INTRODUCTION
Liver cancer is one of the most common malignancies worldwide, especially in Asia and Africa[1]. Hepatocellular carcinoma accounts for about 80%-90% of all liver cancer and is the fourth most common cause of cancer mortality[2]. Major risk factors for liver cancer include hepatitis viral infection, food additives, alcohol, aflatoxins, environmental and industrial toxic chemicals, and air and water pollutants[34]. Diethylnitrosamine (DENA) is a well known potent hepatocarcinogenic agent present in tobacco smoke, water, cured and fried meals, cheddar cheese, agricultural chemicals, cosmetics and pharmaceutical products[5–7]. DENA is known to induce damage in many enzymes involved in DNA repair and is normally used to induce liver cancer in experimental animal models[8]. Although there are many strategies for the treatment of liver cancer[9–11], the therapeutic outcome of this cancer remains very poor. Therefore, prevention seems to be the best strategy for lowering the incidence of this disease. Recently, considerable research has been carried out in the search for natural or synthetic compounds as a means of chemically preventing liver cancer[12–18]. In this regard, many compounds have been tested with proved efficacy against experimentally-induced hepatocarcinogenesis. These compounds include morin[12], silymarin[13], garlic[14], star anise[15], ganfujian granule[1], apigenin[16] and the crude extracts of agarcus blazei[17].
L-carnitine is a naturally occurring compound that is primarily located in mitochondria and possesses potential protective effects against many mitochondrial toxic agents[18–20]. It is derived from two sources: endogenous synthesis, in the liver and kidney, and from exogenous dietary sources such as red meat and dairy products[19–21]. L-carnitine is an essential cofactor for the translocation of long-chain fatty acids from the cytoplasmic compartment into mitochondria, where beta-oxidation enzymes are located for ATP production[22]. It has been reported that mitochondrial injury may exert a major influence on carcinogenesis[23]. Consistent with this hypothesis, Chang et al[24] have reported that mitochondrial dysfunction plays an essential role in hepatocarcinogenesis via production of reactive oxygen species (ROS), and that L-carnitine inhibits pre-neoplastic lesions and prevents hepatocarcinogenesis in Long Evans Cinnamon rats as a model of hepatocarcinogenesis. Furthermore, earlier studies have reported that carnitine effectively inhibits mitochondrial injury induced by ROS and mitochondria-dependent apoptosis[25–27]. In addition, L-carnitine prevents the accumulation of free fatty acids and their toxic intermediates, thus preventing their harmful effects on mitochondrial and cell membranes[202628]. Despite the liver being the main organ responsible for endogenous synthesis of L-carnitine, we were unable to find any previously published studies investigating the role of long-term endogenous carnitine depletion and/or carnitine deficiency during induction of hepatic carcinogenesis. Therefore, this study was initiated with the following specific aims: (1) to determine whether or not endogenous carnitine depletion and/or carnitine deficiency is a risk factor during the development of hepatic carcinogenesis and; (2) to gain insights into the possibility of mechanism-based protection by L-carnitine supplementation against DENA-induced hepatocarcinogenesis.
MATERIALS AND METHODS
Animals
Adult male Wistar albino rats, weighing 180-200 g, were obtained from the Experimental Animal Care Center, College of Pharmacy, King Saud University (KSU), Riyadh, Kingdom of Saudi Arabia (KSA) and were housed in metabolic cages under controlled environmental conditions (25°C and a 12 h light/dark cycle). Animals had free access to pulverized standard rat pellet food and tap water unless otherwise indicated. The protocol of this study was approved by the Research Ethics Committee of College of Pharmacy, KSU, Riyadh, KSA.
Materials
DENA and carbon tetrachloride (CCl4) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). DENA was dissolved in saline and injected in a single dose (200 mg/kg, i.p.) to initiate hepatic carcinogenesis, while CCl4 was used in a single dose (2 mL/kg) by gavage as 1:1 dilution in corn oil to stimulate liver cell proliferation and regeneration according to previously published protocols[2930]. L-carnitine, D-carnitine and mildronate (trimethylhydrazinium propionate) were kindly supplied by Dr. Menotti Calvani, (Sigma-Tau Pharmaceuticals, Pomezia, Roma, Italy). All other chemicals used were of the highest analytical grade.
Carnitine-depleted rat model
Animal models of carnitine deficiency have been developed by many investigators[31–33]. In the current study, carnitine deficiency was induced in rats by administration of D-carnitine (200 mg/kg per day) and mildronate (200 mg/kg per day) in drinking water. Depletion of L-carnitine by D-carnitine occurs via an exchange of the D- and L-isomers across the cell membrane where intracellular L-carnitine has been shown to exchange with extracellular D-carnitine[31]. Moreover, mildronate inhibits carnitine biosynthesis by inhibition of the gamma-butyrobetaine hydroxylase enzyme[3233].
Experimental design
To achieve the ultimate goal of this study, a total of 60 adult male Wistar albino rats were divided into six groups with 10 animals in each group. Rats in group 1 (control group) received a single intraperitoneal (ip) injection of normal saline (2.5 mL/kg). Animals in group 2 (carnitine-supplemented group) were given L-carnitine (200 mg/kg per day) in drinking water for 8 wk. Animals in group 3 (carnitine-depleted group) were given D-carnitine (200 mg/kg per day) and mildronate (200 mg/kg per day) in drinking water for 8 wk. Two weeks after the experiment began, the animals in groups 1, 2 and 3 received a single dose of corn oil (0.5 mL/150 g body weight) by gavage. Rats in group 4 (DENA group) were injected with a single dose of DENA (200 mg/kg, i.p.) and 2 wk later received a single dose of CCl4 (2 mL/kg) by gavage as 1:1 dilution in corn oil. Animals in group 5 (DENA-carnitine depleted group) received the same dose of DENA as group 4 and the same dose of D-carnitine-mildronate as group 3. Animals in group 6 (DENA-carnitine supplemented group) received the same dose of DENA as group 4 and the same dose of L-carnitine as group 2. At the end of the treatment protocol, animals were anesthetized with ether and blood samples were drawn from the orbital venous plexus. Serum was separated by centrifugation for 5 min at 1500 g and stored at -20°C until analysis. This was then used to determine ALT, G-GT and ALP activities and total bilirubin. All animals were sacrificed by decapitation and their livers were rapidly excised, weighed, washed with saline, blotted with a piece of filter paper and homogenized in normal saline or 6% perchloric acid, as indicated in the procedures of measurement of each parameter, to yield a 10% (w/v) tissue homogenate, using a Branson sonifier (250 VWR Scientific, Danbury, Conn., USA). Liver specimens from each group were removed for histopathological examination. The specimens were fixed in 10% neutral buffered formalin, sectioned at 3 &mgr;m and stained with hematoxylin and eosin (H & E) stain for light microscopic examination. To avoid any type of bias, the slides were coded and examined by a histopathologist who was blinded to the treatment groups.
Measurements of liver function
The activities of ALT, G-GT and ALP as well as total bilirubin in serum were determined using Randox commercially available kits (Randox Laboratories Ltd., Diamond Road, Crumlin, Co. Antrim, UK).
Determination of reduced glutathione and lipid peroxidation in liver tissues
The tissue levels of the acid soluble thiols, mainly GSH, were assayed spectrophotometrically at 412 nm, according to the method of Ellman[34] using a Shimadzu (Tokyo, Japan) spectrophotometer. The content of GSH was expressed as &mgr;mol/g wet tissue. The degree of lipid peroxidation in liver tissues was determined by measuring thiobarbituric acid reactive substances (TBARS) in the supernatant tissue from the homogenate[35]. The homogenates were centrifuged at 1500 g and the supernatant was collected and used for the estimation of TBARS. The absorbance was measured spectrophotometrically at 532 nm and the concentrations were expressed as nmol TBARS/g wet tissue.
Determination of glutathione peroxidase and catalase activity in liver tissues
The activity of glutathione peroxidase (GSHPx) was determined according to the method of Lawrence and Burk[36]. The changes in the absorbance at 340 nm were recorded at 1 min interval for 5 min and the results were expressed as &mgr;mol/min per g tissue. The catalase (CAT) activity was determined spectrophotometrically by the method of Higgins et al[37] which is the assay of hydrogen peroxide (H2O2). The activity was expressed as &mgr;mol/min per g tissue using the molar absorbance of 43.6 for H2O2.
Determination of total nitrate/nitrite concentrations in liver tissues
Total nitrate/nitrite (NOx), an index of nitric oxide (NO) production, was measured as the stable end product, nitrite, according to the method of Miranda et al[38]. The assay is based on the reduction of nitrate by vanadium trichloride combined with detection by the acidic Griess reaction. The diazotization of sulfanilic acid with nitrite at acidic pH and subsequent coupling with N-(10 naphthyl)-ethylenediamine produced an intensely colored product which was measured spectrophotometrically at 540 nm. The levels of NOx were expressed as &mgr;mol/g wet tissue.
Determination of total carnitine levels in liver tissues
Liver homogenate was prepared in ice-cold 6% perchloric acid and centrifuged at 8000 g for 10 min. Part of the supernatant was used for the estimation of free carnitine, while the remainder was used for the determination of long-chain acyl carnitine after hydrolysis in 1 mol/L KOH at 65°C for 1 h according to Alhomida[39]. Carnitine was determined using HPLC after pre-column derivatization with L-aminoanthracene as previously described by Longo et al[40]. The mobile phase was prepared by mixing 700 mL of 0.1 mol/L ammonium acetate, pH 3.5, with 300 mL of acetonitrile. Chromatographic separation was performed at a flow rate of 1.5 mL/min, using a Kromasil column (C18, 25 cm × 4.6 mm) from Saulentechnik Knayer, Berlin, Germany. The excitation and emission wavelengths of the spectrofluorimeter were 248 and 418 nm, respectively.
Statistical analysis
Differences between obtained values (mean ± SE, n = 10) were carried out by one way analysis of variance (ANOVA) followed by the Tukey-Kramer multiple comparison test. A P value of 0.05 or less was considered statistically significant.
RESULTS
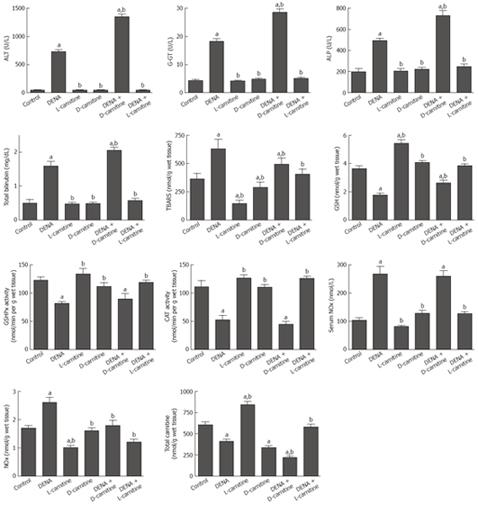
Eight weeks after the experiment began, the administration of DENA resulted in a significant 16.6-fold, 316%, 152%, and 219% increase in serum ALT, G-GT, ALP and bilirubin, respectively, as compared to the control group. Long-term administration of either L-carnitine or D-carnitine alone for 8 wk showed a non-significant change. In carnitine-depleted rats, DENA resulted in a significant 85%, 56%, 49% and 29% increase in serum ALT, G-GT, ALP and bilirubin, respectively, as compared to DENA alone. Interestingly, administration of L-carnitine in combination with DENA resulted in a complete reversal of DENA-induced increases in serum ALT, G-GT, ALP and bilirubin, compared to the control values (Figure 1).
Figure 1 Effect of DENA on serum liver function indices, oxidative stress biomarkers, activities of antioxidant enzymes, NOx concentration and total carnitine levels in liver tissues from carnitine supplemented and depleted rats.
Data are presented as mean ± SE (n = 10). aP < 0.05 vs control; bP < 0.05 vs DENA, at P < 0.05 using ANOVA followed by Tukey-Kramer as a post ANOVA test.
The effects of DENA on the oxidative stress biomarkers, TBARS and GSH, in liver tissues from carnitine-supplemented and -depleted rats are shown in Figure 1. DENA resulted in a significant 73% increase in TBARS and a significant 52% decrease in GSH, as compared to the control group. Long-term administration of either L-carnitine or D-carnitine alone for 8 wk resulted in a significant decrease in TBARS and a significant increase in GSH as compared to either the control or the DENA groups. Moreover, L-carnitine resulted in a complete reversal of the DENA-induced decrease in GSH and increase in TBARS in liver tissues, compared to the control values.
Administration of DENA resulted in a significant 34% and 52% decrease in GSHPx and CAT, respectively, as compared to the control group. Long-term administration of either L-carnitine or D-carnitine alone for 8 wk showed a non-significant change, as compared to the control group. In carnitine-supplemented rats, L-carnitine resulted in a complete reversal of the DENA-induced decrease in GSHPx and CAT in liver tissues compared to the control values (Figure 1).
Administration of DENA resulted in a significant 157% and 54% increase in the levels of NOx in serum and liver tissues, respectively, as compared to the control group. Long-term administration of either L-carnitine or D-carnitine-mildronate alone for 8 wk showed a non-significant change. In carnitine-supplemented rats, L-carnitine resulted in a complete reversal of the DENA-induced increase in NOx levels in serum and liver tissues compared to the control values (Figure 1).
Treatment with DENA resulted in a significant 32% decrease in total carnitine level in liver tissues, whereas long-term administration of D-carnitine-mildronate resulted in a significant 44% decrease in total carnitine level in liver tissues, as compared to the control group. Administration of L-carnitine resulted in a significant 40% increase in total carnitine level in liver tissues as compared to the control group. Administration of DENA to D-carnitine-mildronate-treated rats resulted in a significant 64%, 47% and 36% decrease in total carnitine content in liver tissues as compared to the control, DENA and D-carnitine-mildronate treatments, respectively. In carnitine-supplemented rats, L-carnitine resulted in a complete reversal of the DENA-induced decrease in total carnitine content in liver tissues compared to the control values (Figure 1).
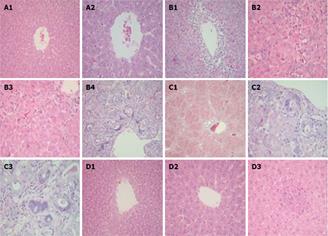
Liver sections from control rats showed normal liver histology with unremarkable central veins (Figure 2A). Animals treated with DENA showed central veins surrounded by extensive necrosis and inflammatory infiltrate, clusters of hepatocyte necrosis and the portal tract with bile duct proliferation and marked atypia (Figure 2B). In carnitine-depleted rats, DENA-induced progressive histopathological and pre-neoplastic lesions that manifested as diffuse bridging fibrosis connecting central veins to portal tracts and nodule formation, bile ducts with marked reactive atypia showing nuclear enlargement, high nuclear/cytoplasmic ratio and prominent nucleoli (Figure 2C). Liver sections from rats treated with DENA and L-carnitine showed normal liver histology with unremarkable central veins, hepatic lobule with few scattered foci of inflammation, no evidence of necrosis, cirrhosis and no reactive atypia (Figure 2D).
Figure 2 Photomicrographs of liver specimens stained with H&E.
A: Liver from control rat showing normal liver histology with unremarkable central vein (A1, × 20 and A2, × 40); B: Liver from rat treated with DENA showing central vein surrounded by extensive necrosis and inflammatory infiltrate (B1, × 20), considerable hepatocyte necrosis represented with arrows (B2 and B3, × 40) and portal tract with bile duct proliferation and marked atypia (B4, × 20); C: Liver from rat treated with DENA plus D-carnitine-mildronate showing diffuse bridging fibrosis and nodule formation (C1, × 10) and bile ducts with marked reactive atypia showing nuclear enlargement, high nuclear/cytoplasmic ratio and prominent nucleoli (C2 and C3, × 40); D: Liver from rat treated with DENA and L-carnitine showing normal liver (D1, × 20) with unremarkable central vein (D2, × 40) and hepatic lobule with a focus of inflammatory infiltrate but no necrosis (D3, × 40).
DISCUSSION
Chemoprevention is defined as the use of naturally occurring and/or synthetic compounds in cancer therapy in which the occurrence of cancer can be entirely prevented, slowed or reversed[13]. Using Long Evans Cinnamon rats as a model of hepatocarcinogenesis, Chang et al was the first to report that L-carnitine inhibited pre-neoplastic lesions and prevented hepatocarcinogenesis[24]. In our laboratory, earlier and more recent studies have demonstrated that carnitine deficiency is a risk factor and should be viewed as a mechanism in cisplatin-induced nephrotoxicity[4142], cardiomyopathy[43] and hepatotoxicity[44]. Although, the liver is the main organ responsible for endogenous synthesis of L-carnitine, we were unable find any studies investigating the role of carnitine deficiency during DENA-induced hepatic carcinogenesis. Taken together, this prompted us to study whether or not long-term endogenous carnitine depletion is a risk factor during DENA-induced hepatic carcinogenesis.
The data presented here demonstrated that DENA increased serum indices of liver function including ALT, G-GT, ALP and total bilirubin (Figure 1) and caused severe histopathological lesions in liver tissues (Figure 2B). It is well known that the elevation of ALT and G-GT activities is repeatedly credited to hepatocellular damage[45]. Also, the increase in ALP reflects a pathological alteration in biliary flow[46]. G-GT is an enzyme embedded in the hepatocyte plasma membrane, mainly in the canalicular domain, and its liberation into serum indicates damage to the cell and thus injury to the liver[1246]. It is important to point out that serum G-GT activity is considered to be one of the best indicators of liver damage[46]. In the current study, this observed increase in serum indices of liver function due to DENA could be a secondary event following DENA-induced lipid peroxidation of hepatocyte membranes, with a consequent increase in the leakage of ALT, G-GT, ALP and total bilirubin from liver tissues. An elevated level of serum indices of hepatocellular damage has been previously reported in many models of DENA-induced hepatocellular degeneration[12174748]. Interestingly, long-term administration of L-carnitine prevented the increase in hepatic enzymes, suggesting that L-carnitine may have a potential protective effect against DENA-induced liver damage. This effect could be due to stabilization of hepatocyte membranes by L-carnitine with the consequent decrease in the leakage of liver enzymes. Indeed, the interaction of L-carnitine with sarcolemmal phospholipids and mitochondrial membranes has been previously reported[49]. In carnitine-depleted rats, DENA produced a progressive increase in the activities of liver enzymes as well as massive degenerative changes and evidence of pre-neoplastic lesions in liver tissues (Figure 2C). Our results are consistent with the data presented by Hytiroglou et al[50], which showed that precancerous lesions which may be detected in chronically diseased livers included clusters of hepatocytes with atypia and an increased proliferative rate.
Data from this study revealed that DENA significantly increased NOx and TBARS and decreased GSH, GSHPx and CAT in liver tissues, suggesting that reactive oxygen and nitrogen species induced by DENA play an important role in DENA-induced hepatic carcinogenesis. Increased generation of ROS and decreased antioxidant enzymes in liver tissues has been reported in many models of DENA-induced hepatocellular carcinoma[12–15]. It has been reported that ROS play a major role in tumor promotion through interaction with critical macromolecules including lipids, DNA, DNA repair systems, and other enzymes[51]. Moreover, NOx is known to inhibit DNA repair proteins, thereby inhibiting the ability of the cell to repair damaged DNA[5253]. Data presented here demonstrated that long-term administration of both L-carnitine and D-carnitine completely reversed the increase in TBARS and NOx and the decrease in GSH, CAT and GSHPx induced by DENA in liver tissues. Our results are consistent with previous studies that have reported that the D- and L-forms of carnitine and its short-chain derivatives have similar non-enzymatic free-radical scavenging activity[415455]. Although, L-carnitine and D-carnitine have similar anti-lipid peroxidation activity, our study indicated that L-carnitine prevented the progression of DENA-induced hepatic carcinogenesis, while D-carnitine-Mildronate aggravated these lesions. Therefore, it is suggested that oxidative stress is not the only cause of DENA-induced hepatic carcinogenesis and that carnitine deficiency plays an important role.
The data presented here showed that DENA significantly decreased total carnitine in liver tissues. This effect could be a secondary event following inhibition of endogenous carnitine biosynthesis and/or decreased carnitine transport in DENA-induced hepatocellular damage. This hypothesis is consistent with data presented by Krahenbuhl et al[56] which showed that biosynthesis of carnitine is decreased in rats with liver cirrhosis. It seems that our results are unique since there is no available data on the effect of DENA on hepatic carnitine content. The level of carnitine in hepatocytes is controlled by the specific carnitine transporter (OCTN-2) and endogenous synthesis[1819]. Decreased expression of OCTN-2 has been reported in the acute hepatitis phase in Long Evans Cinnamon rats as a model of hepatocarcinogenesis[24]. Also, OCTN-2 located on hepatocyte membranes might be destroyed when exposed to ROS induced by DENA.
Although, D-carnitine-mildronate alone decreased hepatic carnitine content more than DENA, only DENA increased ALT, G-GT, ALP and total bilirubin. This argues against carnitine deficiency as a risk factor in DENA-induced hepatic carcinogenesis. A possible explanation for this is that D-carnitine, via its non-enzymatic antioxidant activity, causes stabilization of hepatic membranes and prevents the leakage of liver enzymes, whereas DENA, via the increasing generation of ROS, causes irreversible modification of membrane structures and functions with the consequent increase in liver enzymes. This marked decrease (64%) of carnitine level in liver tissue after combined treatment with DENA and D-carnitine-mildronate was parallel to the marked increase in ALT, G-GT, ALP and the massive histopathological lesions in liver tissues, which may point to carnitine deficiency as a possible risk factor during DENA-induced hepatic carcinogenesis. Most probably, D-carnitine-mildronate via its depletion of L-carnitine, and DENA partly through generation of ROS and partly due to carnitine depletion produced such aggravated hepatocellular damage.
In this study, the chemopreventive effects achieved by long-term administration of L-carnitine against DENA-induced hepatic carcinogenesis is in good agreement with the data presented by Chang et al[24] which showed that L-carnitine inhibits pre-neoplastic lesions and prevents hepatocarcinogenesis in Long Evans Cinnamon rats as a model of hepatocarcinogenesis. Administration of L-carnitine will facilitate beta-oxidation, thereby minimizing the toxic effects of the free form of long-chain fatty acids and their intermediates in mitochondria. Consistent with this hypothesis is a report indicating that administration of L-carnitine decreased free fatty acids in serum and tissues, and prevented tissue injury in mice with juvenile visceral steatosis that lacked a carnitine transporter[5758]. In conclusion, data from this study suggest for the first time that: (1) carnitine deficiency is a risk factor and should be viewed as a mechanism in DENA-induced hepatic carcinogenesis; (2) oxidative stress plays an important role but is not the main cause of DENA-related hepatic carcinogenesis; and (3) long-term L-carnitine supplementation prevents the development of DENA-induced liver cancer. Therefore, carnitine supplementation alone or in combination with other natural chemopreventive compounds could be used to prevent, slow or reverse the occurrence of liver cancer.
COMMENTS
Background
Liver cancer is one of the most common malignancies worldwide, especially in Asia and Africa. In the last few years, considerable research has been carried out in the search for natural materials or foods as a means of chemically preventing liver cancer. L-carnitine is a naturally occurring compound which is primarily located in mitochondria and possesses potential protective effects against many mitochondrial toxic agents. It is derived from two sources: endogenous synthesis in the liver and from exogenous dietary sources such as red meat and dairy products. Despite the liver being the main organ responsible for endogenous synthesis of L-carnitine, the role of endogenous depletion and/or supplementation of L-carnitine during induction of hepatocarcinogenesis has not yet been studied.
Research frontiers
Absolute or relative carnitine deficits develop in chronic congestive heart failure, acute myocardial ischemia, diseases of peripheral blood vessels, and disturbances of lipid metabolism. However, the role of carnitine deficiency or supplementation during the development of hepatocarcinogenesis has not been addressed. In this study, the authors demonstrate that carnitine deficiency is a risk factor and should be viewed as a mechanism during the development of hepatic cancer.
Innovations and breakthroughs
Recent studies have highlighted the importance of L-carnitine in protecting against anticancer-drug-induced fatigue and multiple organ toxicity. This is the first study to report that carnitine deficiency is a risk factor and should be viewed as a mechanism during the induction of hepatic carcinogenesis, and that long-term L-carnitine supplementation prevents the development of liver cancer. Therefore, carnitine supplementation alone or in combination with other natural chemopreventive compounds could be used to prevent, slow or reverse the occurrence of liver cancer.
Applications
By understanding that carnitine deficiency provokes hepatocarcinogenesis, this study may represent a future strategy in preventing the occurrence of liver cancer by carnitine supplementation.
Peer review
This is a well written and well structured paper that provides new insights on the protective action of L-carnitine in the development of liver cancer. Interestingly, the scavenger action of L-carnitine against reactive oxygen species was not the only mechanism in exerting the protection.
Supported by Operating grant from Research Center, College of Pharmacy, King Saud University (CPRC 207)