imaging and appears to be able to distinguish benign from malignant pancreatic masses and lymph nodes with a high sensitivity, specificity and accuracy. It might be reserved as a second line examination to help characterise pancreatic masses after negative EUS-FNA and might increase the yield of EUS-FNA for lymph nodes.
- Citation: Giovannini M, Botelberge T, Bories E, Pesenti C, Caillol F, Esterni B, Monges G, Arcidiacono P, Deprez P, Yeung R, Schimdt W, Schrader H, Szymanski C, Dietrich C, Eisendrath P, Van Laethem JL, Devière J, Vilmann P, Saftoiu A. Endoscopic ultrasound elastography for evaluation of lymph nodes and pancreatic masses: A multicenter study. World J Gastroenterol 2009; 15(13): 1587-1593
- URL: https://www.wjgnet.com/1007-9327/full/v15/i13/1587.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.1587
A major limitation of endoscopic ultrasound (EUS) examination is its limited capacity to determine the exact nature of a lesion. Differential diagnosis between benign and malignant lymph nodes and focal pancreatic masses based on the EUS appearance is difficult and frequently requires EUS-guided fine needle aspiration (EUS-FNA) for confirmation of malignancy[1–4].
Elastography has recently been presented as a novel technique that can be applied during ultrasound (US) examination to assess and measure tissue elasticity. Knowing that malignant tissues are generally harder than normal surrounding tissue, elastography might provide interesting clinical information to help distinguish benign from malignant tissue based on their specific tissue consistency. Clinical research has shown promising results in differentiating between benign and malignant tissue in the thyroid gland[5], breast[6–8], prostate[910] and to assess liver fibrosis[11–14]. Recently, elastography has also been introduced during EUS examination[15–19]. The current study is a continuation of previous research[15] to validate the potential role of elastography in distinguishing benign from malignant lymph nodes and focal pancreatic lesions in a large retrospective trial. The aim of this multicenter study was to classify lymph nodes and pancreatic masses during EUS examination as benign or malignant based on the real time (qualitative) elastography patterns and to compare the results with a classification based on the conventional B-mode EUS images and with the final diagnosis obtained by EUS-FNA and/or by surgical pathology.
Every patient (n = 222) who underwent EUS examination with evaluation of a pancreatic mass (n =121) or lymph nodes (n = 101), between October 2006 and February 2007, was included. The study was conducted in seven different centers throughout Europe. Only one lesion per patient was examined and by one single endoscopist per center. Each center started the study after six months experience of EUS elastography. The EUS examinations were performed with conventional linear EUS probes (Pentax EG38-UT and EG38-70UTK, Hamburg, Germany). The examined lesion was first classified as benign or malignant based on the conventional B-mode images. Subsequently, elastography was carried out in real time using a commercially available module incorporated into the Hitachi EUB-8500 system (Hitachi Medical Systems Europe, Zug, Switzerland). The technology measures the degree of tissue deformation after compression as an indicator for the stiffness of tissue. This compression during EUS examination is naturally obtained by arterial pulsations and respiratory movements. Detailed reviews on the technical aspects of elastography have been previously published[152021]. The sample area was adjusted to the region of interest and the suitability of the elastographic signal was indicated by a numeric scale within the image. Tissue elasticity was shown superimposed on the conventional B-mode EUS image by colors reflective of stiffness. Hard tissue areas were marked with blue, intermediate areas with green, medium soft areas with yellow and soft areas with red. Elastographic and B-mode images were displayed simultaneously side by side. The complete spectrum from blue to red was applied to each elastographic image and represented the graduation of relative elasticity within the sample area. Elastographic images were interpreted during the examination and a 60 s video loop was recorded for an interobserver study. After assessing the elastographic images, EUS-FNA was performed in all cases for clinical reasons using a 22-gauge needle (Wilson-Cook Medical, Winston-Salem, North Carolina). The technique of EUS-FNA is described elsewhere[22]. In all participating centers, the specimen were examined using the monolayer cytology technique[23]. An on-site pathologist was present in only four centers during the examination. In the the remaining centers, the endoscopist assessed the sample to ensure sufficient tissue was obtained based on the presence of tissue filaments in the conservation solution and repeat punctures were performed if necessary. The pathologist was blinded to the elastography results. The final diagnosis was based on histology obtained by EUS-FNA and surgical specimen when available. If EUS-FNA was found to be negative (after at least one repeat examination), a 12 mo clinical and imaging follow-up was carried out in the absence of surgical specimens. The following parameters were recorded in a protocol: the classification as benign or malignant based on the B-mode images, the elastography score based on the elastographic pattern and the classification as benign or malignant based on this pattern and the final result based on histology.
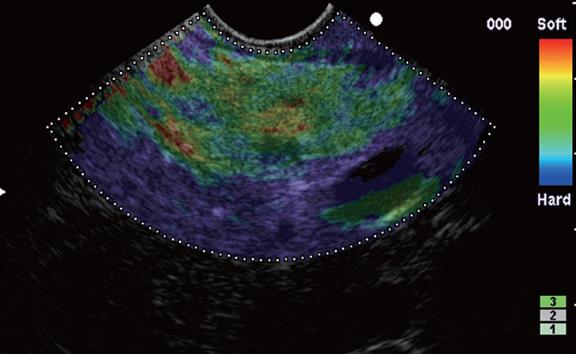
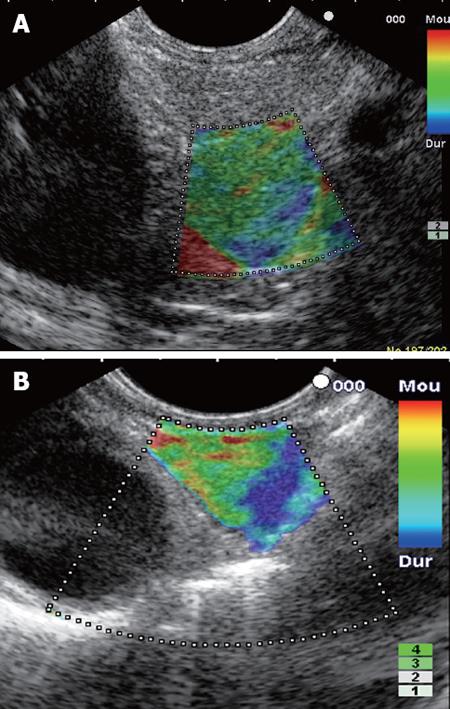
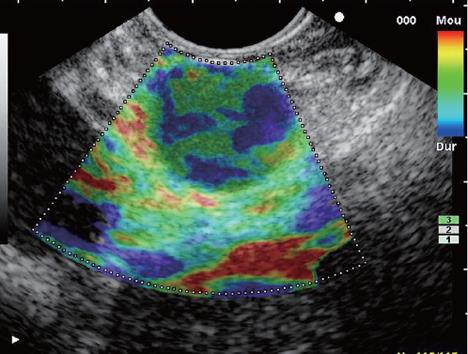
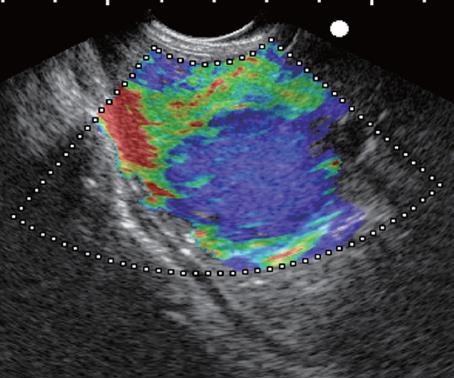
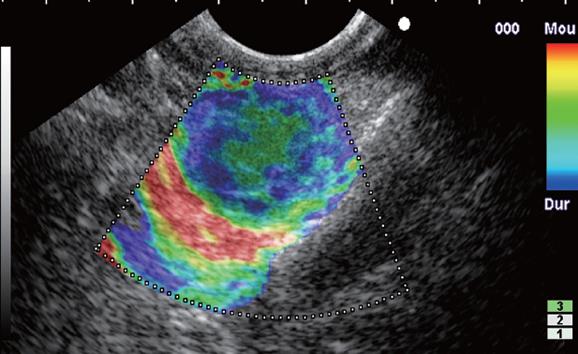
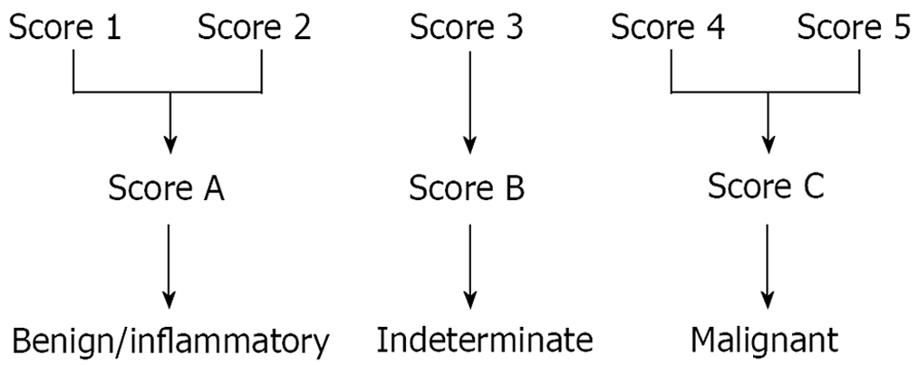
The elastographic images were scored according to elastographic patterns based on previous research[15]: a score equal to 1 was assigned when the image showed a homogenous soft tissue area (green) corresponding to normal tissue (Figure 1), a score equal to 2 when the image indicated heterogenous soft tissue (green, yellow, and red) corresponding to fibrosis or inflammatory tissue (Figure 2A and B), a score equal to 3 when the image dislayed mixed colors or a honeycombed elastography pattern indicative of mixed hard and soft tissue making the interpretation difficult (Figure 3), a score equal to 4 when the image displayed a small soft (green) central area surrounded by mainly hard (blue) tissue corresponding to a malignant hypervascularized lesion (Figure 4) and a score equal to 5 was assigned to lesions representing mainly hard (blue) tissue with areas of heterogeneous soft tissue (green, red) representing zones of necrosis in an advanced malignant lesions (Figure 5). For the study purpose and to facilitate the use in clinical practice, we subsequently classified an elastography score equal to 1 and 2 as: (A) representing normal tissue or a benign tumor; a score equal to 4 and 5 was classified as (C) representing a malignant lesion; and a score equal to 3 was classified as (B) which represented tissue difficult to classify as benign or malignant based on the elastographic pattern. However a score equal to (B) was considered as malignant for statistical analysis (Figure 6).
The results are presented as means plus or minus standard deviation or as medians with ranges, depending on the data distribution. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and accuracy were calculated as appropriate.
An interobserver study was performed on a statistically representative and blinded selection of 30 videos (15 of a pancreatic mass and 15 of a lymph node). These videos were each evaluated by five endoscopists experienced in EUS and elastography. The elastographic images were scored with a whole number from 1 to 5 using the previous described criteria, constituting an ordered variable. The agreement between two different examiners was measured by an adapted kappa coefficient. The interobserver study also evaluated the agreement between two examiners to classify the examined tissue as benign or malignant. The result is a binary variable (“malignant” or “benign”). The results being “inconclusive” were considered as missing data for the calculation of the agreement. This agreement was measured by the Cohen kappa coefficient.
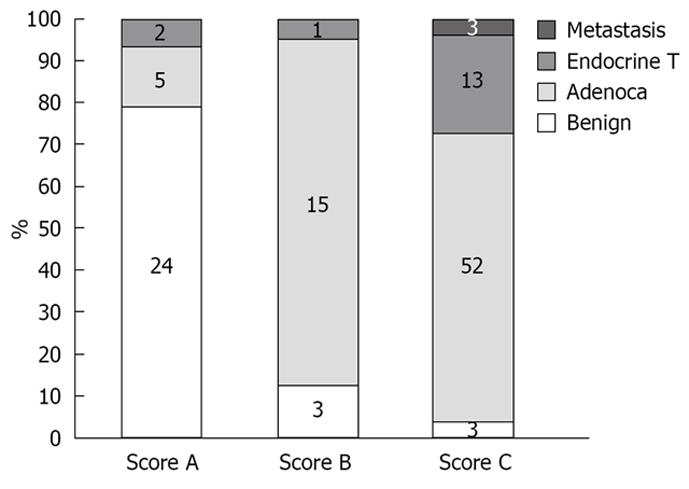
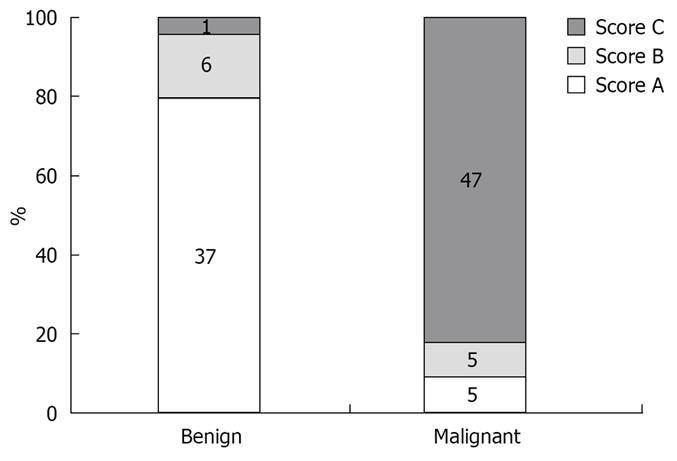
One hundred and twenty-one patients (77 M and 44 F, mean age 63 years) underwent EUS examination with elastography for evaluation of a pancreatic mass (mean diameter 29.5 mm, range 7-80 mm). The masses were located in the pancreatic head (n = 48), isthmus (n = 17), body (n = 29), tail (n = 13) and uncinate process (n = 14). No complications occurred during the study. The final histological assessment was based on the FNA results in 82 cases and on surgical pathology in 39 cases. The final diagnosis of the pancreatic masses included pancreatic adenocarcinoma (n = 72), malignant endocrine tumor (n = 16), benign endocrine tumor (n = 2), benign chronic pancreatitis related nodules (n = 28) and pancreatic metastasis (n = 3) (Figure 7). The elastographic images were interpreted as benign (score 1 + 2 = A) in 31 cases, indeterminate (score 3 = B) in 19 cases and malignant (score 4 + 5 = C) in 71 cases. Considering the “indeterminate” result equal to score (B) as malignant, the calculated sensitivity, specificity, positive and negative predictive values of EUS elastography to differentiate benign from malignant pancreatic masses were, respectively, 92.3%, 80.0%, 93.3% and 77.4% with a global accuracy of this new technology of 89.2%. The calculated sensitivity, specificity, positive and negative predictive values of conventional B-mode images to differentiate benign from malignant pancreatic masses were, respectively, 92.3%, 68.9%, 75.8% and 22.2% with an accuracy of 71.9% (Table 1).
| Histology | |||
| Malignant (n) | Benign (n) | ||
| Elastography/conventional B-mode | Malignant | 84/85 | 6/27 |
| Benign | 7/7 | 24/2 | |
One hundred and one patients (56 M and 45 F, mean age 61.1 years) underwent EUS examination of a lymph node for staging of lung cancer (n = 25), oesophageal carcinoma (n = 25), gastric cancer (n = 13), pancreatic cancer (n = 13), for suspicion of lymph node relapse of kidney cancer (n = 2) and of breast cancer (n = 8). EUS examination was also performed for evaluation of isolated lymph nodes (n = 15). Lymph nodes (mean diameter 20.1 mm, range 7-50 mm) were located in the mediastinum (n = 51), in the cervical area (n = 4), in the celiac or mesenteric area (n = 44), and in the perirectal space (n = 2). No complications occurred during the study. The final histological assessment was based on FNA and classified the lymph nodes as malignant in 57 cases, including metastasis of an adenocarcinoma (n = 35), metastasis of a squamous cell carcinoma (n = 13), metastasis of an endocrine tumor (n = 3), metastasis of a melanoma (n = 1), lymphomas (n = 5), and benign in 44 cases (including three cases of sarcoidosis) (Figure 8). The elastographic images were interpreted as benign (score 1 + 2 = A) in 38 cases, indeterminate (score 3 = B) in 10 cases and malignant (score 4 + 5 = C) in 53 cases. Considering the “indeterminate” result equal to score (B) as malignant, the calculated sensitivity, specificity, positive and negative predictive values were, respectively, 91.8%, 82.5%, 88.8% and 86.8% with a global accuracy of this new technology of 88.1%. The calculated sensitivity, specificity, positive and negative predictive values for the conventional B-mode images were respectively 78.6%, 50.0%, 70.5% and 60.6% with an accuracy of 67.3% (Table 2).
| Histology | |||
| Malignant (n) | Benign (n) | ||
| Elastography/conventional B-mode | Malignant | 51/48 | 2/20 |
| Benign | 10/13 | 38/20 | |
The kappa coefficient of the sonoelastography score for pancreatic masseswas 0.524, for the lymph nodes 0.519, and 0.520 for all cases confound.
The kappa coefficient for the differentiation between benign and malignant tissue was 0.785 for the pancreatic masses, 0.657 for the lymph nodes and 0.725 for all cases confound.
The aim of this multicenter study was to evaluate the ability of EUS elastography to distinguish benign from malignant focal pancreatic masses and lymph nodes and to compare the results with the conventional B-mode images and final histology.
Our study shows that EUS elastography has high sensitivity, specificity and accuracy and a much higher specificity than conventional B-mode images to differentiate between benign and malignant focal pancreatic lesions. Using our current scoring system, 15.7% of the cases still obtain an elastography score equal to 3 indicating tissue difficult to classify as benign or malignant. However, 84% of these cases with an elastography score equal to 3 turned out to be malignant and we believe that the soft tissue parts of these focal lesions on elastography represent necrotic areas in an adenocarcinoma (n = 15) or a hypervasccularised area in an endocrine tumor (n = 1). Hence, an elastography score equal to 3 should be considered as malignant, in our opinion.
There were seven false negative cases (five adenocarcinoma and two neuroendocrine tumors) that may be explained in a similar way: the presence of abundant necrotic or vascular tissue resulted in an elastographic pattern mainly consisting of soft tissue. By contrast, the false positive cases in our study (n = 6) might represent patients with (early) chronic pancreatitis having areas of hard fibrotic nodules. Unfortuntaly, lack of surgical specimens in these patients cannot confirm this hypothesis. However, in a recent publication by Janssen et al[16], the elastographic patterns of the normal pancreas and the pancreas affected by inflammatory or focal disease were studied. They concluded that elastography does not distinguish between chronic pancreatitis and tumors because of their similar fibrous structure. This implies that EUS elastography will not be able to help target suspicious lesions and improve the rather low accuracy of EUS-FNA in patients with chronic pancreatitis.
In distinguishing benign from malignant focal pancreatic lesions, EUS elastography does not replace tissue confirmation and we believe that EUS elastography should not be used as a first line examination in the evaluation of focal pancreatic lesions. However, when facing (repeated) negative EUS-FNA or technical problems in performing EUS-FNA, the interpretation of the EUS elastographic images could help orientate the diagnosis and influence the decision making for surgery when the lesion is suspicious on elastography, or justify a follow-up when the elastographic images are in favour of a benign lesion.
Our data also shows that EUS elastography has high sensitivity, specificity and accuracy in distinguishing benign from malignant lymph nodes and seems to be superior to conventional B-mode images. Whether the false negative and false positive cases in this study are due to the presence of necrotic and fibrotic areas in lymph nodes, respectively, is less certain. Our results confirm comparable results obtained by Săftoiu et al[18] using similar elastography pattern criteria to differentiate benign from malignant lymph nodes in 42 patients with a reported sensitivity, specificity and accuracy of, respectively, 91.7%, 94.4% and 92.86%. The role of EUS elastography to distinguish benign from malignant lymph nodes should be considered as complementary to other imaging techniques rather than a replacement for tissue confirmation. Based on a high PPV, EUS elastography might help in selecting more suspicious lymph nodes for tissue sampling, especially in patients presenting multiple lymph nodes, such as in oesophageal or lung cancer. Based on a high NPV, it might be used to reduce the number of unnecessary biopsies. As for focal pancreatic lesions, EUS elastography might offer an alternative for differential diagnosis in the case of negative EUS-FNA of a lymph node, as well as in situations where EUS-FNA is not possible (technical problems, interposed malignant tissue or interposed vascular structure).
The current results are different from the results obtained during our previous research[15]. In this previous study, EUS elastography was shown to have a sensitivity of 100% and specificities of 67% and 50% for diagnosing malignant pancreatic masses and lymph nodes, respectively. Although false positive results in both study groups were reported, it should be recalled that the number of benign lesions in the previous study was relatively small.
For both pancreatic masses and lymph nodes, EUS elastography might also help in guiding the puncture in a non necrotic part of the suspicious lesion when necrotic tissue is present, as in advanced cancer.
One of the main criticisms of EUS elastography is the variability of the elastographic images and the difficulty of interpretation[19]. However, our interobserver study showed a satisfying interobserver concordance for the differentiation between benign and malignant pancreatic masses and lymph nodes (κ = 0.725).
In the absence of pathologic assesment of surgical specimens, we considered the EUS-FNA result as a gold standard. Although the specificity of EUS-FNA is close to 100%[24–28], it has the potential to miss micro-invasion of malignancy into lymph nodes or to give false negative results for a necrotic pancreatic lesion. However, we consider it as representative of daily practice, particularly when it is combined with an adequate clinical and imaging follow-up period.
To overcome the difficulty in classifying the EUS elastography score equal to 3 or (B) as benign or malignant, we are currently evaluating the next generation of elastography software. This new software provides a quantitative histogram analysis of the elastographic images and has already proven to be useful in the evaluation of lymph nodes[18].
The potential role of EUS elastography to help detect and differentiate submucosal tumors as well as any other solid masses situated nearby the gastrointestinal tract has still to be evaluated. The exact role of EUS elastography in patients manifesting symptoms suggestive of chronic pancreatitis with equivocal EUS (3 features or fewer) has still to be validated[29].
EUS elastography is a new application in the field of the endosonography and seems to be able to differentiate benign from malignant lymph nodes and pancreatic lesions with a high sensitivity, specificity and accuracy. EUS elastography is superior compared to conventional B-mode imaging and the interobserver reproductibility is satisfying. The goal is not to replace tissue confirmation. Instead, the information obtained by EUS elastography should be considered as complementary to the conventional EUS imaging. It should be reserved as a second line examination to orientate further decision making after repeat negative EUS-FNA for pancreatic lesions. It may increase the yield of FNA and reduce the number of unnecessary biopsies when assessing lymph nodes. However, further research is necessary to improve our current elastography scoring system. The second generation of elastography software providing quantitative analysis of tissue elasticity might be able to increase the accuracy of this technique.
Elastography has recently been presented as a novel technique that can be applied during ultrasound examination to assess and measure tissue elasticity. Clinical research has shown promising results in differentiating between benign and malignant tissue in the thyroid gland, breast, prostate and to assess liver fibrosis.
endoscopic ultrasound (EUS) elastography is a new application in the field of the endosonography and seems to be able to differentiate benign from malignant lymph nodes and pancreatic lesions with a high sensitivity, specificity and accuracy. EUS elastography is superior compared to conventional B-mode imaging and the interobserver reproductibility is satisfying.
In distinguishing benign from malignant focal pancreatic lesions, EUS elastography does not replace tissue confirmation and should not be used as a first line examination in the evaluation of focal pancreatic lesions. However, when facing (repeated) negative EUS-guided fine needle aspiration (EUS-FNA) or technical problems to perform EUS-FNA, the interpretation of the EUS elastographic images could help orientate the diagnosis and influence the decision making for surgery when the lesion is suspicious on elastography, or justify a follow-up when the elastographic images are in favour of a benign lesion.
The goal is not to replace tissue confirmation. Instead, the information obtained by EUS elastography should be considered as complementary to the conventional EUS imaging. It should be reserved as a second line examination to orientate further decision making after repeat negative EUS-FNA for pancreatic lesions. It might increase the yield of FNA and reduce the number of unnecessary biopsies when assessing lymph nodes.
The importance of the research and the significance of the research contents are high. Presentation and readability of the manuscript is highly acceptable.
| 1. | Bhutani MS, Hawes RH, Hoffman BJ. A comparison of the accuracy of echo features during endoscopic ultrasound (EUS) and EUS-guided fine-needle aspiration for diagnosis of malignant lymph node invasion. Gastrointest Endosc. 1997;45:474-479. |
| 2. | Tamerisa R, Irisawa A, Bhutani MS. Endoscopic ultrasound in the diagnosis, staging, and management of gastrointestinal and adjacent malignancies. Med Clin North Am. 2005;89:139-158, viii. |
| 3. | Vazquez-Sequeiros E, Levy MJ, Clain JE, Schwartz DA, Harewood GC, Salomao D, Wiersema MJ. Routine vs. selective EUS-guided FNA approach for preoperative nodal staging of esophageal carcinoma. Gastrointest Endosc. 2006;63:204-211. |
| 4. | Eloubeidi MA, Chen VK, Eltoum IA, Jhala D, Chhieng DC, Jhala N, Vickers SM, Wilcox CM. Endoscopic ultrasound-guided fine needle aspiration biopsy of patients with suspected pancreatic cancer: diagnostic accuracy and acute and 30-day complications. Am J Gastroenterol. 2003;98:2663-2668. |
| 5. | Lyshchik A, Higashi T, Asato R, Tanaka S, Ito J, Mai JJ, Pellot-Barakat C, Insana MF, Brill AB, Saga T. Thyroid gland tumor diagnosis at US elastography. Radiology. 2005;237:202-211. |
| 6. | Itoh A, Ueno E, Tohno E, Kamma H, Takahashi H, Shiina T, Yamakawa M, Matsumura T. Breast disease: clinical application of US elastography for diagnosis. Radiology. 2006;239:341-350. |
| 7. | Thomas A, Fischer T, Frey H, Ohlinger R, Grunwald S, Blohmer JU, Winzer KJ, Weber S, Kristiansen G, Ebert B. Real-time elastography--an advanced method of ultrasound: First results in 108 patients with breast lesions. Ultrasound Obstet Gynecol. 2006;28:335-340. |
| 8. | Thomas A, Kümmel S, Fritzsche F, Warm M, Ebert B, Hamm B, Fischer T. Real-time sonoelastography performed in addition to B-mode ultrasound and mammography: improved differentiation of breast lesions? Acad Radiol. 2006;13:1496-1504. |
| 9. | Cochlin DL, Ganatra RH, Griffiths DF. Elastography in the detection of prostatic cancer. Clin Radiol. 2002;57:1014-1020. |
| 10. | Sommerfeld HJ, Garcia-Schürmann JM, Schewe J, Kühne K, Cubick F, Berges RR, Lorenz A, Pesavento A, Scheipers U, Ermert H. [Prostate cancer diagnosis using ultrasound elastography. Introduction of a novel technique and first clinical results]. Urologe A. 2003;42:941-945. |
| 11. | Castéra L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, Darriet M, Couzigou P, De Lédinghen V. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343-350. |
| 12. | Corpechot C, El Naggar A, Poujol-Robert A, Ziol M, Wendum D, Chazouillères O, de Lédinghen V, Dhumeaux D, Marcellin P, Beaugrand M. Assessment of biliary fibrosis by transient elastography in patients with PBC and PSC. Hepatology. 2006;43:1118-1124. |
| 13. | Foucher J, Chanteloup E, Vergniol J, Castéra L, Le Bail B, Adhoute X, Bertet J, Couzigou P, de Lédinghen V. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut. 2006;55:403-408. |
| 14. | Gómez-Domínguez E, Mendoza J, Rubio S, Moreno-Monteagudo JA, García-Buey L, Moreno-Otero R. Transient elastography: a valid alternative to biopsy in patients with chronic liver disease. Aliment Pharmacol Ther. 2006;24:513-518. |
| 15. | Giovannini M, Hookey LC, Bories E, Pesenti C, Monges G, Delpero JR. Endoscopic ultrasound elastography: the first step towards virtual biopsy? Preliminary results in 49 patients. Endoscopy. 2006;38:344-348. |
| 16. | Janssen J, Schlörer E, Greiner L. EUS elastography of the pancreas: feasibility and pattern description of the normal pancreas, chronic pancreatitis, and focal pancreatic lesions. Gastrointest Endosc. 2007;65:971-978. |
| 17. | Saftoiu A, Vilman P. Endoscopic ultrasound elastography-- a new imaging technique for the visualization of tissue elasticity distribution. J Gastrointestin Liver Dis. 2006;15:161-165. |
| 18. | Săftoiu A, Vilmann P, Hassan H, Gorunescu F. Analysis of endoscopic ultrasound elastography used for characterisation and differentiation of benign and malignant lymph nodes. Ultraschall Med. 2006;27:535-542. |
| 19. | Fritscher-Ravens A. Blue clouds and green clouds: virtual biopsy via EUS elastography? Endoscopy. 2006;38:416-417. |
| 20. | Ophir J, Cespedesa EI, Garrab BS, Ponnekantia H, Huanga Y, Maklada N. Elastography: Ultrasonic imaging of tissue strain and elastic modulus in vivo. Eur J Ultrasound. 1996;3:49-70. |
| 21. | Frey H. [Realtime elastography. A new ultrasound procedure for the reconstruction of tissue elasticity]. Radiologe. 2003;43:850-855. |
| 22. | Janssen J, Johanns W, Luis W, Greiner L. [Clinical value of endoscopic ultrasound-guided transesophageal fine needle puncture of mediastinal lesions]. Dtsch Med Wochenschr. 1998;123:1402-1409. |
| 23. | Monges G. [Fine needle aspiration biopsy of the pancreas]. Ann Pathol. 2002;22:416-421. |
| 24. | Eloubeidi MA, Jhala D, Chhieng DC, Chen VK, Eltoum I, Vickers S, Mel Wilcox C, Jhala N. Yield of endoscopic ultrasound-guided fine-needle aspiration biopsy in patients with suspected pancreatic carcinoma. Cancer. 2003;99:285-292. |
| 25. | Shin HJ, Lahoti S, Sneige N. Endoscopic ultrasound-guided fine-needle aspiration in 179 cases: the M. D. Anderson Cancer Center experience. Cancer. 2002;96:174-180. |
| 26. | Ardengh JC, Lopes CV, de Lima LF, de Oliveira JR, Venco F, Santo GC, Modena JL. Diagnosis of pancreatic tumors by endoscopic ultrasound-guided fine-needle aspiration. World J Gastroenterol. 2007;13:3112-3116. |
| 27. | Iglesias-Garcia J, Dominguez-Munoz E, Lozano-Leon A, Abdulkader I, Larino-Noia J, Antunez J, Forteza J. Impact of endoscopic ultrasound-guided fine needle biopsy for diagnosis of pancreatic masses. World J Gastroenterol. 2007;13:289-293. |
| 28. | Eloubeidi MA, Chen VK, Eltoum IA, Jhala D, Chhieng DC, Jhala N, Vickers SM, Wilcox CM. Endoscopic ultrasound-guided fine needle aspiration biopsy of patients with suspected pancreatic cancer: diagnostic accuracy and acute and 30-day complications. Am J Gastroenterol. 2003;98:2663-2668. |
| 29. | Hirooka Y, Itoh A, Kawashima H, Hara K, Kanamori A, Uchida H, Goto J, Nonogaki K, Matsumoto Y, Ohmiya N. Preliminary results in the diagnosis of early stage chronic pancreatitis using EUS-elastography. Gastrointest Endosc. 2006;63:AB258. |