Published online Apr 28, 2009. doi: 10.3748/wjg.15.2041
Revised: March 17, 2009
Accepted: March 24, 2009
Published online: April 28, 2009
Chemical ablation of the gallbladder is effective in patients at high risk of complications after surgery. Percutaneous gallbladder drainage is an effective treatment for cholecystitis; however, when the drain tube cannot be removed because of recurrent symptoms, retaining it can cause problems. An 82-year-old woman presented with cholecystitis and cholangitis caused by biliary stent occlusion and suspected tumor invasion of the cystic duct. We present successful chemical ablation of the gallbladder using pure alcohol, through a percutaneous gallbladder drainage tube, in a patient who developed intractable cholecystitis with obstruction of the cystic duct after receiving a biliary stent. Our results suggest that chemical ablation therapy is an effective alternative to surgical therapy for intractable cholecystitis.
- Citation: Lee TH, Park SH, Kim SP, Park JY, Lee CK, Chung IK, Kim HS, Kim SJ. Chemical ablation of the gallbladder using alcohol in cholecystitis after palliative biliary stenting. World J Gastroenterol 2009; 15(16): 2041-2043
- URL: https://www.wjgnet.com/1007-9327/full/v15/i16/2041.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.2041
Tumor obstruction of the cystic duct is a known risk factor for the development of cholecystitis following biliary stent placement. Percutaneous cholecystostomy is an effective treatment for such cholecystitis[12]. However, recurrent cholecystitis or retractable symptoms may be troublesome. Recently, chemical ablation of the gallbladder has been shown to be effective in patients at high risk for complications after surgery[3]. Absolute alcohol or 95% ethanol causes necrosis and fibrosis in the gallbladder epithelium, which reduces the gallbladder to a shrunken fibrous remnant[4].
However, until now there have been few human studies of which sclerosants are safe and feasible, and for how long the sclerosant has to be in contact with the mucosa.
In this report, we describe the successful chemical ablation of the gallbladder in a patient who developed intractable cholecystitis with obstruction of the cystic duct, after undergoing palliative stenting for the management of a malignant biliary obstruction.
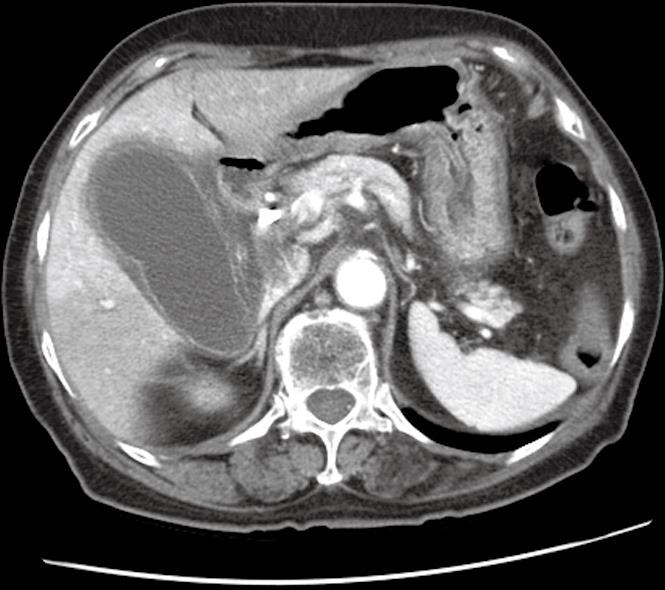
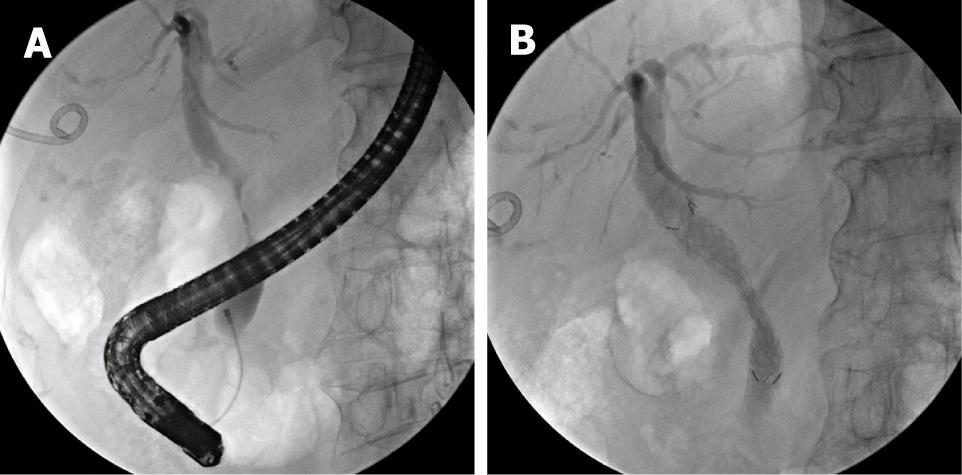
An 82-year-old woman presented with gradually aggravated right upper-abdominal pain after undergoing biliary stent implantation for the palliative management of a cholangiocarcinoma 2 wk previously. Upon presentation, clinical examination revealed severe tenderness of the right upper abdomen without rebound tenderness. Laboratory tests revealed the following: white blood cell count, 14.380 × 109/L (normal 4.0-10.8 × 109/L); total bilirubin, 5.4 g/dL (normal 0.1-1.0 g/dL); aspartate aminotransferase, 118 IU/L (normal < 40 IU/L); alanine aminotransferase, 118 IU/L (normal < 40 IU/L); alkaline phosphatase, 118 IU/L (normal 39-117 IU/L); and carbohydrate antigen 19-9, 98 U/mL (normal < 34 U/mL). Abdominal computed tomography (CT) revealed a markedly enlarged and distended gallbladder with a thickened wall (Figure 1). Clinically, both cholecystitis and cholangitis were suspected, based on CT and laboratory data. Following decompression of the gallbladder via percutaneous cholecystostomy, endoscopy using a duodenoscope (TJF 240; Olympus, Tokyo, Japan) was performed, which showed a completely occluded plastic biliary stent. The occluded stent was subsequently removed. Cholangiography showed a severe irregular segmental stricture at the mid common bile duct (CBD), without visualization of the cystic duct, a finding that indicated cystic duct occlusion caused by tumor invasion (Figure 2A).
For the management of cholecystitis and malignant stricture, the percutaneous drainage tube was left in place and a covered metal biliary stent (Niti-S; Taewoong Medical Co., Ltd., Seoul, Korea), 60 mm in length, was implanted through the peroral route (Figure 2B). Seven days later, the percutaneous cholecystostomy was draining less than 50 mL/d; therefore, removal of the drain tube was attempted. However, the patient complained of recurrent abdominal pain and discomfort whenever the drain tube was closed, and the amount of fluid draining continued at a rate of > 40 mL/d. Consequently, removal of the drain tube failed. As a result of the patient’s advanced age and her refusal of palliative cholecystectomy, medical ablation of the gallbladder was considered to be a good option for treating the symptoms and to allow the removal of the percutaneous drain tube.
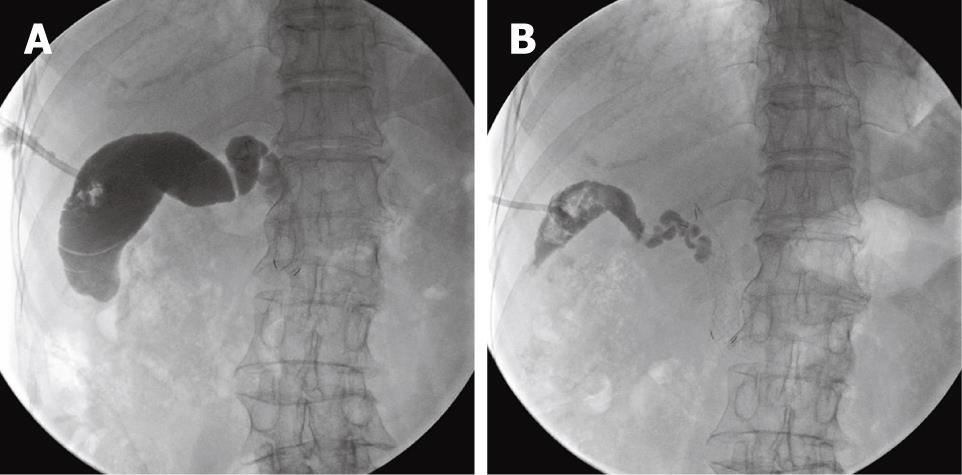
After informed consent from the patient and approval by the ethics committee of our hospital, 99% absolute ethanol was used as a sclerosant for chemical ablation of the gallbladder. The volume of the gallbladder was measured by filling it with contrast medium, followed by aspiration (Figure 3A). Absolute ethanol, 1-2 mL less than the volume of the gallbladder, was infused into the gallbladder through the drain tube. Initially, a total of 55 mL of ethanol was infused and the drainage tube was closed. Then, the patient changed positions every 10 min for a total of 30 min, and the sclerosant was drained. Cholecystography was performed 1 wk after the first chemical ablation, and it showed a decrease in the size of the gallbladder. The same method was repeated twice more in weekly sessions with 40 and 25 mL of ethanol, respectively. During the final week, a small amount of bright yellow fluid (< 10 mL/d) was draining, and cholecystography showed a marked collapse in the lumen of the gallbladder (Figure 3B). During each procedure, the patient’s vital signs were closely monitored. The patient had no abdominal pain or other complications related to the procedure. The cholecystostomy drain tube was removed, and there were no complications in the following 8 wk, during which she remained under outpatient observation.
Endoscopic insertion of biliary stents is a well-established palliative treatment for obstructive jaundice caused by unresectable malignant disease[5–7]. As a result of increased use, complications such as cholangitis, cholecystitis, pancreatitis, stent migration, and stent occlusion are being reported increasingly[8]. In particular, cholecystitis has been reported in 1.9%-12% of stent insertion cases[910]. For several reasons, obstruction of the cystic duct by a tumor is a risk factor for the development of cholecystitis following biliary stent placement[2].
Although cholecystectomy is a safe and effective treatment in patients with cholecystitis, the morbidity and mortality of this operation increases considerably in the elderly and unfit patients who often have concomitant diseases. Percutaneous gallbladder drainage or aspiration, transpapillary gallbladder drainage, and endoscopic-ultrasound-guided gallbladder drainage have been reported for the management of cholecystitis after stent placement or for cystic duct invasion by a tumor[1211]. However, in cases such as those reported here, when the drain tube cannot be removed because of recurrent symptoms, retaining it causes problems for the patient, and its experimental removal may cause other complications.
Chemical ablation of the gallbladder may be a useful alternative to cholecystectomy in high-risk patients or in those who refuse surgery. Recently, experimental studies on chemical ablation of the gallbladder in vitro and in vivo have demonstrated that many sclerosants, including 95% ethanol, 3% sodium tetradecyl sulfate, 5% tetracycline, and 5% trifluoroacetic acid, ablate gallbladder mucosa[312–14]. Oh et al[15] used 99.9% ethanol for the chemical ablation of cystic tumors of the pancreas. Xu et al[3] reported that minicholecystostomy followed by chemical ablation of the gallbladder was safe and effective. In that study, 95% ethanol was in contact with the gallbladder mucosa for 30 min every 4 h, for a total of eight times after occlusion of the cystic duct. A suitable chemical for gallbladder mucosal ablation must be safe, effective, and require brief contact time with the mucosa. However, there have been few human studies to determine which sclerosants are feasible and the duration for which the sclerosant must be in contact with the mucosa. Some studies have reported complications, including mucocele, gallbladder hydrops, abscess formation, and perforation; however, there have been no serious, life-threatening complications[3412–14].
In our case, we used absolute ethanol as a chemical sclerosant. In animal and human studies, alcohol has been found to be safe and has resulted in few complications; however, it requires more treatments of longer duration than other sclerosants. More studies are needed to determine which sclerosants are suitable, how often they need to be applied and at what interval, and the contact duration required for chemical ablation. Absolute ethanol is a safe sclerosant and procedure-related complications did not develop during the procedure or follow-up period. Especially in the case of cystic duct obstruction by tumors, this method is easy and feasible, and is not affected by the presence of additional biliary stents. However, if the cystic duct is patent, the use of chemical agents is restricted and another approach is necessary.
In summary, following development of cholecystitis after stent placement, with tumor obstruction of the cystic duct, or in patients with recurring symptoms, chemical ablation using absolute ethanol may be an alternative to percutaneous cholecystostomy or surgical cholecystectomy. Further investigation of this technique and the identification of suitable sclerosants are necessary, and long-term follow-up should be conducted.
| 1. | Dolan R, Pinkas H, Brady PG. Acute cholecystitis after palliative stenting for malignant obstruction of the biliary tree. Gastrointest Endosc. 1993;39:447-449. |
| 2. | Suk KT, Kim HS, Kim JW, Baik SK, Kwon SO, Kim HG, Lee DH, Yoo BM, Kim JH, Moon YS. Risk factors for cholecystitis after metal stent placement in malignant biliary obstruction. Gastrointest Endosc. 2006;64:522-529. |
| 3. | Xu Z, Wang L, Zhang N, Ling X, Hou C, Zhou X. Chemical ablation of the gallbladder: clinical application and long-term observations. Surg Endosc. 2005;19:693-696. |
| 4. | Uchiyama N, Stridbeck H, Stenram U. Chemical sclerosis of the gallbladder. An experimental study in pigs of the effect of absolute ethanol and polidocanol on gallbladder epithelium. Acta Radiol. 1989;30:427-431. |
| 5. | Cotton PB. Endoscopic methods for relief of malignant obstructive jaundice. World J Surg. 1984;8:854-861. |
| 6. | Kaassis M, Boyer J, Dumas R, Ponchon T, Coumaros D, Delcenserie R, Canard JM, Fritsch J, Rey JF, Burtin P. Plastic or metal stents for malignant stricture of the common bile duct? Results of a randomized prospective study. Gastrointest Endosc. 2003;57:178-182. |
| 7. | O'Brien S, Hatfield AR, Craig PI, Williams SP. A three year follow up of self expanding metal stents in the endoscopic palliation of longterm survivors with malignant biliary obstruction. Gut. 1995;36:618-621. |
| 8. | Kahaleh M, Tokar J, Conaway MR, Brock A, Le T, Adams RB, Yeaton P. Efficacy and complications of covered Wallstents in malignant distal biliary obstruction. Gastrointest Endosc. 2005;61:528-533. |
| 9. | Bezzi M, Zolovkins A, Cantisani V, Salvatori FM, Rossi M, Fanelli F, Rossi P. New ePTFE/FEP-covered stent in the palliative treatment of malignant biliary obstruction. J Vasc Interv Radiol. 2002;13:581-589. |
| 10. | Schöfl R, Brownstone E, Reichel W, Fortunat W, Doblhofer F, Samec HJ, Brandstätter G, Stupnicki T, Pamperl H, Schreiber P. Malignant bile-duct obstruction: experience with self-expanding metal endoprostheses (Wallstents) in Austria. Endoscopy. 1994;26:592-596. |
| 11. | Lee SS, Park do H, Hwang CY, Ahn CS, Lee TY, Seo DW, Lee SK, Kim MW. EUS-guided transmural cholecystostomy as rescue management for acute cholecystitis in elderly or high-risk patients: a prospective feasibility study. Gastrointest Endosc. 2007;66:1008-1012. |
| 12. | Majeed AW, Reed MW, Stephenson TJ, Johnson AG. Chemical ablation of the gallbladder. Br J Surg. 1997;84:638-641. |
| 13. | Soulen MC, Sokol MC, Sullivan KL. Chemical ablation of the gallbladder: evaluation of multiple agents in vitro. J Vasc Interv Radiol. 1994;5:765-769. |
| 14. | Soulen MC, Sullivan KL. Chemical ablation of the gallbladder: is it feasible? J Vasc Interv Radiol. 1995;6:553-558. |
| 15. | Oh HC, Seo DW, Lee TY, Kim JY, Lee SS, Lee SK, Kim MH. New treatment for cystic tumors of the pancreas: EUS-guided ethanol lavage with paclitaxel injection. Gastrointest Endosc. 2008;67:636-642. |