Published online Feb 28, 2008. doi: 10.3748/wjg.14.1302
Revised: December 6, 2007
Published online: February 28, 2008
Gastrointestinal stromal tumour (GIST) is a rare tumour of the gastrointestinal tract which does not generally originate in the rectum. The authors describe a case of a 70-year-old man who underwent an anterior resection of the rectum for a low-risk GIST. The patient was not given adjuvant chemotherapy with imatinib and is still disease-free 30 mo after surgery. The authors conclude that although rectal GIST is extremely uncommon, it should be included in differential diagnosis when a tumour in the rectum is detected. Biopsy of the tumour is essential, since this makes it possible to reach a sure preoperative diagnosis based on the immunohistological features of the CD117 and CD34. Although complete surgical resection with negative tumour margins is the principal curative procedure for primary and non-metastatic tumours, further studies are still needed for the determination of the most effective treatment strategy for patients with rectal GIST.
- Citation: Grassi N, Cipolla C, Torcivia A, Mandalà S, Graceffa G, Bottino A, Latteri F. Gastrointestinal stromal tumour of the rectum: Report of a case and review of literature. World J Gastroenterol 2008; 14(8): 1302-1304
- URL: https://www.wjgnet.com/1007-9327/full/v14/i8/1302.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.1302
Gastrointestinal stromal tumour (GIST) is a rare tumour involving the gastrointestinal tract and expresses CD117, a c-kit proto-oncogene, which can be detected immunohistochemically[12]. The most common GIST sites are the stomach (60%-70%) followed by the small intestine (20%-25%), whereas only about 5% of all GISTs start in the rectum[34]. Rectal GISTs make up 0.1% of all tumours originating in the rectum. According to a recent epidemiological investigation conducted in the USA, their incidence is 6.8 per million people annually[35]. In this report, the authors describe a rare case of GIST of the rectum.
A 70-year-old man was referred to our hospital complaining of a dragging sensation in the rectum and several episodes of rectal pain, without any problems of the alvus. A clinical examination did not detect any palpable abdominal mass. Digital investigation of the rectum revealed a mass of approximately 4 cm in diameter on the left lateral rectal wall at about 2 cm above the dentate line. The mass was hard, elastic and immobile, with an irregular surface. No blood was found on the exploratory finger. A provisional diagnosis of rectal carcinoma was made and a colonoscopy was programmed. This examination confirmed the presence of a mass, probably of submucous origin, measuring about 4 cm in its maximum diameter, attached to the lateral left rectal wall and at 2 cm above the dentate line. The surrounding mucosa proved to be healthy. Endoscopic observation suggested that the lesion probably originated in the connective tissue, transrectal ultrasound was therefore performed, together with an echo-guided needle-biopsy for the histological examination and a computed tomography (CT) with contrast medium of the abdomen and the chest and an assay of the carcinoembryonic antigen (CEA) and Ca19.9 tumoral markers.
Laboratory investigations revealed microcytic hypochromic anaemia, with haemoglobin of 11.9 g/dL, while CEA and Ca19.9 values were normal.
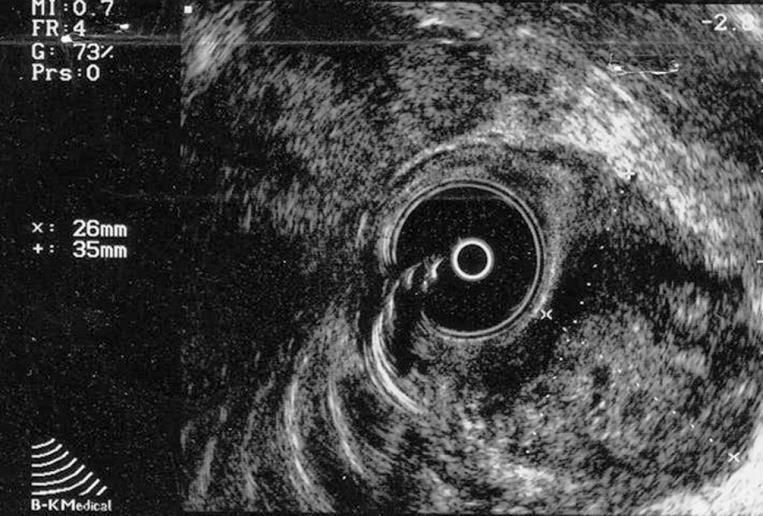
Transrectal ultrasound confirmed a predominantly exophytic, heterogenous, hypoechoic submucosal mass (measuring 35 mm × 26 mm) on the lateral left rectal wall (Figure 1). The histological examination of the biopsy samples led to a diagnosis of GIST due to the immunohistochemical positivity for CD117 and CD34. Furthermore, there was also a mitotic activity of a 3 × 50 high power field (HPF), together with moderate cytological atypia and absence of necrosis.
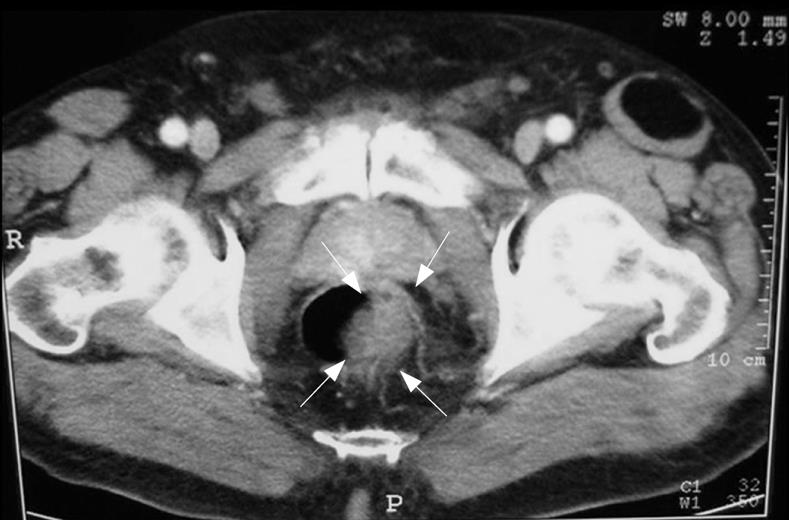
TC confirmed the sonographic findings of the presence of a mass with a marked, irregular, eccentric thickening of the lateral left wall of the lower third of the rectum, but there was no evidence for either pelvic lymphadenopathy or distant metastasis (Figure 2).
The patient underwent nerve-sparing, anterior surgical resection of the rectum, with removal of the mesorectum, followed by colo-anal anastomosis and the formation of a protective colostomy. The post-operative period was perfectly normal, with no complications, and the patient was sent home on d 10. The colostomy was closed up two months after surgery.
Histological examination showed a submucous, intraparietal mass measuring 4 cm × 3 cm × 2 cm, with a central necrotic area and a completely healthy mucosa. The morphological aspect suggested a spindle-cell GIST, a diagnosis confirmed by the immunohistochemical investigation. Both CD117 and CD34 were, in fact, extremely positive (+++), and the mitotic index was < 5 × 10 HPF. The neoplasia was 5 cm away from the margin of the distal resection.
The patient did not undergo adjuvant therapy with imatinib. After 30 mo of follow-up, he showed no signs of either local regression or distant metastases.
GIST is an uncommon mesenchymal tumour located throughout the gastrointestinal tract and expresses CD117, a tyrosine-kinase growth factor receptor and the most important GIST marker[1]. CD117 also serves as the target for drug therapy with imatinib, a selective tyrosine-kinase receptor inhibitor that is at present the only promising chemotherapeutic drug for the treatment of patients with advanced GIST, although complete surgical resection remains the most effective treatment for such a tumour[6].
GISTs are most commonly found in the stomach (60%-70%), followed by the small intestine (20%-25%), only about 5% of all GISTs originate in the rectum[34].
The symptoms of GIST in the rectum do not generally differ from those of other rectal tumours and diagnostic work-up is also similar to that for any other rectal mass. Digital examination of the rectum, colonoscopy and transrectal ultrasound are essential for its diagnosis, together with preoperative biopsy, which plays a key role in the diagnosis of GIST, since it provides information on the immunohistochemical features and mitotic count. GIST typically expresses CD117, often CD34 and sometimes SMA and S-100, but its expressions vary depending on different sites. Miettinen et al[7] found that CD34 expression in rectal GIST is 92%, but only 50% in small intestinal GIST. Smooth muscle antigen (SMA) is most frequently seen in the small intestinal GISTs (47%), whereas it has been observed in only 14% of rectal GISTs. The reason for these variations has not yet been explained. Perhaps the differences in origin and their correlation with prognosis will be explained in the future.
Although endoscopy provides the diagnosis and biopsy material, a definite diagnosis is difficult if there is no mucosal invasion or extrinsic deformity. Most GISTs originate within the muscularis propria and most commonly have an exophytic growth pattern[8–10], seen on CT and MRI images. A focal, well-circumscribed mural mass is the most common finding and an infiltrated layer can be clearly assessed[11]. Furthermore, CT or MRI scanning is needed for the definition of local invasion level and for the detection of the presence of possible metastases.
Since the incidence of rectal GIST is much lower than that of GIST in the stomach or small intestine, the clinicopathological profiles of rectal GIST have not yet been accurately characterised, and there is therefore the tendency to validate the same prognostic factors for the latter as for such tumours at other sites, particularly gastric GIST. The most important and easily applicable histological criteria for prediction of GIST are its size and mitotic rate[12–15]. A rate of ≤ 5 mitoses per 50 HPF is commonly used as a limit for a tumour with expected benign behaviour, and according to a large study, this can discriminate between benign and malignant tumours, especially gastric GISTs[12]. Tumours of 2 cm in diameter are generally expected to behave in a benign fashion. Tumours of < 5 cm in diameter are associated with a better survival rate than those of 5 cm-10 cm in diameter, which in turn have a better prognosis than those of > 10 cm in diameter. Degrees of cellularity and atypia have also been suggested as useful criteria, but their reproducibility is more problematic. The epithelioid phenotype, which seems to lead to a worse outcome, together with symptoms lasting for at least a year, might be considered as further prognostic factors.
It is generally agreed that complete surgical resection with negative tumour margins is the principal curative procedure for primary and non-metastatic tumours, particularly for those at a low risk[816–18]. For rectal GIST, various surgical procedures may be considered, including local excision, anterior resection of the rectum and abdomino-perineal resection. The choice of procedure depends on tumour size and location[419]. Although not essential for the purpose of oncological radicality, we ourselves prefer to remove the mesorectum, which makes it much easier to spare the nerves of the lower hypogastric plexus, reduces intraoperative bleeding and guarantees more locoregional radicality, and this, in our opinion, also reduces the rate of local relapse. Neoadjuvant imatinib may enhance the resectability of inoperable malignant GIST and may allow for optimal surgical timing. Therapy with imatinib is also used in the adjuvant post-operative treatment of tumours at a high risk or in cases of incomplete surgical resection. It has now been more or less accepted that imatinib is a valid treatment for advanced or metastatic tumours, but further evidence for the efficiency of this drug is needed in the case of high risk tumours and for the neoadjuvant therapy.
In conclusion, rectal GIST should be included in differential diagnosis when a tumour in the rectum is detected although it is extremely rare. The diagnostic work-up of rectal GIST is exactly the same as that advised for any other type of rectal neoplasia. There is no doubt that biopsy of the tumour is an essential diagnostic instrument, since it can reach a certain preoperative diagnosis by means of the immunohistochemical characterisation of CD117 and CD34. Finally, the lack of a large series of patients under long-term follow-up observations makes it difficult to assess the necessary extent of surgical resection for rectal GIST and the indication for treatment with imatinib. Further studies are necessary to establish the most effective treatment strategy for patients with rectal GIST.
| 1. | Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O'Leary TJ, Remotti H, Rubin BP. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459-465. |
| 2. | Joensuu H, Fletcher C, Dimitrijevic S, Silberman S, Roberts P, Demetri G. Management of malignant gastrointestinal stromal tumours. Lancet Oncol. 2002;3:655-664. |
| 3. | Tran T, Davila JA, El-Serag HB. The epidemiology of malignant gastrointestinal stromal tumors: an analysis of 1,458 cases from 1992 to 2000. Am J Gastroenterol. 2005;100:162-168. |
| 4. | Miettinen M, Furlong M, Sarlomo-Rikala M, Burke A, Sobin LH, Lasota J. Gastrointestinal stromal tumors, intramural leiomyomas, and leiomyosarcomas in the rectum and anus: a clinicopathologic, immunohistochemical, and molecular genetic study of 144 cases. Am J Surg Pathol. 2001;25:1121-1133. |
| 5. | Conlon KC, Casper ES, Brennan MF. Primary gastrointestinal sarcomas: analysis of prognostic variables. Ann Surg Oncol. 1995;2:26-31. |
| 6. | DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231:51-58. |
| 7. | Miettinen M, Sobin LH, Sarlomo-Rikala M. Immunohisto-chemical spectrum of GISTs at different sites and their differential diagnosis with a reference to CD117 (KIT). Mod Pathol. 2000;13:1134-1142. |
| 8. | Eisenberg BL, Judson I. Surgery and imatinib in the management of GIST: emerging approaches to adjuvant and neoadjuvant therapy. Ann Surg Oncol. 2004;11:465-475. |
| 9. | Richmond JA, Mount SL, Schwarz JE. Gastrointestinal stromal tumor of the stomach with rhabdoid phenotype: immunohistochemical, ultrastructural, and immunoelectron microscopic evaluation. Ultrastruct Pathol. 2004;28:165-170. |
| 10. | Lo SS, Papachristou GI, Finkelstein SD, Conroy WP, Schraut WH, Ramanathan RK. Neoadjuvant imatinib in gastrointestinal stromal tumor of the rectum: report of a case. Dis Colon Rectum. 2005;48:1316-1319. |
| 11. | Levy AD, Remotti HE, Thompson WM, Sobin LH, Miettinen M. Gastrointestinal stromal tumors: radiologic features with pathologic correlation. Radiographics. 2003;23:283-304, 456; quiz 532. |
| 12. | Miettinen M, Sarlomo-Rikala M, Lasota J. Gastrointestinal stromal tumors: recent advances in understanding of their biology. Hum Pathol. 1999;30:1213-1220. |
| 13. | Franquemont DW. Differentiation and risk assessment of gastrointestinal stromal tumors. Am J Clin Pathol. 1995;103:41-47. |
| 14. | Lerma E, Oliva E, Tugues D Prat J. Stromal tumours of the gastrointestinal tract: a clinicopathological and ploidy analysis of 33 cases. Virchows Arch. 1994;424:19-24. |
| 15. | Rudolph P, Gloeckner K, Parwaresch R, Harms D, Schmidt D. Immunophenotype, proliferation, DNA ploidy, and biological behavior of gastrointestinal stromal tumors: a multivariate clinicopathologic study. Hum Pathol. 1998;29:791-800. |
| 16. | Connolly EM, Gaffney E, Reynolds JV. Gastrointestinal stromal tumours. Br J Surg. 2003;90:1178-1186. |
| 17. | Roberts PJ, Eisenberg B. Clinical presentation of gastrointestinal stromal tumors and treatment of operable disease. Eur J Cancer. 2002;38 Suppl 5:S37-S38. |
| 18. | Cipolla C, Fulfaro F, Sandonato L, Fricano S, Pantuso G, Grassi N, Vieni S, Valerio MR, Lo Dico R, Gebbia N. Clinical presentation and treatment of gastrointestinal stromal tumors. Tumori. 2006;92:279-284. |
| 19. | Shibata Y, Ueda T, Seki H, Yagihashi N. Gastrointestinal stromal tumour of the rectum. Eur J Gastroenterol Hepatol. 2001;13:283-286. |