INTRODUCTION
The small intestine is the most difficult part to examine of the gastrointestinal (GI) tract because of its length and tortuous course. The traditional investigations with small bowel enteroclysis and small bowel follow-through reveal information sparingly, and unfortunately involve radiation exposure of the patient. Although it is an organ that is spared from frequent disease, more precise and patient-friendly methods are needed. In the last three decades, new imaging techniques have been developed that have proven useful. Computerized tomography (CT), magnetic resonance imaging (MRI), wireless capsule endoscopy and double-balloon endoscopy are all relatively new additions to the diagnostic armamentarium.
Compared with these methods, transabdominal bowel sonography (TABS), has the advantage of being cheap, portable, flexible and user- and patient-friendly. There are challenges with depth penetration and intestinal air precluding optimal image quality, and the flexibility of ultrasonography (US) warrants a systematic approach by the examiner. However, the development of improved scanner technology and high-resolution transducers has provided the clinician with image data of high temporal and spatial resolution, thus making it a useful tool in the diagnosis of small intestinal diseases[1–3]. This paper presents an overview of established US techniques for examining the small bowel and a description of new, promising scanning techniques.
When using US frequencies in the range of 7.5 to 14 MHz, the wall of the small intestine usually exhibits five different layers that correspond well to the histological layers. When looking at the mesenteric side of the intestine transabdominally, the first layer that is observed is the serosa/subserosa. It can be difficult to define exactly, as it is an interface echo between the surrounding structures (peritoneal wall/intestine/fat) and the serosa. The interface echo is thicker than the actual serosa and extends into the muscularis propria. Thus, the first layer that is clearly defined is echo-poor and corresponds to the muscularis propria. Next, is an echo-rich layer that corresponds to the submucosa, and an interface echo between the submucosa and the mucosa. Subsequently, there is an echo-poor layer followed by an echo-rich layer that corresponds to the mucosa and the interface echo between the mucosa and the luminal content. The wall layers as seen from the luminal side are described in the section on endoscopic sonography. The GI wall has a normal stratification if five US layers are visible, and there is loss of stratification if one or more US layers are missing[45].
BASIC SCANNING TECHNIQUES
Preparations
The patient should be examined in the supine position and usually the examination requires no special preparation. Fasting for > 6 h, however, leads to smaller amounts of fluid and air in the intestine and reduces motility. The intake of fluids orally or through a feeding tube reduces the air content in the intestine and makes it easier to separate the lumen from the wall and different bowel loops from each other. Furthermore, the mesenteric wall of the intestine, which is often hidden behind pockets of air, can be more easily examined with these preparations[6]. Another technique for dealing with the problem of air is graded compression, for which the examiner uses the US transducer to squeeze the air away from the region of interest[7].
High frequency B-mode
It is recommended that the examiner starts performing a regular scan of the abdomen with a 3.5-5 MHz transducer. This might give additional information, a better overview, and larger lesions in the intestine can be imaged completely. For a detailed transabdominal examination of the small intestine, a curved or linear transducer with frequencies in the range 7.5-14 MHz should be used. If pathology is detected, wall thickness, stratification, luminal patency, degree of stenosis or dilatation, and motility pattern should be determined.
Doppler modalities
Duplex scanning of the blood velocity in the superior mesenteric artery (SMA) provides several quantifiable parameters. The peak systolic (PV) and end diastolic velocity (EDV) can be used to calculate the resistive index (RI) = (PV-EDV)/PV, and the estimated mean velocity together with the inner diameter of the SMA is used to calculate the mean blood flow (MBF). The SMA is examined in the long axis. The best place to position the sample area is 2 cm after the SMA branches off from the aorta, and as it runs parallel to the aorta. The examiner should tilt the probe towards the epigastrum to obtain an angle < 60°. Intra- and interobserver agreements are good when the examiners are well trained[8]. Color and power Doppler gives information about the blood flow in smaller vessels that are difficult to find with duplex scanning. The information obtained from these images is qualitative. Triplex scanning can however be used for the estimation of RI in the GI wall.
DISEASE-SPECIFIC FINDINGS
Crohn’s disease (CD)
CD is the most common reason for performing TABS of the small intestine. The typical US findings can be divided into bowel wall changes, focal reactions and complications of the disease. The wall changes are discontinuous and consist of wall thickening, ulcerations, infiltrations, flow alterations, and changes in stratification[9–11]. Around the bowel loops, enlarged lymph nodes and fatty infiltration of the mesentery can be found[1213]. Several studies have also shown that complications such as fistulas, abscesses and stenosis with signs of obstruction can be found using TABS[1415].
The main finding in CD is a marked thickening of the intestinal wall of up to 15 mm. Fraquelli et al[16] have shown in a meta-analysis that, with a cut off of 3-mm, there was 88% sensitivity and 93% specificity for determining CD, while with a cut off of 4 mm, it was 75% and 97% respectively. The conclusion from this analysis should be that when used in primary diagnostics, 4 mm is a sensible lower limit, while in follow-up, a wall thickness > 3 mm should be considered as a sign of disease. Increased wall thickness is also associated with increased risk for surgery and for recurrence of disease after surgery[1718]. Wall thickening is, however, not specific for CD and the final diagnosis should rest on endoscopic and histological findings if possible. In a thickened intestinal segment, loss of stratification is associated with inflammation, while retained stratification suggests fibrosis[111920].
CD is associated with hypervascularity of the bowel wall during active disease. This has been the rationale for performing Doppler studies of regional and local blood flow. Studies with duplex scanning have shown a correlation between disease activity, increased PV, MBF and decreased RI in the SMA[21–23]. Other studies looking at the difference in MBF, pulsatilty index and RI before and after a meal have also found a significant association[2425]. Most of these studies have compared groups of less than 15 patients and have not been confirmed in larger studies. The measurements in the SMA are also be influenced by age, presence of atherosclerosis, and the length of the intestinal segment that is affected[2627]. The most promising parameters, such as MBF, are difficult to assess and have found little use in clinical practice. Other studies have compared disease activity with semi-quantitative and quantitative estimates of vessel density in the GI wall with color and power Doppler. An association has been found in some studies[2829], but the results have been inconclusive in others[3031]. The reason for these conflicting findings might be that the clinical assessment of disease activity with the CD activity index (CDAI) does not reflect vascularity. This theory might be supported by studies using US contrast in combination with power Doppler or harmonic imaging. Several studies have found the expected increase of contrast enhancement in patients with an elevated CDAI, but a subgroup of patients with contrast enhancement with low CDAI[32–34]. There is strong reason to believe that there is a correlation between vascularity and contrast intensity[35]. The hypervascularity in the GI wall of these patients may be a presentation of subclinical disease and it should be followed in prospective studies. There have been some recent studies with Doppler imaging that have investigated risk of relapse and treatment effects, which have indicated that detection of increased flow to and in the small intestine gives prognostic information[2536].
Celiac disease
The associated findings of TABS are fluid-filled distended intestinal loops and continuous peristalsis in the small bowel segments during fasting[337–39]. Furthermore, a reduction in the number of mucosal folds in the jejunum and an increase in the ileum is another typical find[3739]. Some authors have also found intermittent invaginations[3738]. Less specific findings are free fluid, enlarged mesenteric lymph nodes, increased gall bladder size and a slight wall thickening. The studies performed have been mostly of an explorative nature, but in a prospective cohort study in patients with chronic diarrhea, iron deficiency or dyspepsia, dilated fluid-filled bowel loops were the most sensitive finding with a sensitivity of 92% and a specificity of 77%. In combination with increased gall bladder size, thickened small bowel wall (> 3 mm), increased fasting peristalsis, enlarged mesenteric lymph nodes and free abdominal fluid, the sensitivity was reduced to 33%, but specificity rose to 99%[3]. In untreated patients with celiac disease, increased peak velocity, mean velocity and MBF was found, while the RI was decreased as expected[40–42]. These findings disappeared with therapy[42] but reappeared after exposure to gluten[41].
Intussusception
In children with intussusception or intestinal invagination, the typical finding on TABS is a multilayered lesion with concentric circles (onion sign) in the right fossa, when seen in the transversal plane. When the mesentery is involved, this forms an echo-rich crescent open towards the ante-mesenteric side. This is called the crescent in the donut sign. When seen longitudinally, the mesentery is seen as an echo-rich layer between two multilayered structures, the sandwich sign. Using these criteria, a sensitivity of nearly 100% and a specificity around 90% have been found in prospective studies[143]. TABS can also be used as guide in treatment procedures with hydrostatic reduction[44]. Color Doppler examination of children with intussusception shows that absence of flow in the wall of the invaginated intestine makes reduction more difficult, but does not necessarily mean the intestine is necrotic[45].
In adults, TABS has proven useful as a primary diagnostic method, but since intestinal invagination is a rare cause of bowel obstruction in adults, it is often found by other means. The TABS appearance is similar to that in children, but there is often other pathology present that is the pathological lead point of the invagination[4647]. As a result of the low number of incidents, there have been no prospective studies in the literature. Transient intestinal invaginations occur in children and adults, and mostly without symptoms. If there are no pathological lead points, the discovery is incidental, and if the intestinal segment is shorter than 3.5 cm, they are considered harmless[4849].
Malignant tumors
Malignant tumors in the small intestine represent only 2% of GI cancers, and it is mainly the malignant tumors that are diagnosed as result of symptoms. The most frequent is adenocarcinoma (30%-50%), followed by carcinoids (25%-30%) and lymphoma (15%-20%)[50]. Less frequent are mesenchymal tumors of the GI tract, such as malignant GI stromal tumors and leiomyosarcomas[51]. The distribution in the small intestine is mainly duodenal adenocarcinoma, mesenchymal tumor in the jejunum, and lymphoma and carcinoids in the ileum[52]. As a result of the low number of patients, there have been few studies confirming the transabdominal echo characteristics of small bowel tumors. However, explorative studies and case series suggest some echo characteristics. Lymphoma is associated with target lesions caused by gross wall thickening with loss of stratification and sometimes aneurysmal dilatation of the intestine in the affected area[5354]. Carcinoids have been shown to be intraluminal, oval and smoothly shaped. Furthermore they are echo-poor, and homogeneous, with an interruption of submucosa and a thickening of the adjacent muscularis propria[55]. Mesenchymal tumors appear as echo-poor, exophytic or dumbbell-shaped, with little contact with the bowel wall. If the bowel wall is involved, the submucosa is intact over the tumor and the muscularis propria adjacent to the tumor is unaffected. A typical feature might also be a peripheral, echo-rich or anechoic crescent-shaped area that represents necrosis[56].
Ischemic disease
In chronic ischemia of the small bowel, stenotic or occlusive lesions in the celiac and/or mesenteric arteries are found, and the patients typically have postprandial epigastric pain and weight loss. It is considered evidence of significant stenosis if duplex scanning of the celiac artery gives PV > 200 cm/s and EVD > 55 cm/s, and if the SMA PV is > 275-300 cm/s and EDV > 45 cm/s. If the patients have typical symptoms, it is indicative of ischemia, but still angiography must be performed for a definite diagnosis[57–59]. Duplex scanning is not the method of choice for diagnosing acute ischemia of the small bowel. If the ischemia has lasted a few hours, dilated bowel loops and a thickened bowel wall can be seen, but these signs are unspecific, and the examination is often made difficult by increasing amounts of intraluminal air. An arterial occlusion can be found, but the method is unlikely to show distal embolization and has not been studied with non-occlusive disease[60].
CONTRAST ENHANCED ULTRASOUND (CEUS)
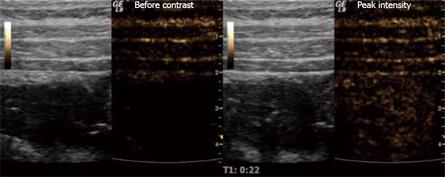
Ultrasound contrast agents consist of micro-bubbles (1-7 &mgr;m), often made of a phospholipid shell with a gaseous content. They are given intravenously and excreted through the lungs. The use of CEUS has led to several new clinical applications[61]. As a result of their size, they stay in the circulation and because of their shape and gaseous content, they produce a non-linear, harmonic response that can be separated from the tissue signal using contrast harmonic US. When examining the intestine, it is preferable to use frequencies above 7.5 MHz, so that the different wall layers can be separated. Since there are fewer micro-bubbles that resonate at these high frequencies, higher contrast doses should be used. When a bolus of contrast is injected it will travel from the peripheral vein via the pulmonary bed and arteries before reaching capillaries in the intestinal wall. This lasts about 10-15 s and the moment contrast can be observed in the capillaries is the time of arrival. The concentration will continue to increase further as the contrast enters the capillaries and reaches its maximum concentration after approximately 30 s (Figure 1). This is the peak intensity, and the period leading up to this is called the arterial phase. In the intestine, it is followed by the venous phase in which the contrast has been distributed to the whole capillary bed and the concentration decreases as result of excretion of the contrast agent in the lungs[62].
Figure 1 Stenosis of the terminal ileum in a patient with CD examined with ultrasound contrast.
The grey-toned images are the B-mode scouts while the brown-toned images portray the harmonic signals that are almost exclusively from micro-bubbles at a low MI. The right image shows the situation before contrast injection, while the left picture is taken at peak intensity.
The simplest way of applying US contrast is to examine the intestinal wall 30 s after injection with power Doppler. This will enhance the Doppler signal, but also destroy all the micro-bubbles in the region of interest, which gives only a momentary flash of enhancement. Using contrast harmonic US with a low mechanical index (MI), the destruction of micro-bubbles is minimal and the arterial and venous phases can be observed continuously. The temporal dimension to this examination gives quantifiable parameters that are independent of attenuation.
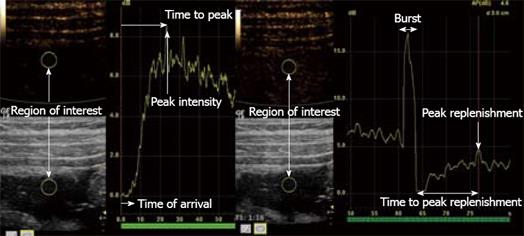
For quantification, there are currently two different methods (Figure 2). First, bolus tracking is a method in which the examiner simply injects a standard dose of contrast for a standardized injection time and registers the arterial and late phases. From the time intensity curve (TIC), time of arrival, peak intensity, time to peak and area under the curve can all be estimated. The problem with this method is that one needs to correct for attenuation, and the parameters are dependent on the injection speed and dose. Second, the burst replenishment method circumvents the problem of injection speed by giving a constant infusion of contrast agent. To observe how the capillary bed fills up in a region of interest, the micro-bubbles are simply destroyed by a burst of high-MI US, and then the replenishment is observed. Time to peak replenishment and the slope of increase are both quantifiable parameters that can be derived from a TIC. The burst replenishment method can however only provide indirect measurements of perfusion, while bolus-tracking potentially can be used for estimation of true perfusion when corrected for the arterial input function and attenuation.
Figure 2 Stenosis of the terminal ileum in a patient with CD.
The right image shows a TIC using bolus tracking. The left image shows burst replenishment without constant infusion in the same patient. When the micro-bubbles are hit by ultrasound with a high MI, this causes them to burst, which produces high-intensity images (Burst). Quantifiable parameters such as time of arrival, peak intensity, time to peak, peak replenishment and time to peak replenishment are shown in the illustration.
STRAIN IMAGING AND ELASTOGRAPHY
Occasionally an intestinal tumor or stenotic intestinal section in patients with CD can be palpated trans-abdominally. Palpation is informative since different tissues have different elastic properties. Elastography or strain imaging are US methods using this information by detecting the elasticity or stiffness of a tissue and providing a visual display[63–65]. The basis of every strain imaging technique is to measure tissue deformation caused by an external stimulus. The derivative of the tissue displacement is called strain, and can be calculated by cross-correlating the radio-frequency data before and after compression. The strain value in each point is color-coded and displayed in an elastogram. This elastogram can then be combined with the B-mode image to display the elastic properties of the tissue to the examiner through color information. Equipment using a quasi-static method of producing strain in the tissue through external compression with the US probe is now commercially available for clinical application[66].
The quasi-static application of elastography of the small intestine represents special difficulties, as the pressure from the US transducer can cause several changes. When adding pressure, one not only deforms the GI wall, but also displaces the luminal content and the intestine itself. The intestinal content can be displaced using a preload, and in some areas, the small intestine can be fixed against the abdominal wall. The terminal ileum can be compressed against the psoas muscle in the right lower quadrant and this may prove useful in CD patients, who often have stenosis in this area[67]. To the best of our knowledge, there have been no human studies using the quasi-static method or other elastography techniques transabdominally on the small intestine, but it has been applied with some success in the endosonographic detection of malignant lymph nodes[6869].
CD with stenotic obstruction is a potential application for elastography. We present two cases with stenosis of the terminal ileum. In one patient, the resection specimen was examined ex vivo (Figure 3), and in the other, the stenosis was examined transabdominally with elastography before surgery (Figure 4). In both patients, elastography indicated harder tissue in the stenotic area compared to the surrounding tissue. The pathologist found a fibrotic stenosis in both surgical specimens.
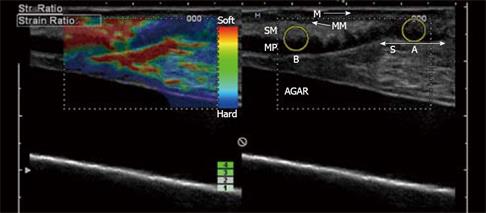
Figure 3 In vitro examination of a stenotic terminal ileum in a patient with CD.
The intestine has been cut open longitudinally and is resting on agar. The image was acquired with a 13-MHz linear probe (Hitachi Medical Systems Europe Holding AG, Zug, Switzerland) applied directly to the mucosa. The left image is an ordinary B-mode image showing the thickened GI wall (> 1 cm in vitro) with the muscularis propria (MP), submucosa (SM), mucosa (M) and a thickened muscularis mucosa (MM). Towards the stenosis (S), the stratification is lost. In the right corresponding elastography image, there is a clear transition between the blue (hard) stenotic area and the red (soft) pre-stenotic area. The strain measured in region A and B (yellow circles) differ with a ratio of 3.77 ( A: 0.27%; B: 1.02%; B/A: 3.77), which indicates a distinct difference in elasticity. Histology confirmed a fibrotic stenosis (Bar indicates 0.5 cm/unit).
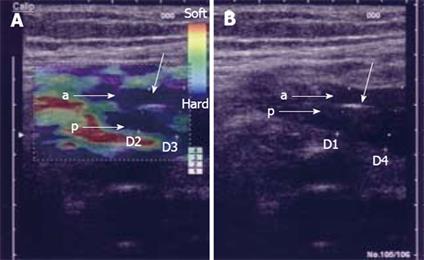
Figure 4 Elastography image acquired transabdominally in a patient with CD and stenosis of the terminal ileum.
In the left image, the bowel wall is thickened without stratification and the anterior (A) and posterior (B) bowel wall is separated by luminal air (unmarked arrow). In the right elastography image, the stenotic area is colored blue relative to the surrounding tissue, which indicates that it is hard. The patient was operated upon and the histology confirmed a fibrotic stenosis. D1: 9.5 mm; D2: 8.8 mm; D3: 10.0 mm; D4: 13.0 mm.
HYDROSONOGRAPHY
High-resolution transabdominal ultrasound for imaging the small intestine is often hampered by intestinal gas. The lumen of the small bowel is collapsed frequently and significant intraluminal pathology can be missed. Moreover, separation of different bowel loops can be difficult. Hydrosonography of the small intestine is a method in which an echo-poor contrast liquid is used for distending the bowel and removing intestinal air, and thereby improving imaging. Most often, an isotonic polyethylene glycol (PEG) solution is used (e.g. Laxabon®). The PEG solution is indigestible and non-absorbable, therefore, it gives a predictable amount of luminal fluid and intestinal distension. In principle, any echo-poor fluid can be used. The amount of PEG solution used in different studies varies between 200 and 2000 mL. Oral intake of PEG solution (small intestine contrast ultrasonography) is the least invasive way of giving the contrast liquid, and probably the preferred method of most patients[70]. However, in patients having trouble drinking large amounts of PEG solution, a nasojejunal feeding tube can be used. The tube is placed at the duodenojejunal flexure by endoscopy, fluoroscopy or ultrasound guidance. These procedures are more time consuming than a regular transabdominal ultrasound examination, and the average examination may vary between 30 and 40 min (range 10-90 min). Examination time is very much dependent on the passage of contrast fluid to the terminal ileum. Sensitivity for diagnosing a suspected disorder in the small intestine in patients referred for a barium study varies as much as 64%-100%, while generally, a near 100% specificity is found[671–73]. In patients with known CD, the sensitivity for detecting a lesion in the small intestine varies between 96% and 100%[73–75]. Hydrosonography of the small intestine is safe. As opposed to enteroclysis and CT, the method can be used for examining the small intestine in patients in whom radiation should be avoided. Particularly, it is useful in the follow-up of patients with inflammatory bowel disease where repeated examinations often are necessary.
ALLERGOSONOGRAPHY
Food hypersensitivity reactions, including GI reactions due to allergy against food items, can be visualized by advanced imaging and visualization modalities such as endosonography[76], TABS and MRI. In Western countries, the rate of perceived food hypersensitivity is around 25% in the general population[77]. Conversely, a diagnosis of true food allergy in adults is confirmed in only 1%-4% of the cases. The difficulty is to diagnose the reactions, which occur by non-allergic mechanisms, or local reactions in the GI tract. They usually have negative allergic test results and when they reach the doctor, the symptoms are already gone. Therefore, a provocation test is often necessary to determine the reactions and symptoms.
Objective tests for diagnosing of food allergy are missing. In previous studies, double-blind placebo-controlled food challenge (DBPCFC) has been considered the gold standard for many years, but the latest literature has criticized the method because the procedure is labor-intensive and time-consuming, and the assessment is based on subjective symptoms such as abdominal discomfort and bloating[78]. In practice, therefore, the diagnosis is based on history and skin prick tests, and DBPCFC is seldom performed.
We have applied US to establish whether food hypersensitivity reactions can be objectively visualized. Not only mucosal swelling, but also luminal fluid can be visualized by TABS. Therefore, we wanted to explore the feasibility of using US to monitor the response of the intestine to direct luminal provocation in 32 patients with food hypersensitivity[79]. A nasoduodenal feeding tube was placed with its tip in the duodenum, aided by a gastroscope. The intestinal mucosa was challenged with the suspected food item dissolved in 10 mL water or saline given through the nasoduodenal tube over a 3-min period. Sonography was performed before (-5 min) and 5, 20, 30 and 60 min after the provocation. The following sonographic features were explored: wall thickness and diameter of the duodenal bulb, wall thickness and diameter of the pars descendens of the duodenum, wall thickness and diameter of the proximal jejunum, number of fluid-filled jejunal loops, air in the small intestine, and peristalsis of the small bowel. Sonographic changes were observed after challenge in 14 (44%) of the 32 patients. A positive sonographic response (increased wall thickness, diameter, peristalsis and/or luminal fluid) was significantly related to positive skin prick test (P = 0.008) and positive DBPCFC (P = 0.03). A significant correlation was found between provocation-induced symptoms and wall thickness of the duodenal bulb (r = 0.50, P = 0.004) or jejunum (r = 0.42, P = 0.02). Intra- and interobserver variation of the tracing procedure revealed low values.
Sonography appeared to be the most sensitive test applied, but the specificity of the new test is not yet known. Based on our findings, we conclude that responses of the proximal small intestines to direct provocation (swelling of the wall and exudation of fluid into the lumen) can be visualized by TABS, and that this new provocation test may become helpful in the evaluation of patients with food hypersensitivity.
ENDOSCOPIC SONOGRAPHY
Intraluminal US (endosonography) is the common denomination of US examination using intracorporal transducers that are inserted under endoscopic guidance or blindly into the GI tract. Thus, the visceral wall and adjacent structures can be imaged in detail. Endoscopic US (EUS) has had an impact on the clinical management of several GI disorders and has become a valuable tool for investigating mucosal and deeper lesions of the GI wall, as well as biomechanical and motility abnormalities[8081].
The small intestine may be examined by relatively new endoscopic techniques such as wireless capsule endoscopy or double-balloon enteroscopy (DBE). DBE also allows therapeutic procedures to be performed and biopsies to be obtained. EUS of the duodenum and terminal ileum has been performed previously, but other parts of the small intestine have been regarded less accessible. However, when transducers are placed in the stomach or colon, it is possible to image loops and limited areas of the small intestine[82].
The normal wall of the small intestine is thin and folded and is displayed by high-frequency US as a layered structure usually consisting of a 5-layer structure but sometimes 3-9 layers are seen. When the visceral wall is imaged with an endoluminal transducer, the interface between the luminal content and mucosa accounts for the first, echo-rich layer. The rest of the mucosa appears as a second, echo-poor layer. The third, echo-rich layer usually includes the interface between the lamina propria and muscularis mucosae, the submucosa, and the interface at the border of the muscularis propria. In a five-layer structure, the rest of muscularis propria is imaged as an echo-poor fourth layer. The fifth, echo-rich layer represents the outer wall delineation and is an interface echo at the serosal level[483].
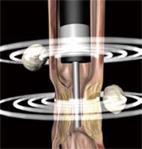
New and longer miniprobes that can be inserted through the working channel of enteroscopes are now available. These miniprobes may be helpful in the examination of small bowel lesions (e.g. CD, lymphoma, ischemic disease, subepithelial masses, tumors, strictures, stenoses and bleeding spots) when used in combination with DBE. Forward-viewing optic echoendoscopes can be combined with transendoscopic miniprobes, which allow endosonographic imaging with both instruments in the same procedure. Thus, imaging of stenotic areas not traversable for echoendoscopes, or other areas not accessible with echoendoscopes (e.g. parts of the jejunum) can be performed (Figure 5). Small bowel ulcers may exhibit a typical EUS pattern that consists of three separate elements: the ulcer crater, the echo-rich ulcer base, and frequently an echo-poor inflammation zone, which can extend beyond the thickness of the wall and more widely than the endoscopic changes (Figure 6). Enlarged vessels running into the ulcerated area can sometimes be demonstrated by EUS and may indicate an increased bleeding risk.
Figure 5 Illustration of EUS performed with an echoendoscope and an ultrasound miniprobe simultaneously.
The echoendoscope can not pass a stenotic area which, however, can be further examined with a miniprobe. Lymph nodes are seen outside the GI wall.
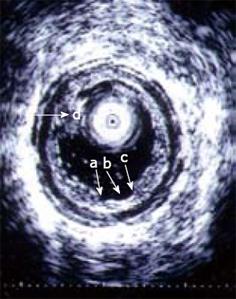
Figure 6 An ultrasound miniprobe (20 MHz) is placed in the ascending part of the partly water-filled jejunal lumen in a patient with CD.
The layers of the wall are seen and also an erosion/superficial ulcer (b) can be observed. The edge of the lesion is indicated (a, c). A mucosal polypoid elevation is also seen (d).
EUS has the potential to image biomechanical changes in all or individual GI wall layers as well as in the surrounding tissue. This information may improve our understanding of pathophysiological mechanisms of gut pathology. Several studies have been performed that combine miniprobe US with manometry to describe the functional role of muscle layers of the GI wall. Recently, a multimodal device that combines bag distension, manometry, high frequency intraluminal US, laser Doppler flowmetry and symptom registration was applied to the esophagus, and demonstrated that these modalities can be used in combination without technical influence on each other[84].
NUTRITIONAL IMAGING
Nutritional imaging is US imaging to study the effects of nutrition such as fat, proteins and glucose on the function of the GI tract. Information concerning movement of luminal contents in humans can be obtained by fluoroscopy, scintigraphy, MRI, and impedance and duplex sonography. Studies based on scintigraphy and standard US of the stomach and duodenum indirectly measure overall rates of gastric emptying, but these methods do not have the temporal resolution to assess the rapid changes of transpyloric flow.
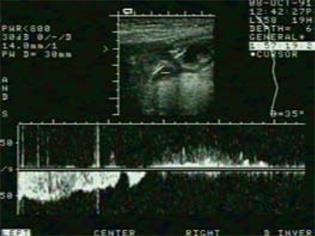
Hausken et al[85] have shown that using pulsed Doppler combined with real-time US (duplex sonography), it is possible to visualize antro-duodenal motility and transpyloric flow simultaneously. Antegrade and retrograde transpyloric flow is visualized using bidirectional velocity curves. Most contractions of the proximal duodenal bulb precede closure of the pylorus (and the terminal antrum), and duodenal bulb contraction is often accompanied by a short burst of duodenogastric reflux that occurs immediately before closure of the pylorus (Figure 7).
Figure 7 Scan of the transpyloric area with velocity curve of gastric emptying episode (left) and duodenogastric reflux episode (right).
Calibration bars indicate cm/s and 0.2 s on the y-axis and x-axis, respectively.
Patients with functional dyspepsia often experience early satiety and discomfort after a meal. Using duplex sonography, it is possible to relate timing of symptoms and early postprandial emptying in patients with functional dyspepsia[86]. Meal-related discomfort is experienced after commencement of transpyloric emptying. An inverse relationship has been found between the duration of the tasting period and symptom intensity, which suggests that the time allowed for duodenal tasting might be too short in patients with functional dyspepsia.
The effects of intraduodenal glucose, fat and protein on blood pressure, heart rate and SMA blood flow have been determined in healthy older subjects. Intraduodenal glucose, fat and protein decrease systolic BP in healthy older subjects, but the onset of the hypotensive response is earlier after glucose, and the effect of protein on SMA blood flow is less than that of the other nutrients[87].
ADVANCED COMPUTATIONAL VISUALIZATION
When a 3D volume data set of the small intestine is acquired using 3D US, CT or MRI, a diagnostic procedure known as virtual endoscopy (VE)[88] can be utilized. VE has been considered to be of interest for several years, mostly because of its potential to (at least partially) substitute standard colonoscopic examinations (and the associated risks). Endoscopic views in the US examinations are nowadays incorporated into some medical workstations[89].
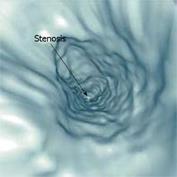
While the colon is especially suitable for VE examinations since it is rather easy to inflate with air, and has a large diameter and a predictable position, the small intestine offer more of a challenge. Previous studies with liquid contrast and special procedures to minimize the presence of air have suggested that VE of the small intestine can be carried out[9091]. Contrasting the interior of the small intestine enables segmentation and the diagnosis of certain pathologies using VE. Figure 8 shows an example of a diagnosis of intestinal stenosis using VE of the small intestine.
Figure 8 VE of the ileum demonstrating stenosis of the intestinal lumen (arrow) (Rogalla et al[90]).
VE generally aims at examining all relevant parts of an inspected tubular structure, such as the colon or small intestine, or areas outside the GI tract, such as the bronchi or, in case of high precision scans, even thin vascular structures. The viewpoint navigation of a complex tubular shape can introduce an unnecessary burden on the examiner’s side. Therefore, an advanced VE approach should compute and suggest a camera path along the tube’s center line to the examiner. The examiner can then easily navigate throughout the entire structure, and if a more fine-tuned viewpoint is needed, detach from the pre-computed path and navigate in freehand mode. In most cases, the computation of the path is based on a skeletonization of segmented tubular structures[92]. The skeleton is a topological structure that is equidistant to the borders of given object. In medical imaging and visualization, it can be computed for particular organs using morphological operators or the distance transform.
Selective visualization techniques
VE does not display the inspected tissue in its original color, and this information is acquired by optical endoscopy only. The advantage of VE, however, is the ability to see structures that are in close vicinity to the tube walls from outside. A tumor, for example, which develops on the outside wall of the examined structure, can be investigated in this way. Using semi-transparency for rendering the inspected organ walls can help with revealing external, potentially pathological structures. This can be achieved, for example, with a two-step first-hit ray-casting approach[93] or possibly with opacity peeling[94] and volume rendering. First-hit ray-casting usually stops ray traversal when a selected density value is reached and displays this boundary as an iso-surface. The two-step extension means that there are two locations where the viewing ray intersects the iso-surface. The inner one is represented with a semi-transparent surface, whereas the second, outer surface is fully opaque. In the case of opacity peeling, the ray traversal accumulates the optical representatives of the density values in the same way as the common direct volume rendering, i.e. using the over operator. The difference is that when the accumulation during the ray traversal reaches nearly full opacity, the accumulation is reset to zero and ray traversal continues until the accumulation reaches full opacity again. This means that the second level of accumulation is shown. When selecting an appropriate transfer function, the tube wall can be peeled away to see structures behind the wall upon the examiner’s request.
VE is essentially a computerized form of the optical screening approach that shares the same basic principles of pursuing a walk-through along the tubular structure. Alternatively, there are other new approaches that aim to present the whole structure in a single image. In some sense, this is analogous to representing the earth on a sphere and by a conformal map projection of the world into a 2D plane. One of these techniques, usually referred to as colon unfolding[95], simulates a cut and a consecutive virtual opening of the tube, eventually flattening it into two dimensions. This technique requires high reconstruction quality during unfolding and good design for the cut (to not dissect a polyp structure, for example). The advantage of easing the complete visual inspection comes with a certain risk of deforming structures of interest, e.g. polyps, which may lead to errors in the interpretation. This issue can be addressed by another technique, originally developed for vascular investigation and recently applied for virtual colonoscopy[96], known as curved planar reformation (CPR). The principle of CPR[97] is that a curved plane that includes the center line of the tubular structure cuts the tube in two halves. Then, both halves can be inspected separately or the plane can be rotated to redefine the cutting geometry. The center line that defines the plane can be estimated from the organ skeletonization[92], for example. For particularly curved tubular structures, as in the case of the small intestine, the plane can be stretched or completely straightened to give a clear view on inside of the tube.
An alternative to the CPR technique is view-dependent section-view rendering such as the VesselGlyph focus + context technique[98]. To view the internal part of the walls, the section is cut starting at the center line, with opening towards the viewer. All these representations, for which all structures are visible on a single image, or the outside-in view is incorporated, can be effectively combined with endoscopy for easy navigation and better and faster diagnosis.
CONCLUSION
In this review, we have presented different scanning techniques and US modalities for examining the small bowel. While TABS of the small intestine is standard in many tertiary centres for GI diseases around the world, some of the modalities reviewed here have not been implemented widely in clinical routine.
The strength of the US examination is high flexibility, repeatability, low patient risk and price, in combination with good spatial and excellent temporal resolution. If the initial US examination is not fully adequate, the operator has the opportunity to continue with more advanced methods, like CEUS or hydrosonography. However, further studies are needed to establish the methods’ effectiveness at detecting small lesions in the small bowel. In patients in whom an invasive procedure is necessary, EUS with biopsy or even local resection is possible. Although the procedure is technically challenging, invasive and time-consuming, these factors are outweighed by the risk of an exploratory laparotomy or laparoscopy.
US can provide a wide range of physiological and pathophysiological parameters from the small bowel. Doppler techniques give information about the speed and direction of blood flow, and also provide indirect measurements of perfusion. The measurements can be difficult to reproduce and the perfusion estimates should be interpreted with care as detection of blood flow in small vessels is a great challenge. However, CEUS enhances the sensitivity of flow signal detection remarkably and enables calculation of true perfusion in a region of interest. This information is important in the characterization of small bowel tumors and lesions of CD.
Some of the techniques presented in this review have shown their potential in explorative studies, but need to be further tested to determine their ultimate clinical utility. Indeed, imaging of the small intestine is a great challenge to the clinician. In future, the clinician should be able to switch easily between the different modalities using the same scanner and with the help of improved visualization techniques, and have all relevant information presented simultaneously. Accordingly, the advance of multimodal and multiparametric imaging of the small intestine will enable better diagnostic performance and subsequently improved patient management.