Published online May 14, 2009. doi: 10.3748/wjg.15.2234
Revised: March 21, 2009
Accepted: March 28, 2009
Published online: May 14, 2009
AIM: To investigate the effect of 5-allyl-7-gen-difluoromethylenechrysin (ADFMChR) on apoptosis of human liver carcinoma HepG2 cell line and the molecular mechanisms involved.
METHODS: HepG2 cells and L-02 cells were cultured in vitro and the inhibitory effect of ADFMChR on their proliferation was measured by MTT assay. The apoptosis of HepG2 cells was determined by flow cytometry (FCM) using propidium iodide (PI) fluorescence staining. DNA ladder bands were observed by DNA agarose gel electrophoresis. The influence of ADFMChR on the proxisome proliferator-activated receptor γ (PPARγ), NF-κB, Bcl-2 and Bax protein expression of HepG2 cells were analyzed by Western blotting.
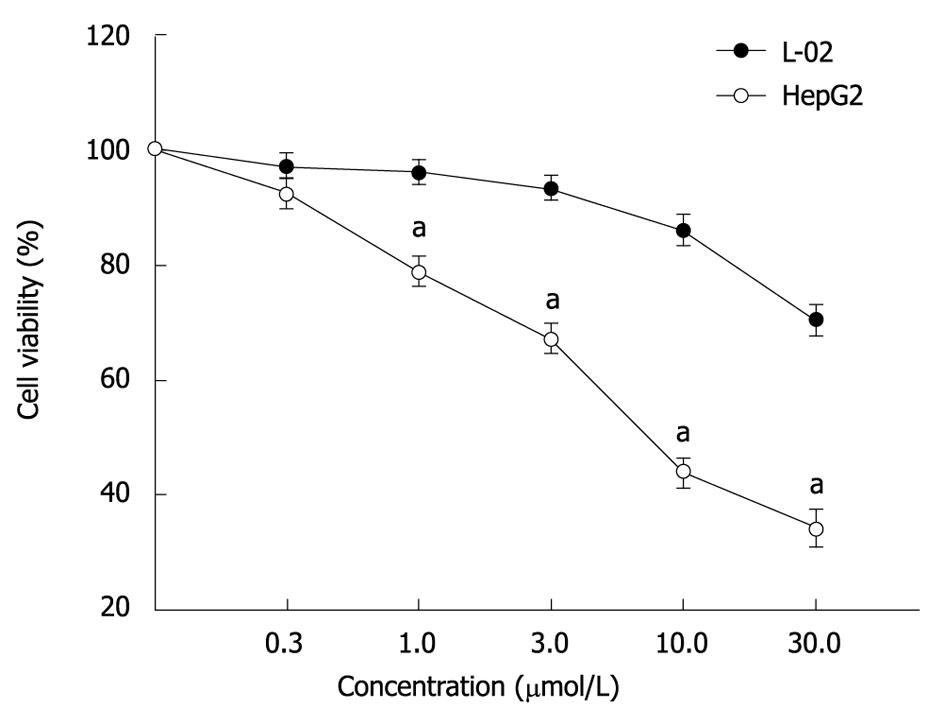
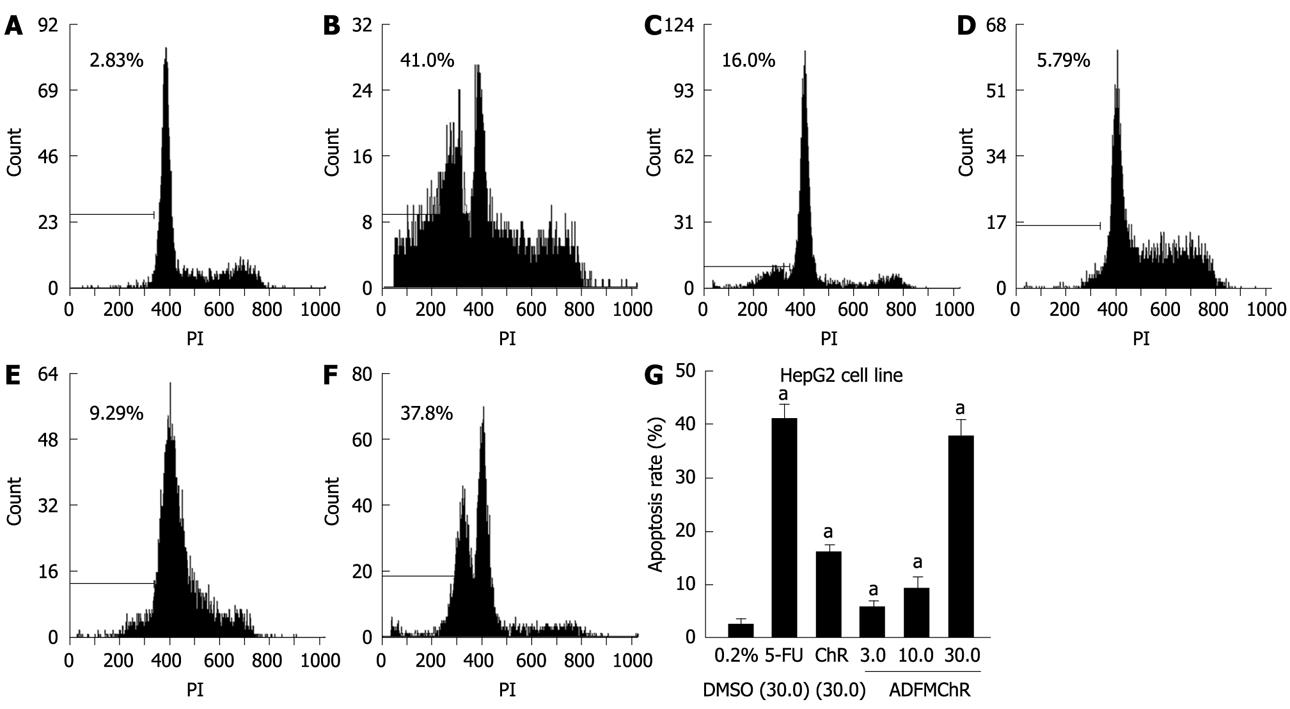
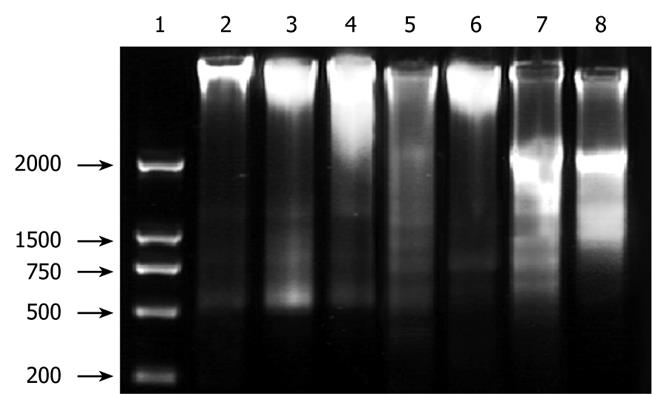
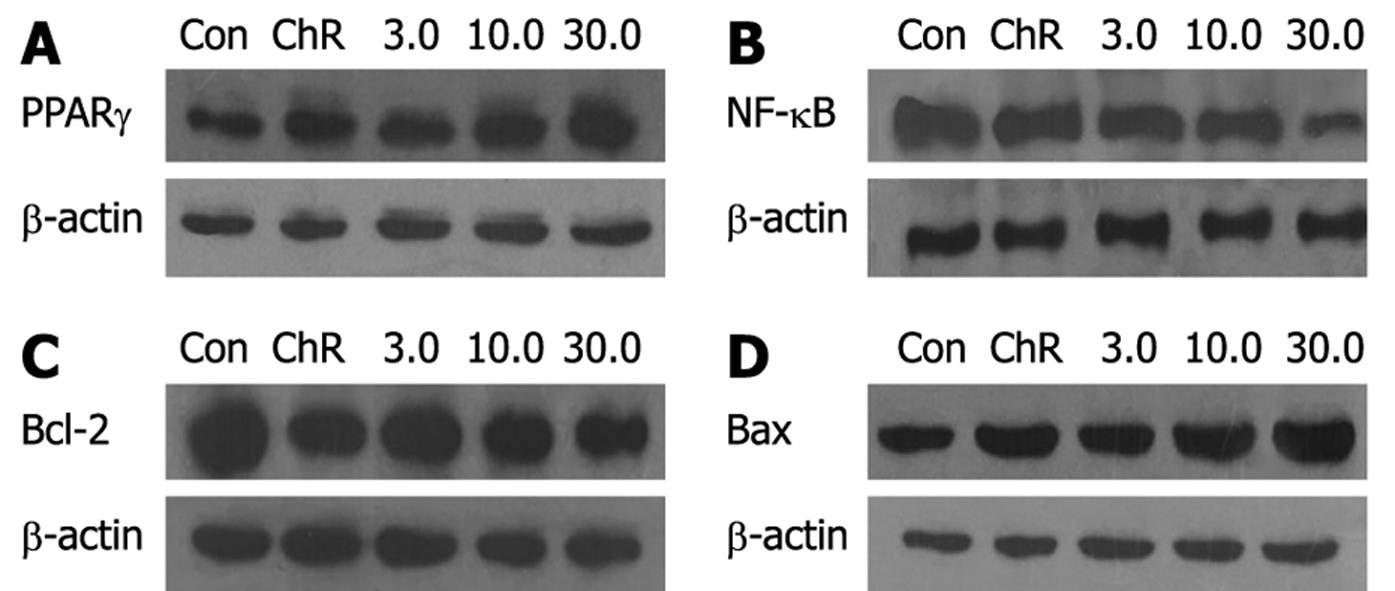
RESULTS: MTT assay showed that ADFMChR significantly inhibited proliferation of HepG2 cells in a dose-dependent manner, with little effect on growth of L-02 cells, and when IC50 was measured as 8.45 &mgr;mol/L and 191.55 &mgr;mol/L respectively, the potency of ADFMChR to HepG2 cells, was found to be similar to 5-fluorouracil (5-FU, IC50 was 9.27 &mgr;mol/L). The selective index of ADFMChR cytotoxicity to HepG2 cells was 22.67 (191.55/8.45), higher than 5-FU (SI was 7.05 (65.37/9.27). FCM with PI staining demonstrated that the apoptosis rates of HepG2 cells treated with 3.0, 10.0 and 30.0 &mgr;mol/L ADFMChR for 48 h were 5.79%, 9.29% and 37.8%, respectively, and were significantly higher when treated with 30.0 &mgr;mol/L ADFMChR than when treated with 30.0 &mgr;mol/L ChR (16.0%) (P < 0.05) and were similar to those obtained with 30.0 &mgr;mol/L 5-FU (41.0%). DNA agarose gel electrophoresis showed that treatment of HepG2 cells with 10.0 &mgr;mol/L ADFMChR for 48 h and 72 h resulted in typical DNA ladders which could be reversed by 10.00 &mgr;mol/L GW9662, a blocker of PPARγ. Western blotting analysis revealed that after 24 h of treatment with 3.0, 10.0, 30.0 &mgr;mol/L ADFMChR, PPARγ and Bax protein expression in HepG2 cells increased but Bcl-2 and NF-κB expression decreased; however, pre-incubation with 10.0 &mgr;mol/L GW9662 could efficiently antagonize and weaken the regulatory effect of 3.0, 30.0 &mgr;mol/L ADFMChR on PPARγ and NF-κB protein expression in HepG2 cells.
CONCLUSION: ADFMChR induces apoptosis of HepG2 cell lines by activating PPARγ, inhibiting protein expression of Bcl-2 and NF-κB, and increasing Bax expression.
- Citation: Tan XW, Xia H, Xu JH, Cao JG. Induction of apoptosis in human liver carcinoma HepG2 cell line by 5-allyl-7-gen-difluoromethylenechrysin. World J Gastroenterol 2009; 15(18): 2234-2239
- URL: https://www.wjgnet.com/1007-9327/full/v15/i18/2234.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.2234
Human liver carcinoma is the fifth most common cancer in the world and is responsible for > 600 000 deaths annually[1]. The majority of patients with hepatocellular carcinoma die within 1 year after the diagnosis. At present, the treatment of hepatocellular carcinoma mainly includes surgery and chemotherapy, but the curative effects of the existing chemotherapeutic drugs are not good enough and they have numerous side effects. Therefore, searching for highly efficient antitumor drugs remains a hot research area.
Peroxisome proliferator-activated receptor γ (PPARγ) is a member of the nuclear hormone receptor superfamily; a ligand-dependent transcription factor that plays an important role in lipid and glucose metabolism[23]. In recent years, over-expression of PPARγ has been found in a variety of tumor cells and PPARγ agonists can induce apoptosis[45]. It has been reported that chrysin (ChR) and its derivatives activate PPARγ to inhibit COX-2 and iNOS activity through various pathways distinguished from thiazolidones[6].
Chrysin (5,7-dihydroxy flavone, ChR) is a kind of flavonoid with pharmacological activities and is widely distributed in the plant kingdom. It has been demonstrated that ChR can markedly inhibit the growth of human thyroid cancer cells[7], and has an effect on the inhibition of proliferation and induction of apoptosis in human myeloid leukemia cells as well[89]. Comte et al[10] reported that, through alkylation, the hydrophobicity of ChR is increased, its KD value decreased, and its binding affinity towards P-glucoprotein (P-gp) enhanced. We confirmed that a series of B-ring trifluoromethylated derivatives of ChR markedly inhibited the growth of HT-29 and SGC-7901 cell lines[11] and that 5, 7-dihydroxy-8-nitrochrysin (NOChR) had an inhibitory effect on subcutaneously transplanted primary Lewis lung carcinoma in mouse and its spontaneous metastasis in a dose-dependent manner[12]. Our previous study showed that the suppressive effect of 5-allyl-7-gen-difluoromethylenechrysin (ADFMChR) on proliferation of the CoC1 cell line was stronger than that of ChR[13]. However, whether ADFMChR has antitumor effects on human liver carcinoma is unknown.
In this study, we aimed to investigate whether ADFMChR induces apoptosis of HepG2 cell line by activation of PPARγ and whether NF-κB, Bcl-2 and Bax are involved in this mechanism, thereby providing a new opportunity for research with regard to the pharmaceutical prevention and cure of human liver cancer.
HepG2 cells and L-02 cells were purchased from the China Center for Type Culture Collection (CCTCC) and were cultured in RPMI-1640 medium with 10% fetal bovine serum. Antibiotics added were 100 units/mL penicillin and 100 &mgr;g/mL streptomycin (Life Technologies, Inc) at 37°C in a 5% CO2 incubator.
ADFMChR was synthesized in the Medical College, Hunan Normal University as previously described[14]; with a molecular weight of 344 ku, characteristic yellow crystals and purity of 99.0%, its molecular formula is C19H14O4F2. ADFMChR was dissolved in dimethyl sulfoxide (DMSO), diluted with phosphate buffer solution (PBS), and finally prepared as 2 mmol/L storage solution after filtration sterilization. RPMI-1640, ChR, MTT and DMSO were purchased from Sigma Company. 5-fluorouracil (5-FU) was from Jinghua Pharmaceutical Corporation Ltd, Nantong. Ladder Apoptotic DNA Ladder Detection Kit was purchased from Bodataike Company, Beijing. Mouse anti-human Bcl-2 monoclonal antibody, mouse anti-human NF-κB monoclonal antibody, mouse anti-human Bax monoclonal antibody and rabbit anti-human PPARγ polyclonal antibody were purchased from Santa Cruz Biotechnology, Inc (U.S.A).
HepG2 cells or L-02 cells were seeded in a 96-well plate at a density of 1.0 × 104 cells/well as previously described[15]. Drugs of different concentrations were added to each well and cultured for 48 h, followed by incubation with 5 mg/L MTT for 4 h. The supernatant was removed after centrifugation. Finally, 100 &mgr;L of DMSO was added and absorbance at 490 nm wavelength (A490) was measured by means of Enzyme-labeling instrument (EX-800 type). Relative cell proliferation inhibition rate (IR) = (1 - average A490 of the experimental group/average A490 of the control group) × 100%.
HepG2 cells were treated with serum-free medium for 24 h, followed by treatment with media containing 3.0, 10.0, 30.0 &mgr;mol/L ADFMChR, 30.0 &mgr;mol/L ChR and 30.0 &mgr;mol/L 5-FU for 48 h, respectively. Cells were collected and prepared as a single cell suspension by mechanical blowing with PBS, washed with cold PBS twice, fixed with 700 mL/L alcohol at 4°C for 24 h, stained with PI and cell apoptosis was detected using FCM (American BD Company, FACS420).
As previously described[16], cells were cultured with 10.0 &mgr;mol/L ADFMChR and 10.0 &mgr;mol/L ADFMChR plus 10.0 &mgr;mol/L GW9662, a PPARγ antagonist, for 0, 24, 48 and 72 h, respectively. Cells were washed twice with PBS and DNA was extracted with an Apoptotic DNA Ladder Detection Kit according to the manufacturer’s instructions. The extracted DNA was kept at 4°C overnight. Then 8.5 &mgr;L of DNA sample was mixed with 1.5 &mgr;L of 6 × Buffer solution, electrophoresed on 20.0 g/L agarose gel containing ethidium bromide at 40 V, and observed through DBT-08 gel image analysis system.
As previously described[17], cells were treated with 3.0, 10.0, 30.0 &mgr;mol/L ADFMChR and 30.0 &mgr;mol/L ChR for 24 h, respectively. Cells were collected, washed three times with PBS, lysed in cell lysis buffer containing 0.1 mol/L NaCl, 0.01 mol/L Tris-Cl (pH 7.6), 0.001 mol/L EDTA (pH 8.0), 1 &mgr;g/mL Aprotinin, 100 &mgr;g/mL PMSF, and then centrifuged at 13 000 ×g for 10 min at 4°C. The extracted protein sample (25 &mgr;g total protein/lane) was added in the same volume of sample buffer and subjected to denaturation at 100°C for 10 min, then electrophoresed on 100 g/L or 60 g/L SDS-PAGE at 100 mA for 3 h, and finally transferred onto PVDF membrane. The PVDF membrane was treated with TBST containing 50 g/L skimmed milk at room temperature for 2 h, followed by incubation with the primary antibodies PPARγ, NF-κB, Bcl-2 and Bax (1:500 dilution), respectively, at 37°C for 2 h or at 4°C overnight. After being washed with TBST for 30 min, the corresponding secondary antibody was added and incubated at room temperature for 1 h. The membrane was then washed three times for 15 min each with TBST. Fluorescence was visualized with enhanced chemiluminescence (Amersham, Arlington Heights, IL). The results were analyzed with Image analyzer and the product of area and optical density was expressed as integral absorbance (IA).
Experimental data in each group were presented as mean ± SD. Analysis of variance was performed with SPSS software for windows 15.0 by using one way ANOVA and pairwise comparison with Student’s t test. P < 0.05 was considered statistically significant.
MTT assay showed that ADFMChR markedly inhibited proliferation of HepG2 cells in a dose-dependent manner (Figure 1), with little effect on growth of L-02 cells, and when IC50 were measured as 8.45 &mgr;mol/L and 191.55 &mgr;mol/L, respectively, the potency of ADFMChR to HepG2 cells was found to be similar to 5-fluorouracil (5-FU, IC50 was 9.27 &mgr;mol/L). The selective index of ADFMChR cytotoxicity to HepG2 cells was 22.67 (191.55/8.45), higher than 5-FU (SI was 7.05 (65.37/9.27).
FCM with PI staining demonstrated that the apoptosis rates of HepG2 cells treated with 3.0, 10.0 and 30.0 &mgr;mol/L ADFMChR for 48 h were 5.79%, 9.29% and 37.8%, respectively, and were significantly higher when treated with 30.0 &mgr;mol/L ADFMChR than when treated with 30.0 &mgr;mol/L ChR (16.0%) (P < 0.05) and were similar to those obtained with 30.0 &mgr;mol/L 5-FU (41.0%) (Figure 2).
DNA agarose gel electrophoresis showed that treatment of HepG2 cells with 10.0 &mgr;mol/L ADFMChR for 48 h and 72 h resulted in typical DNA ladders, which could be eliminated or attenuated by treating with 10.0 &mgr;mol/L ADFMChR plus 10.0 &mgr;mol/L GW9662 for 48 h and 72 h (Figure 3).
Western blotting analysis showed that the relative densities of PPARγ, NF-κB, Bcl-2 and Bax protein bands of HepG2 cells treated with 3.0, 10.0, 30.0 &mgr;mol/L ADFMChR for 24 h were 109.3%, 126.4%, 147.7% and 92.9%, 89.0%, 72.4% and 94.1%, 85.5%, 77.3% and 106.8%, 116.3%, 125.7% of the HepG2 cells not treated with ADFMChR, respectively (P < 0.05) (Figure 4). This indicates that ADFMChR can increase the PPARγ and Bax protein expression and decrease NF-κB and Bcl-2 protein expression.
Western blotting analysis demonstrated that when HepG2 cells were pre-incubated with 10.0 &mgr;mol/L GW9662, a blocker of PPARγ, for 30 min, the effects of 3.0, 30.0 &mgr;mol/L ADFMChR on PPARγ protein expression and NF-κB protein expression were antagonized or weakened (Figure 5), suggesting that the effects of ADFMChR on up-regulation of PPARγ protein expression and down-regulation of NF-κB protein expression were associated with the activation of PPARγ.
Tumorigenesis and tumor progression are strongly associated with abnormal apoptosis. A number of antitumor drugs exert their therapeutic effects by inducing or promoting apoptosis. Enhancing the antitumor effect of existing anticancer drugs, but not to increase its toxicity, is the aim of current anticancer research. There is evidence to support the concept that luteolin, apigenin and chrysin have great potential to be developed into novel cancer preventative agents[18]. Our previously research showed that ADFMChR potently inhibited the proliferation of ovarian cancer CoC1 cells in a dose-dependent manner[19], and could induce apoptosis of SMMC-7721 cells in vitro, with its mechanism possibly associated with G1 phase cell cycle arrest[20]. Li et al[19] and Xu et al[21] found that the ability of ADFMChR to induce induction of apoptosis in CoC1 cells may be mediated by activation of PPARγ, sequentially accompanied by reducing NF-κB and Bcl-2 levels and increasing Bax expression. Our experiment was to investigate the apoptosis of human liver carcinoma HepG2 cell line induced by ADFMChR and to provide experimental evidence for its application as an antitumor drug.
Apoptosis usually results in typical morphological and biochemical characteristics, including condensed chromatin in cells, appearance of apoptotic bodies, presence of hypodiploid peak in FCM analysis and DNA ladder bands on agarose electrophoresis[2223]. In this study, treatment of HepG2 cells with ADFMChR resulted in formation of DNA ladder bands and the appearance of marked hypodiploid peak. Thus, this experiment suggested that ADFMChR can induce apoptosis of human liver carcinoma HepG2 cell line in vitro.
PPARγ is a kind of ligand-activated nuclear transcription factor belonging to a nuclear receptor superfamily and has been implicated in metabolic diseases and is associated with cell proliferation, differentiation and apoptosis[24]. NF-κB inhibits apoptosis, promotes cell survival and reduces the expression of Bcl-2[25]. Chen et al[26] confirmed that PPARγ ligands may markedly inhibit NF-κB expression and reduce Bcl-2 expression leading to inhibited cell growth and induction of apoptosis of colonic cancer HT-29 cell line by activation of PPARγ. Liang et al[6] have recently shown that ChR is activated in different ways with thiazolidinones, and PPARγ inhibits activation of cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase. 8-bromo-7-methoxychrysin (BrMChR) or 5,7-dihydroxy-8-nitrochrysin (NOChR) induce apoptosis of SGC2-7901 cell line by activating PPARγ[2728]. In order to find out whether ADFMChR decreases NF-κB and Bcl-2 protein expression to induce apoptosis of HepG2 cells by activation of PPARγ, we pre-incubated HepG2 cells with GW9662, a selective antagonist of PPARγ, and observed the effect of ADFMChR on apoptosis and PPARγ and NF-κB protein expression of HepG2 cells. Our results showed that preincubation with GW9662 could effectively antagonize ADFMChR-induced apoptosis of HepG2 cells and down-regulation of NF-κB protein expression, suggesting that apoptosis of HepG2 cells induced by ADFMChR is dependent on activation of PPARγ.
Apoptosis is a complex process involving several genes, such as Bcl-2, Bax, and great attention has been given to the Bcl-2 family. The Bcl-2 family can positively and negatively regulate apoptosis[29]. Bcl-2 and Bax are two members of the Bcl-2 family, and play different roles in programmed cell death[30]. When Bax is over-expressed in cells, apoptosis in response to death signals is accelerated, leading to its designation as a death agonist[31]. When Bcl-2 is over-expressed it heterodimerizes with Bax and death is repressed[31]. Therefore, the ratio of Bcl-2 to Bax is important in determining susceptibility to apoptosis[30]. The results in this study confirmed that Bcl-2 expression in non-treated HepG2 cells was higher than in those treated with 3.0, 10.0, 30.0 &mgr;mol/L ADFMChR for 24 h; in contrast, Bax expression was lower. Thus, the ratio of Bcl-2 to Bax in HepG2 cells treated with ADFMChR was lower than that of non-treated HepG2 cells, which indicated that ADFMChR-induced HepG2 cells apoptosis was associated with down-regulation of Bcl-2 expression, up-regulation of Bax expression and reduction of the ratio of Bcl-2 to Bax.
In summary, ADFMChR possesses stronger anti-hepatic cancer effect in vitro than parent compound ChR, and was similar to 5-FU, and it exerts its apoptotic effect by activation of PPARγ, down-regulation of NF-κB and Bcl-2 protein expression, up-regulation of Bax protein expression, and reduction of the ratio of Bcl-2 to Bax. ADFMChR might be a promising candidate for the development of antitumor drugs.
Human liver carcinoma is the fifth most common cancer in the world. Unfortunately, the disease is often diagnosed at a late stage. For these patients, medical treatments, including chemotherapy, chemoembolization, ablation, and proton beam therapy, are not adequate. Most patients show disease recurrence that rapidly progresses to the advanced stages with multiple intrahepatic metastases and their 5-year relative survival rate is only 7%. Clearly, there is an urgent need for new therapies for this disease.
Enhancing the antitumor effect of existing anticancer drugs, but not to increase its toxicity, is the aim of current anticancer research. Natural compounds have been extensively studied and have shown anti-carcinogenic activities by interfering with the initiation, development and progression of cancer through the modulation of various mechanisms including cellular proliferation, differentiation, apoptosis, angiogenesis, and metastasis. Flavonoids are a group of polyphenolic substances widely distributed in the plant kingdom and present in human diets. Previous reports have shown that flavonoids (such as chrysin, apigenin) could inhibit the proliferation and induce apoptosis in tumor cells. In this study, the authors demonstrate that 5-allyl-7-gen-difluoromethylenechrysin (ADFMChR) could induce apoptosis of human liver carcinoma HepG2 cells in vitro by activation of PPARγ.
Recent research has shown that chrysin and its derivatives possess a strong anticancer effect. This is the first study to report that ADFMChR, the derivative of chrysin, has a greater suppressive effect on proliferation of HepG2 cells than that of chrysin, and induces apoptosis of HepG2 cells. These data support the idea that ADFMChR has great potential to be developed into novel cancer preventative agents.
This finding may provide a molecular basis for the clinically observed cancer-preventive effects of 5-allyl-7-gen-difluoromethylenechrysin (ADFMChR) and new clues for research about pharmaceutical prevention and cure of human liver carcinoma.
ADFMChR, a Chrysin derivative, which was taken as the principle compound to design and synthesize, was prepared by alkylation, methylation, and gen-difluoromethylation of chrysin, and was found to have stronger anticancer activities than parent compound chrysin.
The authors demonstrate that the effects of ADFMChR on induction of apoptosis in HepG2 cells may be associated with activation of PPARγ, sequentially accompanied by inhibition of protein expression of NF-κB and Bcl-2 and reduced ratio of Bcl-2 to Bax. The results provide a new idea for cure of human liver carcinoma.
| 2. | Hamm JK, el Jack AK, Pilch PF, Farmer SR. Role of PPAR gamma in regulating adipocyte differentiation and insulin-responsive glucose uptake. Ann N Y Acad Sci. 1999;892:134-145. |
| 3. | Rahimian R, Masih-Khan E, Lo M, van Breemen C, McManus BM, Dube GP. Hepatic over-expression of peroxisome proliferator activated receptor gamma2 in the ob/ob mouse model of non-insulin dependent diabetes mellitus. Mol Cell Biochem. 2001;224:29-37. |
| 4. | Leung WK, Bai AH, Chan VY, Yu J, Chan MW, To KF, Wu JR, Chan KK, Fu YG, Chan FK. Effect of peroxisome proliferator activated receptor gamma ligands on growth and gene expression profiles of gastric cancer cells. Gut. 2004;53:331-338. |
| 5. | Li M, Lee TW, Mok TS, Warner TD, Yim AP, Chen GG. Activation of peroxisome proliferator-activated receptor-gamma by troglitazone (TGZ) inhibits human lung cell growth. J Cell Biochem. 2005;96:760-774. |
| 6. | Liang YC, Tsai SH, Tsai DC, Lin-Shiau SY, Lin JK. Suppression of inducible cyclooxygenase and nitric oxide synthase through activation of peroxisome proliferator-activated receptor-gamma by flavonoids in mouse macrophages. FEBS Lett. 2001;496:12-18. |
| 7. | Yin F, Giuliano AE, Van Herle AJ. Growth inhibitory effects of flavonoids in human thyroid cancer cell lines. Thyroid. 1999;9:369-376. |
| 8. | Ko WG, Kang TH, Lee SJ, Kim YC, Lee BH. Effects of luteolin on the inhibition of proliferation and induction of apoptosis in human myeloid leukaemia cells. Phytother Res. 2002;16:295-298. |
| 9. | Woo KJ, Jeong YJ, Park JW, Kwon TK. Chrysin-induced apoptosis is mediated through caspase activation and Akt inactivation in U937 leukemia cells. Biochem Biophys Res Commun. 2004;325:1215-1222. |
| 10. | Comte G, Daskiewicz JB, Bayet C, Conseil G, Viornery-Vanier A, Dumontet C, Di Pietro A, Barron D. C-Isoprenylation of flavonoids enhances binding affinity toward P-glycoprotein and modulation of cancer cell chemoresistance. J Med Chem. 2001;44:763-768. |
| 11. | Zheng X, Cao JG, Meng WD, Qing FL. Synthesis and anticancer effect of B-ring trifluoromethylated flavonoids. Bioorg Med Chem Lett. 2003;13:3423-3427. |
| 12. | Xu YY, Zheng X, Zhu BY, Cao JG. Synthesis and antitumor effect of 5,7-dihydroxyl-8-nitriochrysin. Nanhua Daxue Xuebao (Medical Edition). 2004;32:283-289. |
| 13. | Li JL, Xie WY, Cao JG. The Effect of 5-allyl-7-gen-difluoromethylenechrysin on proliferation and apoptosis in ovarian cancer Cell Cultured in Vitro. Am J Chin Clin Med. 2005;7:323-326. |
| 14. | Zheng X, Cao JG, Liao DF, Zhu BY, Liu HT. Synthesis and anticancer effect of gem- difluoromethylenated chrysinderivatives. Chin Chem Lett. 2006;17:1439-1442. |
| 15. | Mauceri HJ, Hanna NN, Beckett MA, Gorski DH, Staba MJ, Stellato KA, Bigelow K, Heimann R, Gately S, Dhanabal M. Combined effects of angiostatin and ionizing radiation in antitumour therapy. Nature. 1998;394:287-291. |
| 16. | Leslie EM, Mao Q, Oleschuk CJ, Deeley RG, Cole SP. Modulation of multidrug resistance protein 1 (MRP1/ABCC1) transport and atpase activities by interaction with dietary flavonoids. Mol Pharmacol. 2001;59:1171-1180. |
| 17. | Liu H, Zang C, Fenner MH, Liu D, Possinger K, Koeffler HP, Elstner E. Growth inhibition and apoptosis in human Philadelphia chromosome-positive lymphoblastic leukemia cell lines by treatment with the dual PPARalpha/gamma ligand TZD18. Blood. 2006;107:3683-3692. |
| 18. | Chen D, Chen MS, Cui QC, Yang H, Dou QP. Structure-proteasome-inhibitory activity relationships of dietary flavonoids in human cancer cells. Front Biosci. 2007;12:1935-1945. |
| 19. | Li HZ, Cao JG, Deng YA, Xu JH, Xie WY. Induction of apoptosis of human ovarian cancer CoC1 cells by 5-allyl-7-gen-difluoromethylenechrysin through activation of peroxisome-proliferator activated receptor-gamma. Zhonghua Yixue Zazhi. 2007;87:2914-2918. |
| 20. | Wang Y, Zhou XT, Cao JG, Zhou XT, Zhang L. Induction of growth inhibition and apoptosis in human liver cancer SMMC-7721 cell line by 5-allyl-7-gen-difluoromethylenechrysin. Changzhi Yixueyuan Xuebao. 2007;21:165-168. |
| 21. | Xu JH, Zheng X, Li HZ, Cao JG. Induction of apoptosis of human ovarian cancer CoCl cells by 5-allyl-7-gem-difluoromethylenechrysin. Zhongguo Bijiao Yixue Zazhi. 2008;18:5-9. |
| 22. | Chen YC, Shen SC, Lee WR, Hsu FL, Lin HY, Ko CH, Tseng SW. Emodin induces apoptosis in human promyeloleukemic HL-60 cells accompanied by activation of caspase 3 cascade but independent of reactive oxygen species production. Biochem Pharmacol. 2002;64:1713-1724. |
| 23. | Darzynkiewicz Z, Bedner E, Smolewski P. Flow cytometry in analysis of cell cycle and apoptosis. Semin Hematol. 2001;38:179-193. |
| 24. | Zhang YQ, Tang XQ, Sun L, Dong L, Qin Y, Liu HQ, Xia H, Cao JG. Rosiglitazone enhances fluorouracil-induced apoptosis of HT-29 cells by activating peroxisome proliferator-activated receptor gamma. World J Gastroenterol. 2007;13:1534-1540. |
| 25. | Deeb D, Jiang H, Gao X, Al-Holou S, Danyluk AL, Dulchavsky SA, Gautam SC. Curcumin [1,7-bis(4-hydroxy-3-methoxyphenyl)-1-6-heptadine-3,5-dione; C21H20O6] sensitizes human prostate cancer cells to tumor necrosis factor-related apoptosis-inducing ligand/Apo2L-induced apoptosis by suppressing nuclear factor-kappaB via inhibition of the prosurvival Akt signaling pathway. J Pharmacol Exp Ther. 2007;321:616-625. |
| 26. | Chen GG, Lee JF, Wang SH, Chan UP, Ip PC, Lau WY. Apoptosis induced by activation of peroxisome-proliferator activated receptor-gamma is associated with Bcl-2 and NF-kappaB in human colon cancer. Life Sci. 2002;70:2631-2646. |
| 27. | Xiang HL, Zheng X, Cao JG. Induction of apoptosis of human gastric carcinoma SGC-7901 cell line by 8-bromo-7-methoxychrysin. Zhongguo Yaolixue Tongbao. 2008;24:1370-1373. |
| 28. | Ai XH, Zheng X, Tang XQ, Sun L, Zhang YQ, Qin Y, Liu HQ, Xia H, Cao JG. Induction of apoptosis of human gastric carcinoma SGC-7901 cell line by 5, 7-dihydroxy-8-nitrochrysin in vitro. World J Gastroenterol. 2007;13:3824-3828. |
| 29. | Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322-1326. |
| 30. | Kirkin V, Joos S, Zornig M. The role of Bcl-2 family members in tumorigenesis. Biochim Biophys Acta. 2004;1644:229-249. |