INTRODUCTION
Although metastatic tumours are the commonest malignant lesions affecting the liver, the incidence of ‘primary’ liver malignancies has significantly increased over the last 20 years. This is particularly true for hepatocellular carcinoma (HCC), which is globally the commonest liver primary, and for cholangiocarcinoma, the second commonest primary liver tumour[1]. HCC is now one of the commonest causes of cancer death worldwide and is the fifth most common cancer worldwide[2]. Cholangiocarcinoma accounts for 3% of all gastrointestinal cancers[3] but has increased relatively rapidly worldwide[4]. Mesenchymal liver tumours are rare, but include hepatic angiosarcoma and primary hepatic lymphoma.
Improvements in imaging technology have allowed exploitation of the dual blood supply of the liver by both the hepatic artery (25%-30%) and portal vein (70%-75%), and the fact that many benign lesions demonstrate characteristic contrast enhancement, due to their vascular supply. Imaging of the liver is often achieved in three distinct phases following intravenous contrast enhancement - the arterial phase, portal venous phase and a late phase.
For the purposes of this review, while we concentrate on the diagnosis and staging of both HCC and cholangiocarcinoma, we also discuss certain characteristics of benign liver lesions. We aim to highlight the advantages of each imaging technique, as well as underscoring potential pitfalls and limitations.
IMAGING MODALITIES USED IN THE ASSESSMENT OF LIVER CANCER
Ultrasound
B mode ultrasound (US) is often the first line investigation in liver disease and its use is outlined in the British Society of Gastroenterologist (BSG) guidelines for diagnosis of both HCC and cholangiocarcinoma in adults[56].
Contrast-enhanced ultrasound (CEUS): Progress in both technical advances by ultrasound manufacturers and in the development of ultrasound contrast agents (UCAs) has allowed the role of UCAs to change from Doppler rescue agents to diagnostic agents, providing an assessment of contrast enhancement patterns of liver lesions in real-time.
UCAs used in diagnostic US are characterized by a microbubble structure, consisting of gas bubbles stabilized by a shell[7]. Current generation microbubbles are based on perfluorocarbons with a phospholipid membrane, providing low solubility and favorable resonance behavior at low acoustic pressures. Microbubble sizes typically range from 3 to 5 &mgr;m and on intravenous injection remain in the vascular compartment for several minutes, being small enough to avoid filtration by the lungs and too large to enter the interstitial fluid. These compounds demonstrate strong non-linear harmonic responses when insonated with low acoustic pressure and generate specific signals without being destroyed when insonated at low mechanical index (MI), thus allowing continuous real-time imaging[7].
UCAs act as blood pool agents allowing the definition and visualization of three overlapping vascular phases-the arterial phase, portal venous phase and late phase, which last until there is clearance of the UCA from the hepatic parenchyma. This late phase differs from the equilibrium phase of extracellular computed tomography (CT) and magnetic resonance imaging (MRI) agents and may reflect sinusoid pooling and reticulo-endothelial system (RES) or Kupffer cell uptake[89].
Minor adverse effects of UCAs are reported in less than 5% of subjects and typically include transient discomfort at the injection site, taste aberrations and vasovagal attacks. UCAs are not nephrotoxic and it is not necessary to check renal function prior to their administration. The incidence of severe hypersensitivity or allergic reaction is lower than current X-ray contrast agents and comparable to that of MR contrast agents[10].
CT
The development of multidetector row helical computed tomography (MDCT), with its superior spatial and temporal resolution, has resulted in improved detection and characterization of focal liver lesions[11]. The acquisition of multiple data sets with each rotation of the x-ray tube in MDCT means the entire liver can be imaged in 10 s or less, compared with 25-30 s for single slice helical CT technology. The short time needed to image the liver allows multiple passes through the liver in different vascular phases following bolus contrast injection and thin-section collimation produces volume data sets with isotropic or near-isotropic voxel dimensions, resulting in superior spatial resolution and the capability to display data in multiple planes.
With single-slice helical CT, a ‘dual-phase’ technique is commonly employed with image acquisition in the hepatic arterial-dominant phase and in the portal venous-dominant phase. The ‘triple-phase’ technique includes an early arterial phase, imaged 18-25 s following bolus injection of contrast[12]. Using multiplanar reconstructions, a 3D CT hepatic-mesenteric angiogram can be obtained.
Hypervascular liver lesions are best appreciated in the late arterial phase as they show maximal enhancement relative to the background liver parenchyma. In the portal venous-dominant phase there is maximal parenchymal enhancement with opacification of the hepatic veins. This phase is extended to include the entire abdomen and, depending on the clinical indication, the pelvis. A delayed or equilibrium phase performed 3-5 min following contrast administration may be helpful in further characterizing focal liver lesions.
MRI
Although MRI is often viewed as the most sensitive and specific technique for evaluating the liver, this is probably debatable, given the recent revolution in multi-detector CT technology[1314]. Nevertheless, lesion/liver contrast is higher for MRI than with CT and the flexibility and range of pulse sequences available in MRI provide a significant advantage over CT.
Hepatobiliary MRI uses several magnetic resonance pulse sequences, each of which produces images that provide unique information about the liver and the biliary tree. Most examinations include a T1-weighted in-phase/out-of-phase spoiled gradient echo sequence and one or more T2-weighted sequences. Combining these sequences with extracellular intravenous contrast agents, usually with a fat-saturated spoiled gradient echo sequence, also allows patterns of tumour enhancement to be determined. In addition, use of tissue-specific contrast agents such as super paramagnetic iron oxide, allows improved detection and characterisation of liver tumours[15–17]. Contraindications to MRI include pacemakers, implantable cardiac defibrillators, cochlear implants and metallic orbital foreign bodies.
Intravenous MR contrast agents: Categories of clinically available liver contrast agents include non-specific extracellular contrast; liver-specific categories of hepatocyte selective; RES-specific; and agents with combined early blood pool and late RES-specific properties.
Extracellular gadolinium chelates are used extensively for liver MRI. Following intravenous injection of a gadolinium-based agent, typically three phases of contrast enhancement are imaged: the arterial, portal venous phase and the equilibrium phase[15]. During the arterial phase, most of the liver does not enhance as the majority of the liver’s blood supply is via the portal vein[15]. Enhancement patterns of liver lesions are similar to those demonstrated on CEUS and contrast-enhanced CT. The equilibrium phase or delayed phase is useful for identifying late enhancement of liver lesions. In addition, washout of contrast from HCC and peripheral or heterogeneous washout from liver metastases are characteristic findings on delayed imaging[15]. Recent concern about nephrogenic systemic sclerosis in renal impairment following intravenous gadolinium may require modifications in imaging protocols[1819].
Liver-specific contrast agents
Hepatocyte-selective contrast agents: Hepatocyte-selective contrast agents undergo uptake by hepatocytes and are eliminated through renal and biliary excretion[20]. All are T1-relaxation enhancing agents and increase the signal intensity in normal liver tissue and hepatocyte containing tumors. Non-hepatocyte containing tumors, such as hemangiomas and metastases, do not take up these agents and are rendered more conspicuous by the increase in signal of the background liver on delayed imaging. Agents such as Gd-BOPTA (Multihance) also exhibit early perfusional information, similar to gadolinium chelates.
Reticuloendothelial agents: Reticuloendothelial agents target the RES, particularly the liver and spleen and reflect the number of functioning macrophages[21]. Reticuloendothelial agents currently in clinical use include superparamagnetic iron oxide (SPIO) particles. SPIO particles act as a negative contrast agent and can be used alone or in combination with gadolinium[2223]. Most liver tumors, whether benign or malignant are deficient in Kupffer cells and do not exhibit SPIO particle uptake[2425]. Hence, most liver tumors appear relatively hyperintense, because the background liver darkens preferentially following SPIO administration. Combining gadolinium and SPIO-enhanced imaging in a ‘dual contrast’ MRI could be the most accurate technique for the detection of liver tumors[26].
Diffusion-weighted MRI: Diffusion-weighted imaging (DWI) uses pulse sequence techniques that are sensitive to the very small scale motion of water protons at a microscopic level and improves the conspicuity of many hepatic and extrahepatic tumors[27].
Positron emission tomography (PET)
The advent of molecular imaging with PET has revolutionized the concept of functional imaging in the management of disease, particularly in the field of oncology, which accounts for 90% of PET applications. Imaging with PET rather than gamma camera SPECT radiopharmaceuticals allows higher spatial resolution and good image quality, with better detection of even small lesions[28]. PET has the advantage over cross-sectional anatomical imaging of providing whole body imaging, allowing the detection of multifocal and metastatic disease. There are many radiopharmaceuticals based on labeled short-lived positron emitters, of which, the most widely used is the fluorinated glucose derivative 18F-fluorodeoxyglucose (18F-FDG). The use of other nuclear medicine techniques in the imaging of liver malignancy, e.g. colloid scintigraphy, has been rendered largely obsolete by improvements in other cross-sectional imaging techniques, principally MRI and US[29].
HCC
The majority of HCC cases develop in the cirrhotic liver. Cirrhosis is the strongest predisposing factor for HCC, with chronic viral hepatitis the commonest underlying etiological cause worldwide[30]. Curative resection, ablation or liver transplantation is possible if the tumor is detected at an early stage. Early detection of HCC is thus critical to achieve effective treatment and prolong survival[31]. However, it is often difficult to diagnose small HCCs, particularly in the presence of cirrhosis. In Europe, patients with chronic liver disease undergo regular surveillance, usually involving both alpha-fetoprotein (AFP) determination and conventional B-mode US examination of the liver[32]. Alpha-fetoprotein, the most commonly used serum tumor marker, is unfortunately not a dependable biomarker for diagnostic and prognostic purposes, having poor sensitivity, specificity, and positive predictive value, emphasizing the importance of high quality imaging techniques[33].
Hepatocarcinogenesis is considered a multi-step process characterized by the development of a spectrum of nodules from benign regenerative nodules (RNs) and dysplastic nodules (DNs) to overt malignant HCC. Although conventional US can reveal the different types of nodules in cirrhosis, the ability to distinguish RNs from DNs or malignant HCC is limited as this necessitates an understanding of hemodynamic changes: The vascular supply of RNs is similar to that of the liver parenchyma[34]; whereas DNs demonstrate a more complex vascular supply with a degree of capillarization ranging from that similar to RNs to the arterial hypervascularization typical of HCC. Thus, a reduction of both portal vein and normal hepatic artery branches with a progressive increase in abnormal hepatic arteries are considered histological features of malignant transformation[34]. It is this process of tumor neoangiogenesis that gives rise to the characteristic appearances of HCC on several different contrast-enhanced imaging techniques (CEUS, contrast-enhanced CT and MR with gadolinium enhancement) and facilitates its differentiation from other benign causes of focal liver lesions.
In patients with liver cirrhosis, the diagnosis of HCC can be based on clinical, laboratory and imaging techniques with an accuracy of up to 99%[35]. If a mass detected on US is ≥ 2 cm in diameter, there is a greater than 95% chance that the lesion is HCC and biopsy is not indicated.
Characteristic appearances of HCC
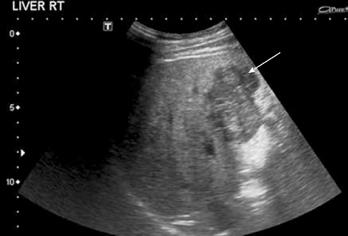
Ultrasound: HCC appearances on B-mode US are variable. Small lesions are usually hypoechoic, but larger lesions may demonstrate heterogeneous echotexture due to necrosis and fibrosis (Figure 1).
Figure 1 HCC.
B-mode US demonstrates a heterogeneous hypoechoic solid liver lesion (arrow) in segment IV, confirmed to be HCC at histological examination. Note the background of an echogenic liver with an irregular surface.
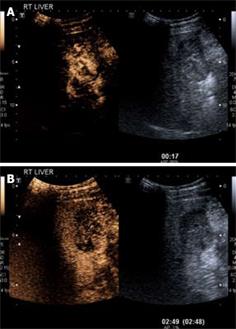
The most common feature of HCC using CEUS is the presence of early, intense and homogenous intratumoural enhancement (Figure 2A). Real-time imaging can demonstrate the ‘basket pattern’ of blood flow with peripheral vessels encircling and internally penetrating the tumor. After the arterial hyperenhancement, HCC show, ‘wash-out’, resulting in an isoechoic or hypoechoic appearance in the portal-venous and delayed phase (Figure 2B). In most cases, this ‘wash-out’ is slower than with other malignant lesions, such as metastases. The degree of late-phase enhancement is determined by the degree of similarity of the nodule to normal liver parenchyma[36]. RNs usually have a hypoechoic or isoechoic appearance in the arterial phase and an isoechoic appearance in the portal and late phases[37]; and the detection of arterial hypervascularization reflects transformation to HCC. Some caution needs to be exercised, however, as up to 30% of HCCs, particularly the small, well-differentiated variety, may be isoechoic rather than hypoechoic on the late phase of imaging and thus be mistaken for a regenerative nodule or dysplastic nodule.
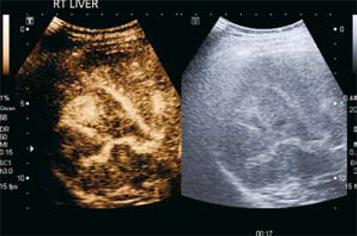
Figure 2 HCC.
A: CEUS in the arterial phase (17 s following injection) demonstrates arterial phase enhancement of the solid lesion (corresponding grey scale image of the lesion is shown on the right hand side; software known as “twin view” which helps the sonologist track the lesion through the phases of enhancement). Note the “basket weave” pattern of angiogenic vessels with haphazard enhancement of this lesion; B: Twin view of the same segment IV lesion in the delayed phase (2 min 49 s following injection) of the same lesion as Figure 2A demonstrates contrast wash-out compared to the surrounding liver parenchyma which are features of a malignant lesion.
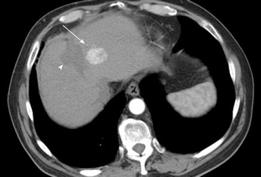
CT: On contrast-enhanced MDCT, the typical HCC demonstrates similar enhancement patterns to those seen on CEUS, with intense inhomogeneous enhancement seen in the hepatic arterial-dominant phase and contrast wash-out in the late portal venous phase[38] (Figure 3). A minority of HCCs are hypovascular tumors and do not demonstrate arterial enhancement, appearing as hypoattenuating lesions relative to the liver parenchyma on the portal venous-dominant phase.
Figure 3 HCC.
Contrast enhanced CT demonstrates heterogeneous arterial phase enhancement of a focal liver lesion (arrow) adjacent to a low attenuation area representing a previous radiofrequency ablation site (arrowhead).
HCC arising in the non-cirrhotic liver is often large, due to its long asymptomatic course and late presentation[3940]. This contrasts with the multi-focal tumors more commonly found in patients with other forms of chronic liver disease. Large HCCs may demonstrate a number of characteristic appearances on MDCT which make differentiation from other causes of focal liver masses relatively straightforward. A mosaic appearance may be seen in large tumors, with fibrous septa separating areas of variable attenuation which represent internal regions of hemorrhage, necrosis, fatty degeneration and fibrosis. Characteristic satellite tumor nodules close to the margins of large tumors may be seen. Well-defined, lobulated margins and a distinct fibrous capsule are also features of large HCCs. The fibrous capsule has low attenuation on unenhanced images and does not enhance in the arterial phase but begins to enhance in the late portal venous phase. Retention of contrast within the capsule increases its conspicuity in the equilibrium phase. HCC has a propensity for early invasion of the portal venous system and biliary tree. The portal vein may be expanded by tumor thrombus that can be differentiated from bland tumor thrombus by the demonstration of arterial enhancement, either diffuse or streaky[41].
A number of benign lesions, including hemangiomas, focal confluent fibrosis, peliosis, benign regenerative nodules and transient hepatic attenuation difference (THAD), can simulate small HCC lesions on CT, and an awareness of these lesions and their imaging characteristics is vital for the radiologist interpreting multi-phasic hepatic CT studies. THAD describes a focal increase in hepatic arterial flow that, in the context of cirrhosis, is most commonly due to arterial-portal shunting, but THAD can also result from intrahepatic thrombosis of hepatic or portal veins. The typical appearance of THAD is a peripheral, often wedge-shaped, area of arterial phase enhancement[42]. In a study of a large screening population with cirrhosis, Brancatelli and colleagues found an 8% false-positive rate upon comparison of pre-transplant CT studies with pathological examination of explanted livers[43]. Both hypo- and hyper-attenuating nodules were incorrectly diagnosed as HCC on CT but most of the lesions were small (< 1.5 cm diameter).
The use of both double arterial phase imaging and delayed phase imaging improve detection of HCC, particularly of small (≤ 2 cm) nodules[44]. However, with radiation dose considerations in mind, most centers adopt a pragmatic approach in combining late arterial phase and portal venous phase imaging to evaluate HCC. Double arterial phase imaging is often reserved for patients who are candidates for surgical resection or chemoembolization, in whom accurate delineation of hepatic arterial anatomy is vital. The addition of an unenhanced phase does not improve detection of HCC in the cirrhotic liver[45], but is used in some centres to differentiate high attenuation siderotic regenerative nodules from arterially-enhancing nodules. Delayed CT, following intra-arterial injection of iodized oil (Lipiodol®, Guerbet, Paris, France), may improve detection of HCC nodules and facilitate targeted biopsy due to the uptake and retention of contrast within the hypervascular tumor nodules[4647].
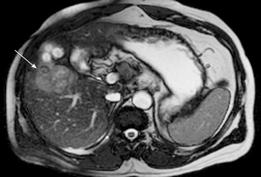
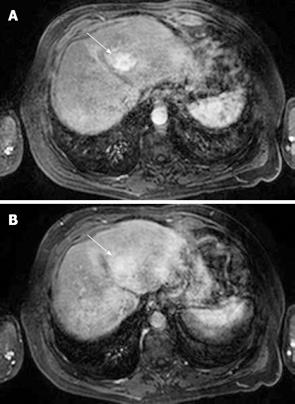
MRI: HCC can have a variable appearance on unenhanced T1-weighted images and typically shows increased signal on T2-weighted images[48] (Figure 4). Following gadolinium administration, HCC demonstrates characteristic early enhancement on arterial phase imaging[49] and washout on the delayed images - resulting in a hypointense lesion compared to the surrounding parenchyma[15] (Figure 5A and B).
Figure 4 HCC.
T2-weighted gradient echo sequence shows a solid lesion in segment V (arrow) of the liver which is of intermediate signal hyperintensity compared to the surrounding liver parenchyma.
Figure 5 HCC.
A: T1-weighted fat-suppressed sequence following gadolinium intravenous injection shows arterial phase enhancement of a focal liver lesion in segment IV (arrow); B: HCC: The same lesion as shown in Figure 5A becomes relatively less conspicuous on the portal phase images (arrow) as the surrounding liver parenchyma begins to enhance.
Fibrolamellar HCC, a rare tumor found in young patients without a history of pre-existing liver disease, is usually seen as a large, well-circumscribed focal lesion with low signal intensity on T1-weighted MR images and high signal on T2-weighted images. There is usually early heterogeneous contrast enhancement and a central radiating scar is seen in 80% of cases, which is usually hypointense relative to the remainder of the tumor[50].
Diffusion-weighted MRI may aid detection and characterization of focal liver lesions. Liver parenchyma is dark on DWI, whereas liver tumors (both benign and malignant) are depicted as high signal intensity masses[2751–53], although malignant liver tumors have lower apparent diffusion coefficients (ADCs) than benign cysts and hemangiomas[5153].
PET
18F-fluorodeoxyglucose (18F-FDG), the most widely used PET radiopharmaceutical in oncological imaging, can demonstrate the presence of malignant cells, because tumor cells utilize more glucose than normal tissue. FDG is phosphorylated via hexokinase to FDG 6-phosphate, which has a much slower rate of dephosphorylation compared to glucose 6-phosphate, and is progressively trapped inside metabolically active cells. The rate of FDG trapping in the cell is therefore proportional to the rate of glycolysis. However, differentiated hepatocytes have a relatively high glucose-6-phosphatase activity which allows dephosphorylation of FDG and subsequent leakage of the tracer from cells. Trojan and colleagues reported a sensitivity of 50% in detecting HCC, with increased FDG uptake associated with moderate or poor differentiation, high serum levels of AFP and large tumor burden[54]. A study by Khan and colleagues demonstrated a similar sensitivity of 55%[55]. Therefore, 18F-FDG is of very limited value in diagnosing HCC. This has led to the search for other PET tracers which may be used to screen for the presence of HCC. In a study by Ho and colleagues, dual imaging using 11C-acetate and 18F-FDG was assessed[56]. These authors found that the poorly differentiated HCCs were detected by 18F-FDG and the well-differentiated HCCs were detected by 11C-acetate, leading to a 100% sensitivity using both tracers. In addition, other liver malignancies such as cholangiocarcinomas and liver metastases did not demonstrate abnormal 11C-acetate uptake, although focal nodular hyperplasia did show mild increased uptake.
HCC detection
Despite technical improvements in all modalities used in the imaging of HCC, difficulties remain in detecting and characterizing small (≤ 2 cm) lesions in the cirrhotic liver.
A recent prospective study comparing imaging findings with pathological examination of the explanted liver in a pre-transplant population, found that US, MRI and CT had similar sensitivities for HCC detection on a lesion-by-lesion basis, although US performed slightly better on a patient-by-patient basis[13]. All three modalities missed small lesions. PET failed to detect any of the pathologically proven HCC lesions.
Currently, B-mode US is recommended in the screening of patients at risk of HCC, including patients with hepatitis B and cirrhosis[5]. In experienced hands, B-mode US can detect 80%-95% of lesions 3-5 cm in diameter and has 60%-80% sensitivity in the detection of lesions of 1 cm[5758]. CEUS can further improve the detection of lesions, even if < 2 cm[59–62], having the same sensitivity as helical CT[59]. Furthermore, the presence of ‘washout’ improves the specificity of CEUS in the detection of HCC by allowing the distinction from hemangiomas or hypervascular DNs and pseudo-nodules that can mimic HCC due to the presence of homogeneous arterial enhancement. In cases of elevated AFP with no nodule visible on US, CT is recommended to investigate the possibility of infiltrative HCC.
In a prospective study comparing CT findings and pathological examination of the explanted liver in a large pre-transplant population with cirrhosis, CT detected tumor in only 44% of patients in whom HCC was found at pathological examination[63]. However, a variety of CT protocols were used and if only triphasic helical CT (comprising non-contrast, hepatic arterial and portal venous phases) was considered, prospective tumor detection improved to 59%. This study employed single slice helical CT technology and current use of MDCT technology is likely to have improved tumor detection rate.
The sensitivity of MRI in the detection of HCC decreases in cases with advanced cirrhosis[14], and the presence of ascites can generate significant artifacts. Additional difficulties can arise in distinguishing well-differentiated HCC from regenerative and DNs[49]. Frequently, patients with cirrhosis have transient foci of enhancement on arterial phase imaging that cannot be visualized on any other pulse sequence[6465]. While these are usually benign and likely to represent small arteriovenous shunts or dysplastic nodules, small HCCs can exhibit an identical appearance in up to 13%[66]. Although some advocate the use of SPIO contrast agents to improve sensitivity and specificity, this has had mixed results[13]. HCCs show variable enhancement with SPIO depending on the degree of differentiation and number of functioning Kupffer cells[15], and often in the context of cirrhosis and small tumors (≤ 1.5 cm) gadolinium enhanced imaging is preferred[67]. The combination of gadolinium and SPIO, however, provides a dual contrast assessment of HCC that is more effective than either contrast agent alone[2268]. Given the rapid doubling time of most HCCs, close follow-up of cases with transient foci of enhancement may provide an additional strategy[69].
CHOLANGIOCARCINOMA
Cholangiocarcinomas are generally classified as intrahepatic, hilar or extrahepatic. Most cholangiocarci-nomas, up to 60%, occur at the liver hilus[6]. Hilar and extrahepatic cholangiocarcinomas typically present with biliary duct obstruction. A multi-modality imaging approach, including endoscopic retrograde cholangiopancreatography (ERCP), US, CT, MRI and magnetic resonance cholangiopancreatography (MRCP), is used in patients with a suspected malignant cause for biliary obstruction.
Although the identification of a mass with B-mode US varies from 37% to 87%[7071], B-mode US is a highly sensitive method for confirming biliary duct dilatation, localization of the site of obstruction and excluding gallstones[72]. Peripheral cholangiocarcinomas can appear as a hyper- or hypoechoic mass similar to HCC, and distal lesions can present with intra- and extra-hepatic duct dilatation[73]. On CEUS, a cholangiocarcinoma can behave in the same way as a hypovascular metastasis and result in an area of hypoenhancement in the delayed phase following contrast administration.
Dual phase (arterial dominant and portal venous dominant) contrast-enhanced CT may be helpful in the assessment of cholangiocarcinoma. Small central tumors are often difficult to detect because of their size, infiltrating nature and iso- or subtle hypo-attenuation compared to adjacent liver parenchyma, and marked dilatation of the intrahepatic biliary tree may be the only evidence of disease on CT. Visualized central tumors are usually hypoattenuating masses that enhance poorly in the arterial and portal venous phases (Figure 6). Enhancement may be seen on delayed images, thought to be due to retention of contrast within the dense fibrous stroma[74]. The peripheral form of cholangiocarcinoma may appear as a nodular, hypoattenuating lesion surrounded by dilated biliary ducts. Previous studies have shown that CT tends to underestimate disease extent, accurately assessing tumor resectability in only 60% of patients in one study[75]. However, a more recent study by Aloia and colleagues showed that high resolution helical CT with 2.5 mm slice reconstructions correctly predicted tumor resectability in 17 of 18 patients who underwent laparotomy for cholangiocarcinoma[76].
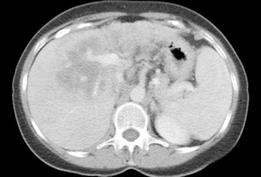
Figure 6 Cholangiocarcinoma.
Contrast enhanced CT in the portal venous-dominant phase demonstrates a poorly-enhancing central liver lesion. The hypodensity appears to follow some of the biliary radicals.
The appearance of intrahepatic cholangiocarcinoma on MRI is non-specific, typically appearing hypointense on T1-weighted and mildly hyperintense on T2-weighted sequences. More than 50% of patients will have satellite or remote intrahepatic tumor nodules[77]. Following gadolinium chelate contrast, peripheral enhancement can be seen initially and delayed equilibrium enhancement can show dense contrast retention adding to the detection and characterisation of these lesions[78]. A central scar may also be evident.
Extrahepatic cholangiocarcinomas have similar signal characteristics to intrahepatic tumors on MRI. The majority of extrahepatic cholangiocarcinomas show heterogeneous enhancement following gadolinium with a gradual increase to a peak on delayed imaging[79]. Peripheral early enhancement is rarely seen. Dynamic contrast-enhanced MRI is comparable to angiography in the assessment of the portal vasculature invasion in patients with cholangiocarcinoma[80].
MRCP is the non-invasive imaging study of choice to investigate the biliary tree. MRCP has an excellent overall sensitivity and specificity for demonstrating the level and the presence of biliary obstruction, with slightly lower sensitivity for detecting stones or differentiating malignant from benign obstruction than ERCP[81]. Evaluation of non-dilated biliary ducts, however, remains problematic as ductal distension is likely an important factor in visualizing subtle biliary abnormalities, particularly given the relatively limited spatial resolution of MRCP techniques.
Cholangiocarcinoma can present as a stenosing/sclerosing process resulting in ductal stricturing, usually with shouldered margins. Cholangiocarcinomas can also result in focal duct wall thickening or nodule formation or as an intraductal papillary lesion.
MRCP permits visualization of ductal irregularity and narrowing as well as intraluminal tumor extent, although bile duct involvement can be underestimated with MRCP[82]. Compared to ERCP, however, MRCP can more accurately determine the suprahilar tumor extension[82]. Furthermore, the combination of MRI and MRCP imaging allows the evaluation of the presence and size of extraluminal extension of the tumor and liver parenchyma invasion, leading to a greater accuracy in preoperative staging (Figure 7A and B).
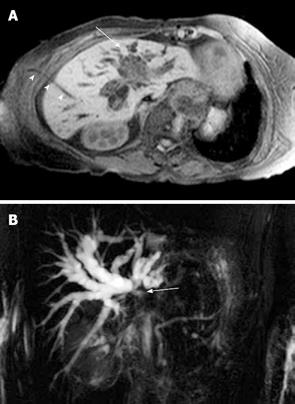
Figure 7 Cholangiocarcinoma.
A: Contrast-enhanced T1-weighted magnetic resonance sequence shows irregular central liver mass (arrow) which enhances poorly in comparison to the adjacent liver parenchyma. There is intrahepatic bile duct dilatation and an external biliary drain (arrowheads); B: Maximum intensity projection image of the MRCP study of the same case as shown in Figure 7A demonstrates marked intrahepatic bile duct dilatation and abrupt cut-off at the liver hilum (due to obstructing tumour) (arrow) with non-visualisation of the extrahepatic ducts.
There are few data regarding the use of 18F-FDG PET in the diagnosis of cholangiocarcinomas. Anderson and colleagues reported a sensitivity of 85% for the nodular morphological type of cholangiocarcinoma but only 18% for the infiltrating type[83]. In addition, sensitivity for metastatic disease was 65%. All of these metastases were unsuspected on other imaging and led to a change in management in all of the patients. Studies have shown that tumors of the tubular pathological type with high cell density and limited mucin production[84], and peripheral[85], nodular[83] lesions are better detected than hilar cholangiocarcinomas, infiltrating lesions and mucinous tumours. Dynamic 18F-FDG PET may have a useful role in the detection of cholangiocarcinoma in patients with primary sclerosing cholangitis where other modalities such as CT, MRI and US are of limited value[86].
LIVER METASTASES
20%-25% of patients with known solid malignant tumors have hepatic metastases at the time of diagnosis. The incidence of solid benign liver tumors is around 20%[87], thus in patients with known malignancy, 20%-25% of lesions under 2 cm are benign[88]. The most frequent benign lesion is hemangioma with a prevalence of 7%-21%, followed by focal nodular hyperplasia (FNH) with a prevalence of up to 3%[87]; other benign lesions are far rarer. Hence, imaging techniques of the liver in patients with malignancy not only require high sensitivity, but also the ability to reliably differentiate malignant from benign tumors.
CEUS improves the sensitivity of US in the detection of individual lesions by about 20% in comparison to baseline, with a resultant sensitivity of 82%-86%, comparable to contrast-enhanced CT and MRI with non-specific gadolinium chelates[89–92]. Although metastases show characteristic features in the three phases after contrast injection, the hypoenhancement of solid lesions or ‘wash-out’ in late phase is the key to distinguishing malignant from benign lesions. Benign lesions demonstrate sustained enhancement in the portal and late phases (Figure 8). The appearance of metastases in the arterial phase of enhancement depends on the extent of arterial perfusion. For example, the hypervascular metastases in neuroendocrine carcinomas demonstrate homogenous enhancement, whereas hypovascular metastases, commonly arising from breast, lung, colonic or pancreatic primaries, may only demonstrate rim enhancement (Figure 9). For benign lesions, the arterial phase is of particular use in the further characterisation of the lesion: hemangiomas characteristically demonstrate peripheral nodular enhancement with gradual filling in of the lesion in the portal venous phase, whereas FNH may demonstrate the typical ‘spoke-wheel’ arterial pattern with centrifugal filling early in the arterial phase via a dominant filling artery[93].
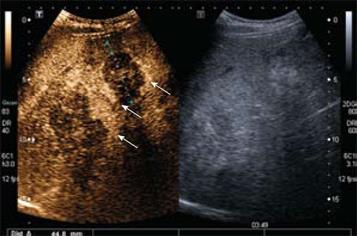
Figure 8 Hepatic metastases.
Delayed phase CEUS (3 min 49 s following contrast injection) demonstrates two hypoechoic liver metastases following contrast washout with a residual peripheral rim of enhancement (arrows). Note that the adjacent image demonstrates that these lesions are echogenic on grey-scale and are poorly defined. The margins are better defined with contrast enhancement (right hand image).
Figure 9 Hepatic metastases.
CEUS obtained in the arterial phase (17 s following contrast injection) demonstrates enhancement of a hypervascular liver metastasis from a colorectal primary tumor. It is the washout with peripheral rim enhancement in the delayed phase (see Figure 8) which helps confirm this to be a metastasis.
Pseudo liver tumors, such as focal fatty infiltration or focal fatty sparing, exhibit the same characteristics as the surrounding liver parenchyma and thus remain isoechoic to normal liver tissue[93].
Appearances of hypovascular liver metastases on MDCT are similar to those seen on CEUS. Lesions are typically rounded and uniformly hypoattenuating on portal venous phase CT imaging. They may demonstrate peripheral rim enhancement on late arterial phase images[94]. Hypervascular hepatic metastases typically demonstrate homogeneous late arterial enhancement on MDCT although inhomogeneous enhancement may be seen due to areas of necrosis or hemorrhage[95].
MRI typically demonstrates liver metastases as hypointense on T1-weighted images and hyperintense on T2-weighted images[48]. Most liver metastases show restricted water diffusion on DW images and therefore appear as hyperintense masses[52]. While it is generally accepted that CE MRI increases the sensitivity for detecting metastases, the sensitivity of unenhanced MRI for liver metastases being in the region of 70% compared to 90% following contrast enhancement and being comparable to, if not better than, CT[26], there is some debate on which contrast agent to use[13], and indeed, on whether detection rates remain comparable to CT with 64 plus multi-detector CT[13].
SPIO-enhanced MRI can be most useful in patients with colorectal carcinoma when being considered for hepatic resection on the basis of limited metastatic disease[96]. In addition, combining gadolinium with SPIO provides advantages for the depiction of liver metastases: hypovascular metastases are better identified on SPIO, whereas hypervascular metastases are better depicted and characterized with gadolinium[97].