THE CLASSIFICATION PROBLEM
Acute recurrent pancreatitis (ARP) is defined as the presence of at least two documented episodes of pancreatitis in a patient without chronic pancreatitis (CP) at imaging. The classification, however, is still a debated and controversial issue. Is this a relapsing acute disease with restitutio ad integrum of the pancreatic gland when the cause is eliminated or does it progress to chronic disease when the cause is not removed? There is also debate about whether ARP is actually a chronic disease ab initio, regardless of whether the cause persists, so that recurrences of pancreatitis are the clinical expression of CP in an early phase.
The term ARP was reported in the first Marseille classification of pancreatitis[1] that strictly divided ARP from CP. ARP is also reported in the classification of TIGAR-Ot[2], so-called from the acronym of the major predisposing risk factors, but was eliminated in the revised classifications of Marseille[3] and Marseille-Rome[4] because of the difficulties of distinguishing between a relapse of acute pancreatitis (AP) and an exacerbation of CP. In the paper referring to the Marseille-Rome classification the majority of the authors commented that the progression of acute to chronic pancreatitis was extremely uncommon.
In contrast, when pancreatitis is interpreted as a “hydraulic” obstructive problem, as a consequence of ductal lesions secondary to periductal inflammatory infiltrates[5] or post-necrotic scars[67], ARP can develop into CP over time if the cause of the disease persists. According to these pathogenic hypotheses, backed by experimental findings, the duration and grade of the pancreatic ductal obstruction are pivotal factors for differentiating the types of pancreatitis: partial, temporary pancreatic ductal obstruction (e.g. biliary lithiasis in humans) can lead to AP, while partial but long-lasting obstruction (e.g. stenosis of Oddi’s sphincter or the Wirsung duct) is a cause of CP. When the ductal obstruction is complete and long-lasting (e.g. neoprene injection after surgery) the consequence is pancreatic atrophy.
AP, ARP and CP, according to this concept of “progression”, are therefore not different diseases but different stages of the same disease. While biliary lithiasis and sphincter of Oddi dysfunction are the most frequent causes of AP and its recurrences, alcohol, smoking, genes and necrosis can all facilitate the progression from ARP to CP. A recent review[8] concluded that probably each of these classification concepts contains part of the truth, each providing one piece of the puzzle named pancreatitis.
ALCOHOLIC PANCREATITIS
Does acute recurrent alcoholic pancreatitis really exist or develop in a pancreas already affected by chronic disease? This question is still much debated. There are two schools of thought: those who sustain that the relapses are an expression of acute disease that evolves to a chronic condition only if repeated over time, and those who maintain that the relapses are the expression of a chronic disease from the very start.
The differences are due to a different reading of the same results, the first more focused on the clinical picture, the second on histological data. The histological evidence of both acute necrotic and chronic lesions in tissue samples from six patients operated for a first acute attack of necrotic pancreatitis seems to support the idea that acute alcoholic pancreatitis develops in a pancreas already suffering from CP[9]. The same group[10] found similar results in 42 patients with alcoholic pancreatitis operated within two years of the clinical onset of the disease, investigated for histological evidence of diffuse or focal acute necrotic pancreatitis. However, only 7% of the patients had necrotic areas in the pancreatic tissue though all had the typical alterations of CP, such as perilobular and intralobular fibrosis, dilated inter- and intralobular ducts and protein plugs within the dilated ducts surrounded by periductal fibrosis, as already described several years ago[1]. These recent histological data confirm the detection of chronic lesions, in addition to the acute changes, previously reported in 12 patients with acute alcoholic pancreatitis, five of them at the first acute episode[11]. The authors suggested that alcoholic pancreatitis begins as a chronic disease and that the idea that repeated episodes of acute necrotic alcoholic pancreatitis cause CP is purely speculative since it is not supported by histology.
Ammann et al[12] conducted a sequential histological study of the pancreas in patients with alcoholic pancreatitis and concluded that it does progress from AP to CP, as suggested first by Comfort[6] and later by Kloppel[7], according to the “necrosis-fibrosis sequence” theory. Further evidence that recurrent pancreatitis can result in CP seems to come from studies of hereditary pancreatitis[1314]. Whitcomb defined “sentinel acute pancreatitis (SAPE)” as an episode of sufficient severity to trigger the series of events that can lead to CP and proposed that a SAPE must occur for the development of CP[13].
The idea that ARP develops into a chronic disease has come under fire because, for example, in Amman’s clinico-morphological study[12] all the patients had mild or severe chronic histologic pancreatic lesions already at their first histologic evaluation. Not only, the necrosis-fibrosis hypothesis fails to explain why some alcoholic patients with recurrent pancreatitis seem to progress to CP despite little evidence of necrosis and consequent pancreatic scarring.
The identification and characterization of stellate cells in the pancreas[15] and their vital role in fibrogenesis has led to further progress in understanding the pathogenesis of fibrosis in AP and CP, especially when caused by alcohol. The pathogenesis of alcoholic pancreatic fibrosis may involve two pathways: (a) a necro-inflammatory pathway involving cytokine release and activation of pancreatic stellate cells; (b) a non-necro-inflammatory pathway involving direct activation of stellate cells by ethanol through its metabolism to acetaldehyde and the generation of oxidant stress[16].
BILIARY PANCREATITIS
Gallstones are the most frequent cause AP. Sometimes patients with biliary ARP have ERCP findings of CP although it is not clear whether the two disease are linked by a cause-effect relationship (lithiasis as an etiological factor for CP) or by a casual association. While there are no data on patients with biliary ARP, abnormalities of the main pancreatic duct have been reported in about half of patients undergoing ERCP for biliary lithiasis[17].
In a retrospective study[18], biliary lithiasis in itself was not an etiological factor for CP: the frequency of main pancreatic duct abnormalities suggestive of CP by ERCP (increased diameter, irregular profiles, stenosis, dilation of the secondary ducts, cysts) was not significantly different in patients with biliary lithiasis, 68.4% of them with bile duct stones, and controls.
“GENETIC” PANCREATITIS
CFTR gene mutations
Recent studies showed that the frequency of cystic fibrosis transmembrane conductance regulator gene (CFTR) mutations is underestimated and that these have an important etiological and pathogenic role in so-called “idiopathic” ARP[19]. The proportion of patients with misdiagnosed cystic fibrosis and pancreatitis is 2%-3%, which is up to 75 times higher than the percentage reported in the general population [2021]. The incidence of a single CFTR gene mutation in patients with ARP is significantly lower than in idiopathic CP (7.4% vs 15.5%, P = 0.007). However, it is difficult to establish whether a single CFTR gene mutation is sufficient to cause the clinical onset of pancreatitis or whether it is only a predisposing factor that needs some other cause, such as biliary microlithiasis, sphincter of Oddi dysfunction or other genetic mutations (SPINK1 or PRSS1).
CP patients with a single CFTR gene mutation had a significantly longer history of recurrent episodes of AP before the diagnosis of CP than patients with CP with a normal CFTR gene (7.4 ± 5.8 vs 2.1 ± 2 years; P < 0.0001). The mean interval between the first and second episode of AP was three years (range 1-6)[20].
The natural history of pancreatitis associated with CFTR gene mutation seems to be characterized by the onset of AP that can recur, leading, over variable periods of time, to dilation of the main pancreatic duct and hence to a diagnosis of CP. The CFTR gene mutation seems to confirm the possibility of progression from AP to CP as in hereditary and obstructive pancreatitis, but in a longer time than usually observed for CP[20]. However, it remains difficult to decide whether ARP associated with CFTR gene mutation is truly an acute condition that evolves to a chronic disease and not already chronic when it starts.
Hereditary pancreatitis (HP)
Patients with (HP) develop repeated episodes of AP that are indistinguishable from pancreatitis due to other causes. In their follow-up, patients with HP often present typical clinical, morphological and functional findings of CP, similar to those of the classic forms of CP[13].
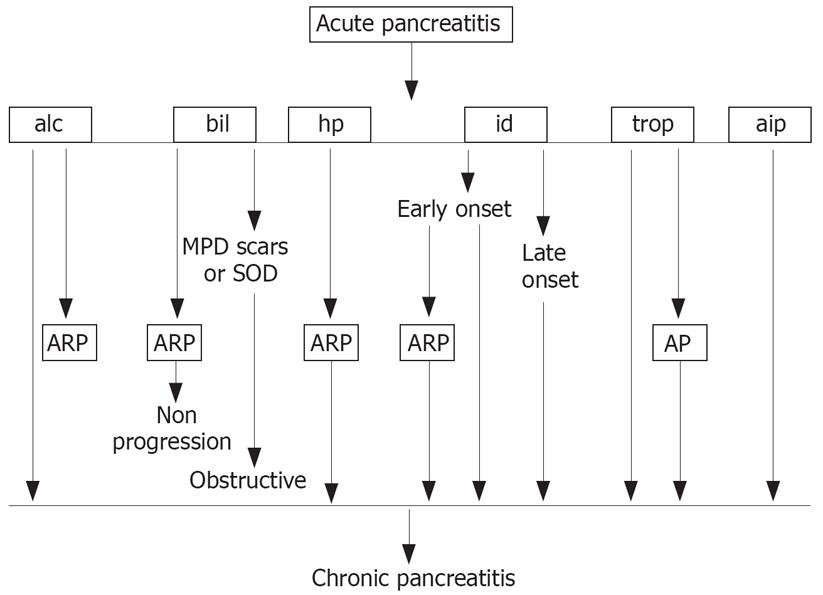
Recurrent attacks of mild or severe AP are more frequent in patients with the N9I mutation[22] and are associated with disabling pain, especially during the first years of the disease. Whitcomb thinks that the necrosis-fibrosis sequence hypothesis has some limitations in explaining why some patients with HP develop CP without significant pancreatic necrosis. He suggested that ARP might progress to CP when cytokines and chemokines are released from acinar cells during the acute episode and stimulate macrophages, with consequent infiltration, differentiation and/or proliferation of pancreatic stellate cells that produce collagen and therefore drive fibrosis. This might help explain the progression from ARP to CP as outlined by Uomo[23] (Figure 1).
Figure 1 Progression from acute (AP) to chronic pancreatitis (CP).
alc: Alcoholic; bil: Biliary; hp: Hereditary pancreatitis; id: Idiopathic pancreatitis; trop: Tropical; aip: Autoimmune; ARP: Acute recurrent pancreatitis; MPD: Main pancreatic duct; SOD: Sphincter of Oddi disfunction. (From ref. 23, with permission).
IDIOPATHIC PANCREATITIS
An etiological diagnosis can be established in about two thirds of patients with ARP. No immediate cause is found in the remainder and ARP is considered “idiopathic”. owever, in some of these patients Endoscopic ultrasound (EUS) investigations have identified some ductal and parenchymal abnormalities suggesting a diagnosis of CP. Most of these patients had fewer than four EUS diagnostic criteria for CP[24] so that only those with pre-study high-risk conditions could be diagnosed as having CP. The recurrences of pancreatitis in these patients are quite likely the expression of a chronic disease ab initio. The frequency of the diagnosis of CP in patients with ARP, on the basis of EUS criteria, ranges from 10% to 30%[2526]. Prospective randomized studies on ARP patients and controls, matched for sex and age, are therefore still needed to further verify the value of these criteria for this diagnosis.
In conclusion, the classification of ARP is still open to discussion. When CP is present, the diagnosis can usually be established on average almost five years after the second episode of AP. Episodes of pancreatic necrosis and gene mutations are suspected risk factors for the progression from ARP to CP, which may explain why, when genetic tests are available, the rate of ARP patients with a diagnosis of CP is higher.
Peer reviewer: Terumi Kamisawa, Dr, Department of Internal
Medicine, Tokyo Metropolitan Komagome Hospital, 3-18-22
Honkomagome, Bunkyo-ku, Tokyo, Japan