Published online Mar 7, 2008. doi: 10.3748/wjg.14.1389
Revised: January 22, 2008
Published online: March 7, 2008
AIM: To determine end-stage pathologic changes in the liver of septic patients dying in the intensive care unit.
METHODS: Needle liver biopsies obtained immediately after death from 15 consecutive patients with sepsis and no underlying liver disease were subjected to routine histological examination. Liver function tests and clinical monitoring measurements were also recorded.
RESULTS: Liver biochemistries were increased in the majority of patients before death. Histology of liver biopsy specimens showed portal inflammation in 73.3%, centrilobular necrosis in 80%, lobular inflammation in 66.7%, hepatocellular apoptosis in 66.6% and cholangitis/cholangiolitis in 20% of patients. Mixed hepatitic/cholestatic type of liver injury was observed in 6/15 (40%) patients and hepatitc in 9/15 (60%). Steatosis was observed in 11/15 (73.3%) patients affecting 5%-80% of liver parenchyma. Among the histological features, the presence of portal inflammation in liver biopsy was associated with increased hospitalization in the ICU prior death (P = 0.026).
CONCLUSION: Features of hepatitis and steatosis are the main histological findings in the liver in the majority of patients dying from sepsis.
- Citation: Koskinas J, Gomatos IP, Tiniakos DG, Memos N, Boutsikou M, Garatzioti A, Archimandritis A, Betrosian A. Liver histology in ICU patients dying from sepsis: A clinico-pathological study. World J Gastroenterol 2008; 14(9): 1389-1393
- URL: https://www.wjgnet.com/1007-9327/full/v14/i9/1389.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.1389
Sepsis is the leading cause of death in patients admitted in intensive care units (ICUs)[1]. Hepatic dysfunction represents a common manifestation during the sepsis process, ranging from a mild elevation of serum bilirubin and/or liver enzymes to severe hepatic failure[2]. The pathophysiology of liver injury in sepsis is multifactorial and involves infection, drugs, metabolic disturbances and a broad spectrum of inflammatory mediators[34]. The detailed histopathological findings in the liver of patients at the late stages of sepsis remain relatively unknown as scarce data are available in humans.
In the present study, we aimed to assess the pathologic changes in liver core-needle biopsies obtained immediately after death from ICU septic patients and to correlate these with clinical and laboratory data.
We evaluated liver core-needle biopsies, obtained immediately (within 5 min) after death, from 15 patients who were admitted because of sepsis in the ICU of the Hippocration General Hospital of Athens. The modified sepsis criteria described by Bone et al[5] were applied, including clinical suspicion of sepsis, hyper- or hypothermia (rectal temperature > 38.5°C or < 35°C), tachycardia, and at least one of the following indications of altered organ perfusion and function: altered mental status, hypoxemia, elevated serum lactate level and oliguria. Exclusion criteria included pre-existing hepatobiliary disease, HIV infection and malignancies. In all patients, the standard treatment for the managing of sepsis was used according to the Surviving Sepsis Campaign guidelines[6]. Death was attributed to multiple organ failure due to sepsis and was defined as the loss of cardiac rhythm which could not be recovered after initial resuscitation. Support was withdrawn from patients with spontaneous cardiopulmonary arrest.
Informed consent was obtained from the patients' relatives in all cases. All liver biopsies were performed by the same ICU consultant physician within first 5 min from resuscitation refractory cardiac arrest as defined by three cycles of defibrillator shock. Biopsy specimens were fixed in 10% neutral buffered formalin and embedded in paraffin.
Causative infective agents, sites of infection, causes of admission in the ICU and/or transfer from another department, initial APACHE II Score as well as patients' demographics are shown in Table 1. Initial laboratory values were measured upon admission to ICU, while the most recent values obtained before the reported time of death, were considered as terminal laboratory values.
| No | Age | Sex | Diagnosis | Cause of hospitalization | APACHE II | Cultured sample | Infective agent |
| 1 | 58 | Male | Respiratory infection | ARD | 17 | Blood | Klebsiella |
| 2 | 70 | Male | Respiratory infection | ARD | 15 | Blood | Klebsiella |
| 3 | 84 | Male | Respiratory infection | Heart failure | 17 | Respiratory (BAL) | Enterobacter |
| 4 | 57 | Male | Respiratory infection | AMI | 23 | Venous catheter | Klebsiella |
| 5 | 50 | Male | Soft tissue infection | Soft tissue infection | 20 | Skin | Staphylococcus epidermidis |
| 6 | 74 | Male | Intra-abdominal sepsis | Peritonitis | 29 | Blood | Enterobacter |
| 7 | 45 | Female | Intra-abdominal sepsis | Peritonitis | 21 | Venous catheter | Klebsiella |
| 8 | 74 | Female | Peritonitis | Peritonitis | 30 | Midstream urine sample | Candida albicans |
| 9 | 86 | Male | Sepsis | Sepsis | 37 | Venous catheter | Pseudomonas |
| 10 | 75 | Male | Respiratory infection | ARD | 27 | Blood | Pseudomonas |
| 11 | 33 | Male | Soft tissue infection | Burn injury | 7 | Blood | Acinetobacter |
| 12 | 69 | Male | Peritonitis | Peritonitis | 17 | Blood | Klebsiella |
| 13 | 72 | Female | Respiratory infection | ARD | 15 | Blood | Klebsiella |
| 14 | 70 | Male | Respiratory infection | AMI | 26 | Venous catheter | Klebsiella |
| 15 | 77 | Male | Respiratory infection | Aneurysm | 20 | Respiratory (BAL) | Candida |
Liver biopsies measured 17-46 (mean 25) mm in length and contained 6-28 (mean 12) portal tracts. Serial sections of liver biopsies were cut at 4-&mgr;m intervals and mounted on slides coated with poly-L-lysine. Sections were stained with hematoxylin and eosin and with special histochemical stains (reticulin, Masson Trichrome, PAS, PAS-diastase, Prussian blue and Gram) for detailed morphological evaluation by two independent pathologists. Type, zonal location and amount of steatosis were recorded for each case. The latter was graded as 0 (< 5% of parenchymal involvement), 1 (5%-33%), 2 (> 33%-66%) and 3 (> 66%), according to previously published criteria[7]. Apoptotic (acidophilic) bodies were evaluated as the total count in the liver specimen and as the number per mm2 of examined liver tissue.
Data were analyzed using SPSS 13.0 software. All continuous variables are reported as the median and range. Relationships between categorical variables were tested with χ2 analysis, with Fisher’s exact test were applicable. Quantitative variables were analysed using non-parametric statistics (Mann-Whitney U test for two independent samples). P < 0.05 were considered statistically significant.
Patients' clinical parameters and laboratory data at entry and before death are shown in Table 2. At entry, 2 patients (13.3%) presented with elevated bilirubin levels, while increased AST was present in 7/15 (46.7%), abnormal ALT in 8/15 (53.3%), γGT in 6/15 (40%) and ALP in 2/15 (13.3%) patients. Before death, elevated serum levels of bilirubin, AST, ALT, γGT and ALP were observed in 8/15 (53.3%), 8/15 (53.3%), 7/15 (46.7%), 14/15 (93.3%) and 11/15 (73.3%) patients, respectively. In summary, an overall deterioration of liver-related biochemical parameters was observed in all our patients. With respect to the type of liver injury, 3/15 (20%) patients showed a clearly cholestatic biochemical profile, with substantial elevation of γGT and ALP and normal or nearly normal AST and ALT levels (< 1.5 the upper normal limit), while 12/15 (80%) patients exhibited a mixed hepatic and cholestatic profile.
| Variable | Initial | Final | P-value |
| Heart rate (/min) | 88 (50-150) | 86 (76-140) | NS |
| Systolic blood pressure (mmHg) | 130 (70-180) | 110 (60-150) | 0.017 |
| Diastolic blood pressure (mmHg) | 70 (45-80) | 50 (40-80) | 0.018 |
| PO2 (mmHg) | 154 (50-579) | 81 (45-120.8) | 0.008 |
| PCO2 (mmHg) | 42 (23-138) | 38 (28.1-81) | NS |
| pH | 7.36 (7.14-7.46) | 7.33 (6.93-7.46) | NS |
| HCO3 (mmol/L) | 24 (11.3-31.2) | 18.5 (11-32.4) | NS |
| Bilirubin (&mgr;mol/L) | 17.1 (6.84-107.73) | 23.94 (8.55-356.13) | NS |
| AST (nKat/L) | 600 (230-2230) | 650 (150-4160) | NS |
| ALT (nKat/L) | 680 (150-4170) | 450 (100-2730) | NS |
| ALP (nKat/L) | 1080 (45-3550) | 2250 (930-4630) | 0.019 |
| γGT (nKat/L) | 850 (180-6700) | 2030 (630-5720) | NS |
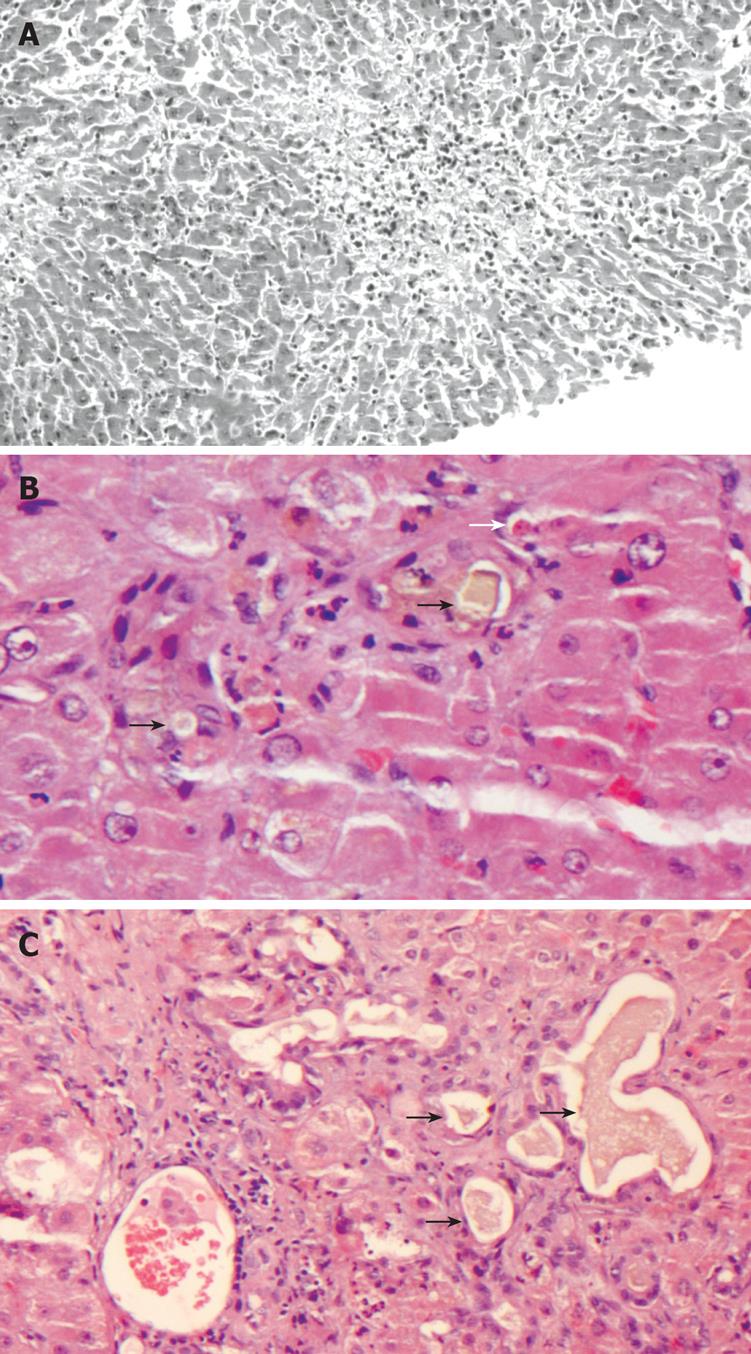
The observed histological findings in liver biopsy specimens were portal inflammation in 11/15 (73.3%, mixed in 8 patients and lymphohistiocytic in 3), centrilobular necrosis in 12/15 (80%), lobular inflammation in 10/15 (66.7%), hepatocellular apoptosis in 10/15 (66.6%), cholangitis in 1/15 (6.6%), cholangiolitis in 2/15 (13.3%) patients, canalicular cholestasis in 1/15 (6.6%) and ductular cholestasis in 2/15 (13.3%) patients (Figure 1A-C). Damage to bile duct epithelium was not observed.
The type of liver injury was defined as mixed or hepatitic according to overall histological features; 9/15 (60%) patients had the “hepatitic” type when only portal/lobular inflammation and/or centrilobular, frequently hemorrhagic, necrosis were present (Figure 1A), while 6/15 patients (40%) had “mixed” histological liver injury characterized by different combinations of biliary lesions (bile duct and/or ductular hyperplasia, cholangitis, cholangiolitis), cholestasis (canalicular and/or ductular) (Figure 1B and C), portal/lobular inflammation and centrilobular necrosis. Gram stains for bacteria and PAS for fungi were negative in all cases.
Steatosis was observed in 11/15 (73.3%) patients, of whom 5 had grade 1 (parenchymal involvement range: 5%-20%), 3 grade 2 (40%-50%) and 3 grade 3 (70%-80%). Four out of 11 patients had macrovesicular, 5/11 microvesicular and 2/11 mixed type of steatosis. Seven of 8 patients (87.5%) who developed hyperbilirubinemia before death had a mixed type of liver injury and one (12.5%) had a hepatitis-like liver injury, whereas those who did not have jaundice (n = 7) had no histological features of cholangitis/cholangiolitis. Apoptotic bodies, observed in 10/15 (66.6%) patients, were mainly seen in those who had lobular inflammation. The association of apoptosis with clinical and biochemical analysis at the closed time of death is seen in Table 3.
| Parameter | Liver apoptosis | P-value | |
| Yes (n = 10) | No (n = 5) | ||
| APACHE II | 17 (7-26) | 18.5 (15-37) | NS |
| Heart rate (min) | 86 (76-124) | 93 (76-140) | NS |
| Systolic blood pressure (mmHg) | 105 (60-150) | 112.5 (85-145) | NS |
| Diastolic blood pressure (mmHg) | 50 (40-80) | 55 (50-65) | NS |
| PO2 (mmHg) | 89.5 (49-120.8) | 77.5 (45-110) | NS |
| pH | 7.34 (7.09-7.46) | 7.29 (6.93-7.38) | NS |
| Bilirubin (mmol/L) | 23.94 (11.97-356.2) | 42.7 (8.55-140.22) | NS |
| Direct bilirubin (mmol/L) | 17.442 (5.13-230.166) | 53.1 (10.26-136.166) | NS |
| AST (nKat/L) | 683.47 (433.42-3717) | 516.8 (216.7-467) | NS |
| ALT (nKat/L) | 733.48 (100-2733.9) | 433.4 (250.1-1150.2) | NS |
| γGT (nKat/L) | 2283.8 (1083.6-5717) | 1467 (633.5-4500) | NS |
| ALP (nKat/L) | 2300.5 (1700.3-4634.3) | 1967.1 (933.2-3133) | NS |
In order to examine whether the histological findings were associated to the duration of ICU hospitalization we performed univariate regression analysis. Patients with portal inflammation in biopsy specimens had a significant long term ICU hospitalization compared to patients without evidence of portal inflammation [no portal inflammation vs portal inflammation: 3 (3-49) vs 25 (4-92) days Mann-Whitney U test, P = 0.026]. None of the other histological findings on liver found to be significantly associated with the length of ICU hospitalization.
In our study, we have observed that liver involvement in sepsis is common and characterized by either a hepatitic-like injury, observed in 60% of our patients, or a mixed, hepatitic and cholestatic, pattern of injury. Furthermore, steatosis was a common finding observed in 74% of our patients. Bacteriaemia and sepsis have been associated with abnormal liver biochemistry[2–48–10]. In the current study, elevated serum ALP and γGT levels were observed in 70% and 93% of our cases before death compared to 13% and 40% at admission, respectively. Similarly, increased bilirubin levels were seen in 53% of our cases before death compared to 13% at admission. In this respect, serum bilirubin, γGT and ALP may serve as indicators of clinical deterioration in septic critically ill patients. Other studies, however, have showed conflicting data regarding the prevalence of abnormal liver tests in patients with bacteraemia[28–10]. These discrepancies can be attributed to differences in patients' selection criteria as well as in the severity of the underlying disease.
Liver histology in sepsis has been evaluated mainly in animal studies and in postmortem human liver tissue where a significant time period had elapsed between autopsy and time of death[4911–13]. In jaundiced septic patients, three histological patterns have been described in a limited number of studies[4911]: canalicular cholestasis, usually most severe in zone 3, ductular cholestasis with inflammation and non-bacterial cholangitis associated with the toxic shock syndrome[12]. Intrahepatic cholestasis in septicemia could be attributed to many factors such as circulating endotoxin causing functional disorders in bile secretion, disturbances in bile canalicular contraction and ischemia[14–17].
The lack of detailed histological data has led clinicians to evaluate sepsis-related liver damage from the serum biochemical markers while no studies have addressed the correlation between laboratory values and pathologic findings. In the current study, we have obtained liver tissue from our septic patients immediately after death leading to the most accurate identification of sepsis-related liver pathology. Our findings showed that sepsis is characterized by a “hepatitic” like liver injury in 60% of our patients and a “mixed”, cholestatic and hepatitic type in 40% of them. Cholangitis and/or cholangiolitis were observed in a few patients. Additionally, ductular cholestasis, a sepsis-specific hepatic lesion[1112], suggesting increased risk of mortality[16] was identified only in two of our patients dying of sepsis, while canalicular cholestasis was present in one. Damage to bile duct epithelium has been previously observed in the liver of a female patient with E. coli septicemia[13]; however, it was not detected in any of our patients. The contrast between our histological findings and that of previous morphological studies where canalicular or ductular cholestasis predominated in the liver of jaundiced patients who died of sepsis, maybe attributed to the small number of cases examined. The presence of mixed, cholestatic and hepatitic, features in the majority of our patients with hyperbilirubinemia may alternatively be the result of the direct effect of endotoxin or a drug-induced injury. Centrilobular hemorrhagic necrosis, which was common in our cases, is a frequent finding in livers from patients with peripheral circulatory failure[19]. Among the above histopathological findings, the presence of portal inflammation, a common finding in chronic hepatitis, was associated with prolonged hospitalization that may reflect the effect of prolonged exposure to treatment medication.
Steatosis was evident in the liver of most of our septic patients. In previous studies, although steatosis was a common finding in the post-mortem liver of septic patients, its extent and type has not been examined in detail or commented on[4911]. The majority of our patients had moderate to severe fatty liver change comprising 40-80% of the liver parenchyma. It is known that sepsis and bacterial toxins may cause macrovesicular[20] or microvesicular steatosis[21] and hypoxia may play a role in these cases. Also, a wide variety of drugs and total parenteral nutrition may be responsible for the development of fatty liver change[20].
Liver apoptosis can be ascribed to a wide variety of individually or simultaneously acting underlying mechanisms. Tissue hypoxia, inflammatory mediators, free radicals, bacterial toxins and drug toxicity are all implicated in the above mechanisms[3202223]. The presence of apoptosis was much more common in patients with more severe liver histology, as defined by the intensity of portal and lobular inflammation as well as the presence of lobular necrosis and the ductular cholestatic lesions. Prognostic determinants cannot be inferred from the current study, since all our patients succumbed due to multiple organ failure. However, the absence of pre-existing liver disease, as well as the fact that all biopsies were optimally performed immediately after death, suggests that the subsequent pathologic evaluation demonstrates terminal sepsis-related histologic changes in the liver parenchyma.
Summarizing our results, the liver of end stage patients mainly shows histopathological features of hepatitis injury, with additionally cholestatic findings along with steatosis. Biopsies were performed almost immediately after each patients death giving the most precise evaluation of sepsis-induced liver injury. Further studies are needed to clarify the role of apoptosis and application of innovative drugs in sepsis-induced liver injury.
Liver histology in sepsis has only been evaluated in animal studies and postmortem autopsies where a significant time period had elapsed between autopsy and time of death. The lack of histological data has led clinicians to evaluate sepsis-related liver damage from the serum biochemical markers while no studies have addressed the correlation between laboratory values and pathologic findings.
In jaundiced septic patients, three histological patterns have been described in a limited number of studies: canalicular cholestasis, usually most severe in zone 3, ductular cholestasis with inflammation and non-bacterial cholangitis associated with the toxic shock syndrome. Intrahepatic cholestasis in septicemia could be attributed to many factors such as circulating endotoxin causing functional disorders in bile secretion, disturbances in bile canalicular contraction and ischemia.
In order to perform a more accurate identification of sepsis related liver pathology, investigators performed liver biopsies immediately after death in fifteen septic patients. In the present study portal/lobular inflammation and/or centrilobular necrosis along with steatosis were the main findings in septic patients. Steatosis, a common finding in the post-mortem liver of septic patients was moderate to severe comprising 40%-80% of the liver parenchyma. Previous studies have shown cholestatic damage in liver parenchyma in post mortem septic specimens in contrast to the present study where cholestatic injury was present but not as frequent. The main advantage of the present study is the identification of the exact nature of sepsis related tissue abnormalities dissociated from the postmortem cellular events.
The results of the present study further add to the understanding of the pathologic changes occurring in the liver of late stage septic patients. Further studies are needed in order to examine the exact role of cellular events in septic liver.
Portal inflammation: infiltration by inflammatory cells in portal tracts; Lobular inflammation: infiltration by inflammatory cells in hepatic lobules; Centrilobular necrosis: features of hepatocyte death in the center of lobules around terminal hepatic veins; Steatosis: accumulation of fat droplets in the cytoplasm of hepatocytes; Ductular cholestasis: accumulation of bile in bile ducts; Canicular cholestasis: accumulation of bile into bile canaliculi; cholestatic injury: damage in bile ducts due to cholestasis.
The exact nature of liver’s histological changes in septic patients described in this study thought to be the hotspot of the article.
| 1. | Jacobi J. Pathophysiology of sepsis. Am J Health Syst Pharm. 2002;59 Suppl 1:S3-S8. |
| 2. | Vermillion SE, Gregg JA, Baggenstoss AH, Bartholomew LG. Jaundice associated with bacteremia. Arch Intern Med. 1969;124:611-618. |
| 3. | Pastor CM, Billiar TR, Losser MR, Payen DM. Liver injury during sepsis. J Crit Care. 1995;10:183-197. |
| 4. | Banks JG, Foulis AK, Ledingham IM, Macsween RN. Liver function in septic shock. J Clin Pathol. 1982;35:1249-1252. |
| 5. | Bone RC, Fisher CJ Jr, Clemmer TP, Slotman GJ, Metz CA, Balk RA. Sepsis syndrome: a valid clinical entity. Methylprednisolone Severe Sepsis Study Group. Crit Care Med. 1989;17:389-393. |
| 6. | Dellinger RP, Carlet JM, Masur H, Gerlach H, Calandra T, Cohen J, Gea-Banacloche J, Keh D, Marshall JC, Parker MM. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004;32:858-873. |
| 7. | Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313-1321. |
| 8. | Sikuler E, Guetta V, Keynan A, Neumann L, Schlaeffer F. Abnormalities in bilirubin and liver enzyme levels in adult patients with bacteremia. A prospective study. Arch Intern Med. 1989;149:2246-2248. |
| 9. | Zimmerman HJ, Fang M, Utili R, Seeff LB, Hoofnagle J. Jaundice due to bacterial infection. Gastroenterology. 1979;77:362-374. |
| 10. | Vermillion SE, Gregg JA, Baggenstoss AH, Bartholomew LG. Jaundice associated with bacteremia. Arch Intern Med. 1969;124:611-618. |
| 11. | Lefkowitch JH. Bile ductular cholestasis: an ominous histopathologic sign related to sepsis and “cholangitis lenta”. Hum Pathol. 1982;13:19-24. |
| 12. | Lysova NL, Gurevich LE, Trusov OA, Shchegolev AI, Mishnev OD. Immunohistochemical characteristics of the liver in patients with peritonitis (early autopsy). Bull Exp Biol Med. 2001;132:1125-1129. |
| 13. | Vyberg M, Poulsen H. Abnormal bile duct epithelium accompanying septicaemia. Virchows Arch A Pathol Anat Histopathol. 1984;402:451-458. |
| 14. | Scheuer PJ, Lefkowitch JH. Liver biopsy interpretation. 7th ed. Philadelphia: Elsevier Saunders 2006; 331-332. |
| 15. | Hirata K, Ikeda S, Honma T, Mitaka T, Furuhata T, Katsuramaki T, Hata F, Mukaiya M. Sepsis and cholestasis: basic findings in the sinusoid and bile canaliculus. J Hepatobiliary Pancreat Surg. 2001;8:20-26. |
| 16. | Crawford JM, Boyer JL. Clinicopathology conferences: inflammation-induced cholestasis. Hepatology. 1998;28:253-260. |
| 17. | Lefkowitch JH. Histological assessment of cholestasis. Clin Liver Dis. 2004;8:27-40. |
| 18. | Moseley RH. Sepsis-associated cholestasis. Gastroenterology. 1997;112:302-306. |
| 19. | Burt AD. Liver pathology associated with diseases of other organs or systems. MacSween’s Pathology of the Liver. 5th ed. London: Churchill Livingstone 2007; 881-932. |
| 20. | Ludwig J, Batts KP. Practical liver biopsy interpretation: diagnostic algorithms. 2nd ed. Chicago: ASCP Press 1998; 53-54. |
| 21. | Cone LA, Woodard DR, Schlievert PM, Tomory GS. Clinical and bacteriologic observations of a toxic shock-like syndrome due to Streptococcus pyogenes. N Engl J Med. 1987;317:146-149. |
| 22. | Ceydeli A, Condon MR, Siegel JH. The septic abscess wall: a cytokine-generating organ associated with portal venous cytokinemia, hepatic outflow fibrosis, sinusoidal congestion, inflammatory cell sequestration, hepatocellular lipid deposition, and focal cell death. Shock. 2003;20:74-84. |
| 23. | James PE, Madhani M, Roebuck W, Jackson SK, Swartz HM. Endotoxin-induced liver hypoxia: defective oxygen delivery versus oxygen consumption. Nitric Oxide. 2002;6:18-28. |