Published online Mar 28, 2009. doi: 10.3748/wjg.15.1493
Revised: January 19, 2009
Accepted: January 26, 2009
Published online: March 28, 2009
AIM: To define the topography of mast cells and their numbers in cases of Hirschsprung’s disease (HD) and non-HD, assess neural hypertrophy using imaging software and to study the relationship between mast cells and nerve fibers.
METHODS: HE stained sections of 32 cases of chronic constipation in the age group of 0-14 years were reviewed for ganglion cells. AChE staining was performed on frozen sections of colonic and rectal biopsies. Based on their findings cases were divided into HD and non-HD and mast cells stained by toluidine blue were evaluated. Image analysis by computerized software was applied to S-100 stained sections for assessment of neural hypertrophy.
RESULTS: Difference between number of mast cells in HD group (mean = 36.44) and in non-HD group (mean = 14.79) was statistically significant. Image analysis morphometry on S-100 stained sections served as a useful adjunct. The difference between number, size, and perimeter of the nerve fibers between HD and non-HD group was statistically significant.
CONCLUSION: Mast cells are significantly increased in HD and their base line values are much higher in Indian children than that reported in Western literature. Their role in HD needs further research. Morphometry of S-100 stained nerve fibers is a useful adjunct to conventional methods for diagnosis of HD.
- Citation: Yadav AK, Mishra K, Mohta A, Agarwal S. Hirschsprung’s disease: Is there a relationship between mast cells and nerve fibers? World J Gastroenterol 2009; 15(12): 1493-1498
- URL: https://www.wjgnet.com/1007-9327/full/v15/i12/1493.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.1493
Constipation is a common presenting complaint in children attending the pediatric outpatient department[1]. Some 13%-15% cases of constipation are due to aganglionosis of bowel segment or Hirschsprung’s disease (HD). HD is a serious disorder and can be fatal if left untreated. The disease is however, surgically correctable, underscoring the need to identify these cases.
HD has an incidence of 1 in 5000 neonates in Western literature and a male to female predominance of 4:1. Although there are no statistical figures available from India, a large number of studies in Indian literature suggest that the disorder is not uncommon in our country[2–4].
Of late there has been a lot of interest in the role of mast cells (MC) in the pathogenesis of Hirschsprung’s disease. Kobayashi et al[5] have found an increased number of mast cells in aganglionic bowel segments and in those with intestinal neuronal dysplasia. The exact role of mast cells in HD is not known. Therefore we undertook this study to evaluate the relationship between mast cells and nerve fibers in HD cases to shed some light on this issue. We have used image analysis morphometry to analyze nerve fibers stained with S-100 and correlated the findings with mast cell numbers.
There are only a couple of studies on mast cells in HD in the literature[5–7]. There is however no study from India on mast cells in children with HD or constipation. As children in a developing country like India are more likely to be exposed to inflammatory stimuli like bacterial and viral antigens and pollutants/allergens as compared to their Western counterparts, an increase in mast cells was expected in the intestines. The cells were assessed in children with constipation, with an emphasis on HD, to ascertain their distribution in the colonic and rectal biopsies.
The study was conducted in the departments of Pathology and Surgery, University College of Medical Sciences and GTB Hospital, New Delhi.
Thirty two cases of chronic constipation (defined as decreased frequency of bowel movements “fewer than three each week” or a difficulty in defecation perceived by the parents as a problem that requires medication or manual intervention[1]) in the age group of 0-14 years were enrolled for the study. Cases of constipation due to local causes like anal fissure, anal stenosis and anterior perineal anus were excluded from the study.
The criterion used for diagnosis of HD in all of the HD cases was absence of ganglion cells upon evaluation of Haematoxylin & Eosin (HE) stained sections of colonic and rectal biopsies. Acetylcholinesterase (AChE) stain for the nerve fiber pattern was carried out in 12 cases. Based on the findings of HE and AChE they were divided into two groups: GroupI- HD and Group II-non-HD. GroupI-HD included 11 rectal biopsies and 7 resection specimens. Group II-non-HD included 14 rectal biopsies. Rectal biopsies obtained were rectal punch biopsies. Mucosa and submucosa was available for review in all the cases. Muscularis propria was available in resection specimens only.
Toluidine blue stain was performed on paraffin sections of all the cases to evaluate mast cells. Immuno-histochemical stain for S-100 was also performed on 12 HD cases & 8 non-HD cases.
Ten micrometer thick sections cut on the cryostat were fixed by dipping in buffered formalin for 1-2 min, preserved by wrapping them in aluminium foil and stored at -20°C in a deep freezer till the time of staining. Stock solutions of various ingredients of incubation medium were kept at 4°C and the incubation medium was prepared just before staining. AChE stain was performed using modified Karnovsky and Roots method as described previously[8]. Incubation medium was prepared by mixing 0.1 mol/L sodium hydrogen maleate buffer 6.5 mL (pH 6.0), 0.1 mol/L sodium citrate 0.5 mL, 30 mmol/L copper sulphate 1.0 mL, 5 mmol/L potassium ferricyanide 1.0 mL, water 1.0 mL and acetylthiocholine iodide 5 mg. The sections were incubated at 37°C for 20 min in the incubation medium. Thereafter, the incubation medium was drained off and re-incubation with rubeanic acid solution was performed for 10 min. Rubeanic acid solution was prepared by mixing absolute alcohol 10 mL, sodium acetate 6.55 g, rubeanic acid 10 mg and distilled water 40 mL. Thereafter, the sections were counterstained with haematoxylin, dehydrated, cleared and mounted in DPX.
Toluidine blue staining on paraffin sections was performed using a simple toluidine blue method that required incubation of sections in 1% aqueous solution of toluidine blue and mounting in a water based medium. Mast cells were counted in five successive high power fields (× 400) in the submucosa.
Immunohistochemical stain for S-100 (Dako Corporation) was performed on HD (12 cases) and non-HD (8 cases) by the standard immunoperoxidase technique. The nerve fibers that stained positive for S-100 were counted in five high power fields (× 400) in the submucosa.
Images of relevant areas in S-100 stained slides were captured using a digital camera. They were subsequently analyzed using the Scion image analysis software (http://www.scioncorp.com). The size and perimeter of the thickest nerve fiber in the submucosa was measured. The number, size and perimeter of nerve fibers were correlated with mast cell number in both the groups.
Statistical analysis was performed by the chi-square and student t-test and correlation assessed by Pearson’s correlation coefficient.
A total of 32 cases of chronic constipation in children below 14 years were studied. Based on the findings of HE and AChE stained sections of colonic and rectal biopsies, they were divided into two groups: GroupI- HD and Group II-non-HD. GroupI-HD included 18 cases and Group II-non-HD included 14 cases.
The age of the patients ranged from 1 mo to 84 mo in the HD group (mean ± 2 SD = 26.78 ± 36.72 mo). Maximum numbers of cases were found in the age group 36-47 mo in this group. In the non-HD group, age ranged from 6 d to 96 mo (mean ± 2 SD = 26.64 ± 49.60 mo), maximum cases in this group being in the age group of 0-11 mo. Male to female ratio was 17:1 in the HD group and 4:3 in the non-HD group.
There was statistically no significant difference in the age distribution between the two groups (P = 0.8). The difference in sex distribution between the two groups was statistically significant, the patient population in the HD group having a distinct male bias (P = 0.01).
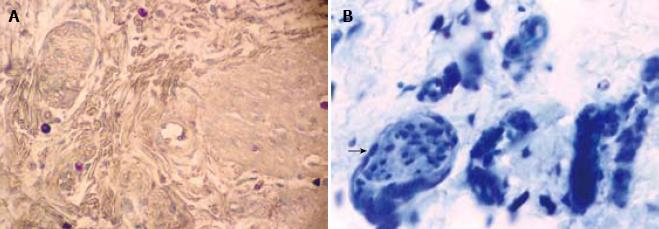
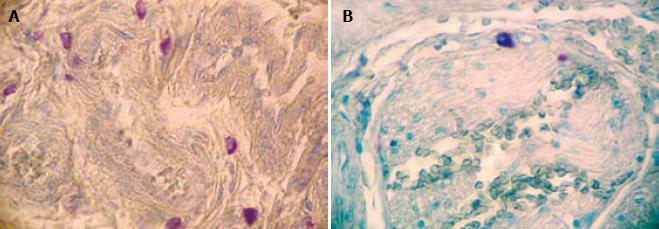
The data obtained after evaluation for mast cells are summarized in Table 1. There was a statistically significant difference in the number of MC between HD (mean ± 2 SD = 36.44 ± 36.02) and non-HD (mean ± 2 SD = 14.79 ± 19.8) (P = 0.0001). The mast cells were distributed transmurally (An observation in resection specimens of HD cases. Statistical analysis could not be done due to small sample size) and were notably present around the nerve fibers (Figure 1) and perivascularly (Figure 2). The number of mast cells in HD as well as in non-HD cases was significantly higher than previous studies (Table 2).
| HD cases | Non-HD cases | ||||||
| S. No. | Age (mo) | Sex | No. of mast cells | S. No. | Age (mo) | Sex | No. of mast cells |
| 1 | 36 | M | 35 | 1 | 30 | F | 1 |
| 2 | 36 | M | 40 | 2 | 24 | M | 15 |
| 3 | 12 | F | 35 | 3 | 36 | M | 25 |
| 4 | 24 | M | 35 | 4 | 36 | F | 5 |
| 5 | 3 | M | 10 | 5 | 4 | M | 10 |
| 6 | 36 | M | 30 | 6 | 48 | M | 3 |
| 7 | 5 | M | 50 | 7 | 1 | M | 1 |
| 8 | 5 | M | 20 | 8 | 1 | M | 25 |
| 9 | 36 | F | 70 | 9 | 96 | M | 15 |
| 10 | 24 | F | 20 | 10 | 1 | F | 32 |
| 11 | 84 | M | 45 | 11 | 2 | M | 15 |
| 12 | 36 | M | 45 | 12 | 30 | F | 25 |
| 13 | 84 | M | 80 | 13 | 36 | M | 15 |
| 14 | 18 | F | 30 | 14 | 48 | M | 20 |
| 15 | 36 | M | 45 | ||||
| 16 | 48 | M | 26 | ||||
| 17 | 48 | M | 13 | ||||
| 18 | 12 | F | 27 | ||||
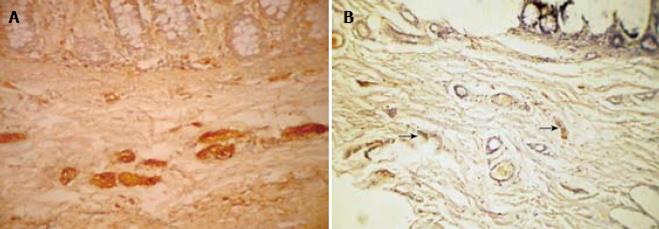
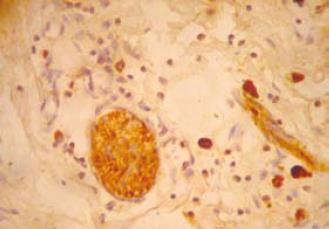
S-100 immunohistochemistry was performed on 20 cases consisting of 12 HD and 8 non-HD cases (Table 3). The number of nerve fibers in the submucosa (Figure 3) of HD (mean ± 2 SD = 11.17 ± 6.18) cases was higher than in non-HD (mean ± 2 SD = 5.13 ± 7.66) cases. The difference between the two groups was statistically significant (P = 0.0001). S-100 also stained the mast cells (Figure 4).
| HD cases | Non-HD cases | |||||||||
| S. No. | Age (mo) | Sex | No. of nerve fibers | Size of nerve fibers (&mgr;m) | Perimeter of nerve fibers (&mgr;m) | Age (mo) | Sex | No. of nerve fibers | Size of nerve fibers (&mgr;m) | Perimeter of nerve fibers (&mgr;m) |
| 1 | 42 | M | 13 | 40.37 | 230.48 | 36 | M | 3 | 42.5 | 215.7 |
| 2 | 12 | F | 8 | 68.0 | 195.9 | 1 | M | 2 | 11.2 | 37.0 |
| 3 | 36 | M | 11 | 65.4 | 172.8 | 5 | M | 4 | 15.3 | 47.3 |
| 4 | 84 | M | 7 | 47.3 | 135.4 | 1 | M | 4 | 15.0 | 51.8 |
| 5 | 48 | M | 16 | 63.5 | 288.75 | 1 | F | 3 | 40.6 | 135.56 |
| 6 | 36 | M | 10 | 77.38 | 237.79 | 30 | F | 3 | 25.0 | 126.67 |
| 7 | 48 | M | 12 | 57.5 | 256.11 | 4 | M | 9 | 28.8 | 83.2 |
| 8 | 36 | M | 14 | 40.7 | 149.3 | 13 | M | 13 | 69.9 | 208.09 |
| 9 | 12 | M | 6 | 55.5 | 190.03 | |||||
| 10 | 24 | M | 11 | 47.6 | 184.08 | |||||
| 11 | 1 | M | 15 | 49.6 | 178.18 | |||||
| 12 | 60 | M | 11 | 73.4 | 203.9 | |||||
Size of the nerve fibers in HD group was mean ± 2 SD = 57.18 ± 24.86 &mgr;m and in non- HD group it was mean ± 2 SD = 31.03 ± 39.08 &mgr;m. The difference between the two was statistically significant (P = 0.0001). The perimeter of nerve fiber in HD group was mean ± 2 SD = 201.89 ± 88.78 &mgr;m and in non-HD group was mean ± 2 SD = 113.26 ± 141.18 &mgr;m. This difference between the two groups was also statistically significant (P = 0.0001) (Table 2).
We correlated each of the parameters obtained by image analysis morphometry of the nerve fibers with MC number. In the HD group it was observed that maximum correlation was seen with number of nerve fibers (correlation coefficient = 0.467). No correlation was seen with size of nerve fibers (correlation coefficient = -0.131) and some correlation was observed with the perimeter of nerve fibers (correlation coefficient = 0.274). In the non-HD group the respective values were 0.406, 0.304 and 0.157.
Recently there has been a lot of interest in the role of mast cells in HD. Kobayashi et al[5] described an increased number of mast cells in the aganglionic segment of the colon in patients with HD. The number of these cells in transitional segments was significantly less compared with ganglionic segments in HD patients and controls. Similar findings were reported by Demirbilek et al[6].
In the present study, mast cells were evaluated in rectal biopsies as well as in resected specimens from cases of HD and non-HD. Although the mast cells can be roughly estimated after HE staining, an exact count is obtained by special histochemical methods. Other authors[5] have used immunohistochemical detection of anti-MC antibody to demonstrate MC. In our study, these were studied by a simple and effective toluidine blue staining which requires a time as short as one minute incubation. A significant increase was noticed in the number of mast cells in HD as compared with the non-HD cases. The HD cases showed a transmural distribution of these cells as described in the previous two studies by Kobayashi et al[5] and Demirbilek et al[6]. However, in a recent study Hermanowicz et al[7] found them to be increased in the mucosa and lamina propria but the increase in mast cells in the submucosa, muscularis propria and serosa was not statistically significantly changed.
The mast cells were characteristically distributed around the nerves and blood vessels in addition to being randomly scattered. In our study the baseline number of the mast cells (i.e. number of mast cells in non-HD cases) was much higher than previous studies[56] (Table 2) and few mast cells were also seen in the ganglionic segment. This may be due to the response of the mast cells to infectious agents or allergens rather than their association with aganglionosis. An increased number of mast cells is also reported in various other gastrointestinal disorders such as acute appendicitis, ulcerative colitis, celiac disease and gluten enteropathy[910]. They are seen to be in apposition to the nerves[11] and are known to secrete substances like nerve growth factor[12].
We also correlated each of the parameters obtained by image analysis morphometry of the nerve fibers with MC number. It was observed that maximum correlation was seen with number of nerve fibers (correlation coefficient = 0.467). No correlation was seen with size of nerve fibers and some correlation was observed with the perimeter of nerve fibers (correlation coefficient = 0.274). This correlation has not been shown in any previous study. This suggests that mast cells via their mediators may cause increased number of nerve fibers and affect size of nerve fibers to some extent. The interactive role of mast cells, their nerve growth factor secretion and neural hypertrophy as suggested by morphology is not explained by the studies to date. The exact role of these cells in the pathogenesis of HD needs further research with focus on the enteric nervous system, its development and the role of MC in cases where ganglion cells are absent.
The neural hypertrophy in the colonic submucosa is associated with aganglionosis and is a surrogate marker for the disease. The thickness of submucosal nerve fibers has been measured by various authors in previous studies[1314]. However, they have used less objective methods for measurement such as image graticules. In this study, we analyzed the status of the nerve fibers after S-100 immuno-staining and examination using an objective technique provided by image analysis software. The number, size and perimeter of the nerve fibers in the colonic submucosa of HD and non-HD cases were measured and results compared between HD and non-HD cases. The difference between the two groups was statistically significant for all the three parameters. Thus image analysis can be a useful adjunct to the available tools for the diagnosis of HD.
In conclusion, mast cells appear to have a significant role in the pathogenesis of Hirschsprung’s disease. Their increased baseline number in Indian children may be a response to additional factors like allergens or antigenic components of infectious agents. The role of anti-mast cell reagents like sodium cromoglycate needs to be explored with regard to constipation in Indian children. The fact that Indian children with HD present at a later age is also significant and emphasizes the need for a different approach to disease in various geographical regions.
Hirschsprung’s disease is an important cause of constipation in children. It is a serious disorder and can be fatal if left untreated. Recently, there has been a lot of interest in the role of mast cells in the pathogenesis of Hirschsprung’s disease. A few studies have found increased number of mast cells in aganglionic bowel segments of Hirschsprung’s disease cases. However the exact role of mast cells in Hirschsprung’s disease is not known.
Mast cells are normally present in small numbers in various organs of the body. They have been reported to be increased in a number of conditions other than Hirschsprung’s disease. Regarding the role of mast cells in Hirschsprung’s disease, the research hotspot is to elucidate the exact role played by them in the pathogenesis of Hirschsprung’s disease and the relationship between mast cells and the neural hypertrophy observed in Hirschsprung’s disease.
Previous studies on the role of mast cells in the pathogenesis of Hirschsprung’s disease have described an increased number of mast cells in the aganglionic segment in patients with Hirschsprung’s disease. Apart from one article, other studies have found mast cells to be increased transmurally. In the present study a significant increase was also noticed in mast cells in Hirschsprung’s disease. However their number was significantly higher than previous studies. This may be due to the response of the mast cells to infectious agents or allergens rather than their association with aganglionosis. The authors also performed image analysis morphometry and correlated each of the parameters obtained with mast cell number. It was observed that maximum correlation was seen with number of nerve fibers. No correlation was seen with size of nerve fibers and some correlation was observed with the perimeter of nerve fibers. This correlation has not been shown in any previous study. This suggests that mast cells via their mediators may cause increased number of nerve fibers and affect size of nerve fibers to some extent.
The study suggests mast cells appear to have a significant role in the pathogenesis of Hirschsprung’s disease. Whether this fact can be exploited to open novel therapeutic options in the management of this disease is something which needs to be looked at in the future.
Hirschsprung’s disease is a congenital condition characterized by dilatation and hypertrophy of colon due to absence (aganglionosis) or marked reduction (hypoganglionosis) of ganglion cells of the myenteric plexus of the rectum and a varying but continuous length of colon above the rectum. Mast cells are connective tissue cells with cytoplasmic coarse metachromatic granules and their normal function is unknown.
This article only contributes minimal new information to the literature (morphometry or S-100 stained nerve fibers), but it reinforces the observation that mast cells are increased in patients with HD and reports this finding in Indian children for the first time.
| 1. | Ghosh A, Griffiths DM. Rectal biopsy in the investigation of constipation. Arch Dis Child. 1998;79:266-268. |
| 2. | Agarwala S, Bhatnagar V, Mitra DK. Long-term follow-up of Hirschsprung’s disease: review of early and late complications. Indian Pediatr. 1996;33:382-386. |
| 3. | Bajpai M, Lall A. Surgical aspects of chronic constipation in children. Indian J Pediatr. 1999;66:S89-S93. |
| 4. | Vijaykumar , Chattopadhyay A, Patra R, Murulaiah M. Soave procedure for infants with Hirschsprung’s disease. Indian J Pediatr. 2002;69:571-572. |
| 5. | Kobayashi H, Yamataka A, Fujimoto T, Lane GJ, Miyano T. Mast cells and gut nerve development: implications for Hirschsprung’s disease and intestinal neuronal dysplasia. J Pediatr Surg. 1999;34:543-548. |
| 6. | Demirbilek S, Ozardali HI, Aydm G. Mast-cells distribution and colonic mucin composition in Hirschsprung’s disease and intestinal neuronal dysplasia. Pediatr Surg Int. 2001;17:136-139. |
| 7. | Hermanowicz A, Debek W, Dzienis-Koronkiewicz E, Chyczewski L. Topography and morphometry of intestinal mast cells in children with Hirschsprung's disease. Folia Histochem Cytobiol. 2008;46:65-68. |
| 8. | Goto S, Ikeda K, Toyohara T. An improved staining technique for acetylcholinesterase activity using rubeanic acid in the diagnosis of Hirschsprung's disease. Jpn J Surg. 1984;14:135-138. |
| 9. | Stead RH, Dixon MF, Bramwell NH, Riddell RH, Bienenstock J. Mast cells are closely apposed to nerves in the human gastrointestinal mucosa. Gastroenterology. 1989;97:575-585. |
| 10. | Krishnaswamy G, Kelley J, Johnson D, Youngberg G, Stone W, Huang SK, Bieber J, Chi DS. The human mast cell: functions in physiology and disease. Front Biosci. 2001;6:D1109-D1127. |
| 11. | Stenton GR, Vliagoftis H, Befus AD. Role of intestinal mast cells in modulating gastrointestinal pathophysiology. Ann Allergy Asthma Immunol. 1998;81:1-11; quiz 12-15. |
| 12. | Leon A, Buriani A, Dal Toso R, Fabris M, Romanello S, Aloe L, Levi-Montalcini R. Mast cells synthesize, store, and release nerve growth factor. Proc Natl Acad Sci USA. 1994;91:3739-3743. |
| 13. | Petchasuwan C, Pintong J. Immunohistochemistry for intestinal ganglion cells and nerve fibers: aid in the diagnosis of Hirschsprung's disease. J Med Assoc Thai. 2000;83:1402-1409. |
| 14. | Monforte-Munoz H, Gonzalez-Gomez I, Rowland JM, Landing BH. Increased submucosal nerve trunk caliber in aganglionosis: a "positive" and objective finding in suction biopsies and segmental resections in Hirschsprung's disease. Arch Pathol Lab Med. 1998;122:721-725. |