Published online Feb 21, 2008. doi: 10.3748/wjg.14.1108
Revised: November 27, 2007
Published online: February 21, 2008
AIM: To examine the ultrastructural changes after ursodeoxycholic acid (UDCA) treatment in hepatocytes from experimentally induced fibrotic livers.
METHODS: Liver fibrosis was induced in male Sprague-Dawley rats with CCl4 for 12 wk, and the rats were divided into two groups. Group I was treated with saline and group II with UDCA (25 mg/kg per day) for 4 wk. All the rats were killed at wk 16. Mitochondria, nuclei, rough endoplasmic reticulum (RER) and smooth endoplasmic reticulum (SER) of hepatocytes were evaluated according to a scoring system.
RESULTS: Mitochondria, nuclei, RER and SER injury scores in group II were significantly lower than those in groupI(P < 0.001).
CONCLUSION: UDCA alleviates hepatocyte organelle injury in CCl4-induced liver fibrosis.
- Citation: Mas N, Tasci I, Comert B, Ocal R, Mas MR. Ursodeoxycholic acid treatment improves hepatocyte ultrastructure in rat liver fibrosis. World J Gastroenterol 2008; 14(7): 1108-1111
- URL: https://www.wjgnet.com/1007-9327/full/v14/i7/1108.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.1108
Liver fibrosis is characterized by an increase in extracellular matrix (ECM) components and occurs as a common sequel of various chronic inflammatory diseases. Certain collagen types deposit around the sinusoidal cell layer in the space of Disse and a molecular re-organization of various ECM molecules results in alterations in the composition of the fibrotic matrix, with deterioration of hepatic functions[1]. Parenchymal cell damage of varying etiology is central to the initiation of fibrogenesis in the liver. Although experimental and clinical trials to establish effective options for prevention or regression of fibrogenesis in the liver are being conducted, it is still an untreatable condition. Some agents that are currently used for other diseases, and several herbal drugs, have recently been reported to have antifibrotic efficacy with different potency in the liver[2].
Ursodeoxycholic acid (UDCA) is the mainstay of therapy of primary biliary cirrhosis (PBC) in humans. UDCA has been reported to have antifibrotic activity in bile duct ligation and CCl4-induced experimental liver fibrosis[34]. Clinically, long-term UDCA therapy improves liver function tests[5] and delays the development and progression of cirrhosis in PBC[67], which leads to prolongation of the time needed for liver transplantation. It has been reported to have antiapoptotic activity on cholangiocytes and hepatocytes[89] and an inhibitory effect on generation of reactive oxygen species[10]. In the present study, we examined the ultrastructural changes after UDCA treatment in hepatocytes from experimentally induced liver fibrosis in the rat. This should show us alterations in hepatocyte structure after treatment of established fibrosis and the possibility of regression of organelle injury.
The Institutional Animal Use and Care Committee of the Gülhane Medical Academy, Ankara, Turkey approved the study, which was also performed in accordance with the National Institute of Health's guidelines for the care and handling of animals. The animals were fed standard rat chow and kept in metabolic cages with controlled temperature and a 12-h light/dark cycle before and during the experiment.
Twenty male Sprague-Dawley rats weighing 250-350 g were injected subcutaneously with 0.2 mL/100 g CCl4 twice weekly for 12 wk to induce liver fibrosis. Then, the rats were randomly divided into two groups. GroupI (n = 10) was treated with saline and group II (n = 10) with UDCA (25 mg/kg per day) for 4 wk. At the end of wk 16, all the rats were killed under anesthesia and the livers were excised under sterile conditions. The specimens were fixed in 2.5% glutaraldehyde for 24 h and consequently washed in phosphate buffer (pH 7.4), post-fixed in 1% osmium tetroxide in phosphate buffer (pH 7.4), and dehydrated in increasing concentrations of alcohol. The tissues were washed with propylene oxide and embedded in epoxy-resin embedding medium. Semi-thin sections approximately 60-90 nm in thickness were cut with a glass knife on an LKB Nova (Sweden) ultramicrotome. Sections were stained with methylene blue and examined by a Nikon Optiphot (Japan) light microscope. Ultrathin 60-90 nm sections were collected on copper grids, stained with uranyl acetate and lead citrate, and examined with a Jeol JEM 1200 Ex (Japan) transmission electron microscope. Twenty cells from each specimen were examined. Mitochondria, nuclei, rough endoplasmic reticulum (RER) and smooth endoplasmic reticulum (SER) of hepatocytes were examined according to a previously described scoring system[1112] (Table 1).
| Nucleus | |
| 0: | Normal |
| 1: | Irregular chromatin distribution |
| -Margination | |
| -Clumping | |
| 2: | Increased heterochromatin |
| 3: | Degenerated nucleus |
| Mitochondria | |
| 0: | Normal |
| 1: | Prominent cristae |
| 2: | Swelling |
| 3: | Collection of amorphous material |
| RER | |
| 0: | Normal |
| 1: | Dilatation |
| 2: | Irregular lamellar organization |
| 3: | Presence of focal breaks |
| SER | |
| 0: | Normal |
| 1: | Dilatation |
| 2: | Presence of vacuole formation (vacuolization) |
| 3: | Presence of focal breaks |
Results were expressed as means ± SEM. The significance of differences in histopathological and ultrastructural fibrosis scores and serum amylase activity were assessed by the Kruskal-Wallis test. P < 0.05 was considered significant.
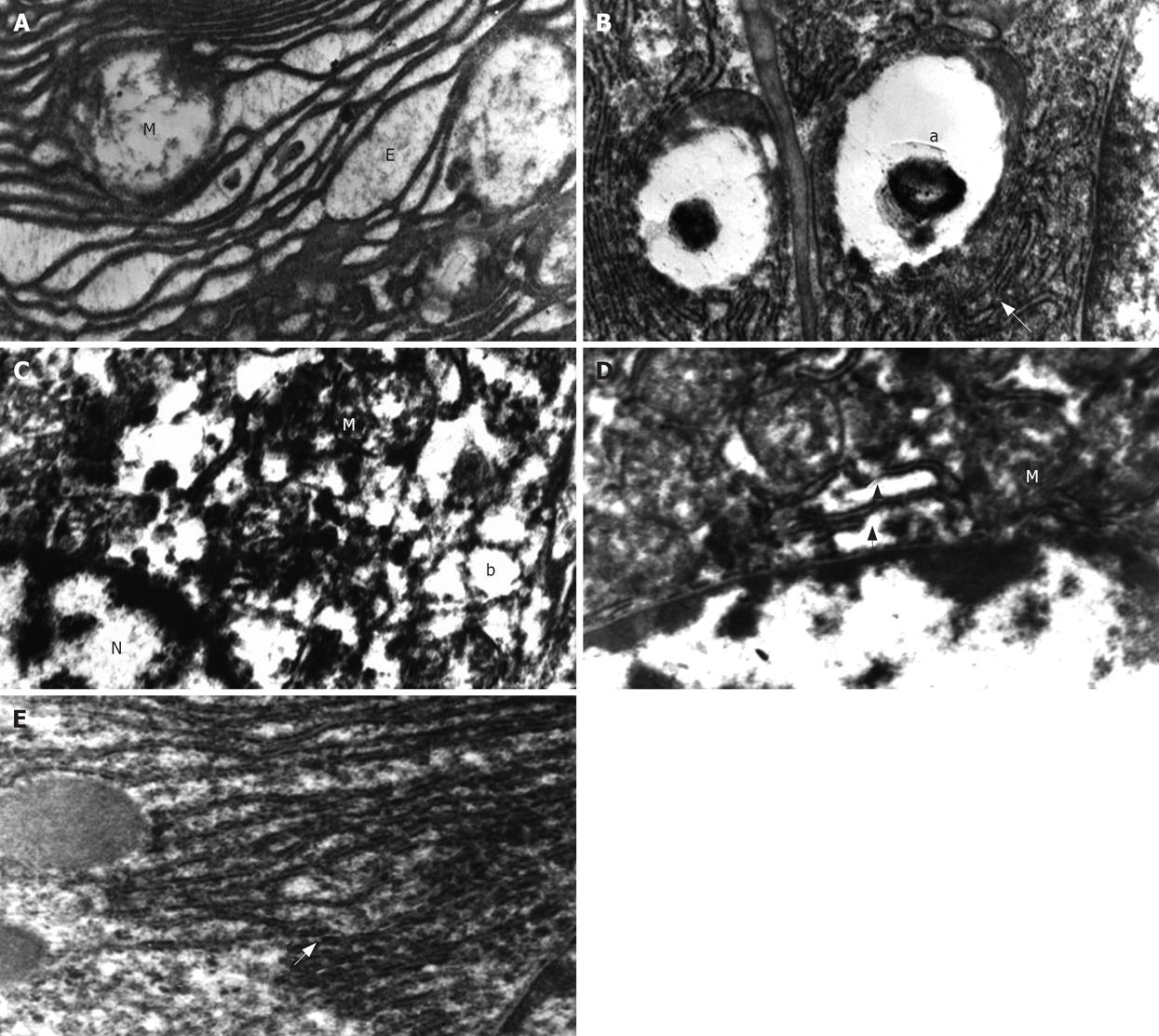
After 4 wk UDCA treatment (by the end of wk 16), the most remarkable finding under electron microscopic examination of tissues from saline-treated animals (groupI) was irregular lamellar organizations of the RER. Large dilatations and focal breaks in the RER were also found in many areas (Figure 1A). Vacuolization in the SER and presence of myelin figures were the other prominent findings in saline-treated animals (Figure 1B). Many of the nuclei had irregular chromatin distribution and margination with clumping. An increase in heterochromatin was seen in some of the nuclei. Almost all of the mitochondria had prominent swelling that was much more severe than that in the UDCA-treated group. Another important finding was the collection of active fibroblasts in some focal areas of the liver.
Ultrastructural findings in the UDCA-treated group (group II) were margination and clumping of the chromatin and a marked increase in the amount of heterochromatin (Figure 1C). Mild swelling of mitochondria was observed (Figure 1D). There were mild dilations in the SER and Golgi apparatus. However, vacuole formation in the SER was seen in only a few areas. There were also some breaks in the RER (Figure 1D and E).
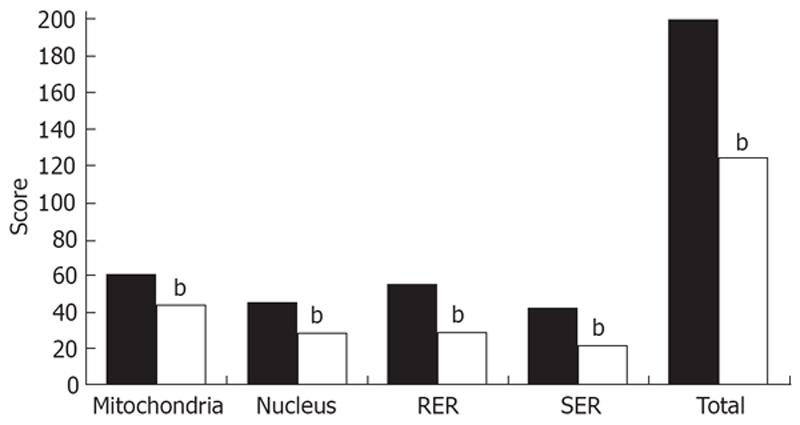
When these findings were scored and evaluated according to the system shown in (Table 1), we found that mitochondria (44 ± 0.5 vs 61 ± 2, t = 7.38, P < 0.001), nuclei (29 ± 0.8 vs 46 ± 1.5, t = 9.3, P < 0.001), RER (30 ± 0.7 vs 56 ± 1, t = 21.7, P < 0.001), and SER (22 ± 0.5 vs 42 ± 1.2, t = 15.3, P < 0.001), and injury scores in group II were significantly improved. Total electron microscopy score in group II was also significantly lower when compared to that in groupI(125 ± 2.3 vs 205 ± 3.7, t = 18.2, P < 0.001) (Figure 2).
The hepatocytes are the parenchyma cells of the liver, whose main function is synthesis of vital substances and removal of various kinds of molecules from the blood and their excretion into bile. Liver fibrosis is the result of deterioration of function of these cells, which results in initiation of many pathogenic events in the liver. As a result of parenchymal cell damage, activation of hepatic stellate cells occurs, which results in excessive production of collagen, and ultimately pathological and clinical features of cirrhosis develop[13].
UDCA has been shown to reduce hepatocellular damage in PBC. Treatment of patients with PBC with UDCA prevents deposition of perisinusoidal collagen[14]. It prevents hepatocyte apoptosis in vitro by inhibiting mitochondrial membrane permeability transition[15]. We performed this work to identify the ultrastructural changes in individual organelles of hepatocytes from UDCA-treated fibrotic livers. Histological changes in hepatic structure after CCl4 treatment have been well documented. Indeed, most studies addressing antifibrotic efficacy of newly tested agents have been supported by direct evidence of histological changes, despite clear biochemical findings. However, electron microscopically supported morphological results are not frequent. Although there have been some ultrastructural studies of the effects of UDCA on the canalicular system, organelle-based activity of this antifibrotic agent may be helpful.
Although the liver has a high regeneration capacity, which can be linked to the phenomenon of spontaneous recovery, we still found severe hepatocyte organelle injury after 4 wk of saline administration in CCl4-treated animals. Disrupted organization of the RER, vacuolization of the SER, and disorganization of nuclear content were indicative of severe organelle damage and established fibrosis. Munoz et al have reported a similar organization of ER with CCl4 treatment in their study which examined the protective effect of adenosine on hepatotoxin-induced liver fibrosis[16]. They have also reported mitochondrial swelling and breaks in the ER after CCl4 treatment.
Altered chromatin distribution in the nuclei was another noticeable finding in the hepatocytes following hepatotoxin exposure, which is suggestive of nuclear events taking place during necrosis and/or apoptosis. Heterochromatin is the condensed form of chromatin, with specific molecular characteristics and nuclear functions. It occurs around the centromeres and is involved in controlling the transcriptional state of the genome by gene silencing and nuclear organization. It lies next to the inner side of the nuclear envelope and is broken up at the sites of nuclear pores. Abundant heterochromatin is seen in resting cells, whereas euchromatin is mostly found in transcriptionally active cells. We found a significant decrease in heterochromatin after 4 wk of UDCA treatment. This may be intranuclear morphological evidence of improving regular cell functions. Mitochondrial swelling, which is characteristic of deteriorated function of this organelle, decreased significantly with UDCA. Taken together with parallel changes seen in the nucleus, we could not conclude any association of this finding with apoptosis, although, antiapoptotic activity of UDCA on hepatocytes has previously been demonstrated[9]. Structural reversal of disrupted ER may also result from a beneficial effect of treatment with UDCA.
In conclusion, structural reversal of injured hepatocyte organelles is possible, and administration of UDCA greatly alleviates CCl4-induced alterations of hepatocyte organelles. Nonetheless, these changes should be inspected at different time points during antifibrotic therapy, to identify the association of such structural recovery of different organelles.
Liver fibrosis and cirrhosis are mostly the results of chronic hepatocyte injury. A drug approved for the management of PBC (UDCA) is the mainstay of therapy of primary biliary cirrhosis (PBC) and has been reported to have antifibrotic activity in the liver. We investigated the ultrastructural changes after UDCA treatment in hepatocytes from experimentally induced liver fibrosis in rats.
Understanding the changes in hepatocyte organelles during injury and recovery may be advantageous in developing new diagnostic and therapeutic tools.
An ultrastructural scoring system based on the specific findings of individual organelles is to be used for the first time on animal hepatocytes.
Transmission electron microscopy may be another tool to follow structural alterations in hepatocytes in the disease state.
UDCA: A drug approved for the management of PBC.
Significant alterations occur in hepatocyte organelles during CCl4 administration and UDCA improves those changes to a great extent. Transmission electron microscopy may be used to follow morphological changes in the liver during injury and healing.
| 1. | Gressner AM. The cell biology of liver fibrogenesis-an imbalance of proliferation, growth arrest and apoptosis of myofibroblasts. Cell Tissue Res. 1998;292:447-452. |
| 2. | Bataller R, Brenner DA. Hepatic stellate cells as a target for the treatment of liver fibrosis. Semin Liver Dis. 2001;21:437-451. |
| 3. | Poo JL, Feldmann G, Erlinger S, Braillon A, Gaudin C, Dumont M, Lebrec D. Ursodeoxycholic acid limits liver histologic alterations and portal hypertension induced by bile duct ligation in the rat. Gastroenterology. 1992;102:1752-1759. |
| 4. | Tasci I, Mas MR, Vural SA, Comert B, Alcigir G, Serdar M, Mas N, Isik AT, Ates Y. Rat liver fibrosis regresses better with pegylated interferon alpha2b and ursodeoxycholic acid treatments than spontaneous recovery. Liver Int. 2006;26:261-268. |
| 5. | Nishio A, Keeffe EB, Ishibashi H, Gershwin EM. Diagnosis and treatment of primary biliary cirrhosis. Med Sci Monit. 2000;6:181-193. |
| 6. | Angulo P, Batts KP, Therneau TM, Jorgensen RA, Dickson ER, Lindor KD. Long-term ursodeoxycholic acid delays histological progression in primary biliary cirrhosis. Hepatology. 1999;29:644-647. |
| 7. | Corpechot C, Carrat F, Bonnand AM, Poupon RE, Poupon R. The effect of ursodeoxycholic acid therapy on liver fibrosis progression in primary biliary cirrhosis. Hepatology. 2000;32:1196-1199. |
| 8. | Koga H, Sakisaka S, Ohishi M, Sata M, Tanikawa K. Nuclear DNA fragmentation and expression of Bcl-2 in primary biliary cirrhosis. Hepatology. 1997;25:1077-1084. |
| 9. | Guicciardi ME, Gores GJ. Is ursodeoxycholate an antiapoptotic drug? Hepatology. 1998;28:1721-1723. |
| 10. | Rodrigues CM, Fan G, Wong PY, Kren BT, Steer CJ. Ursodeoxycholic acid may inhibit deoxycholic acid-induced apoptosis by modulating mitochondrial transmembrane potential and reactive oxygen species production. Mol Med. 1998;4:165-178. |
| 11. | Ates Y, Mas MR, Mas N, Tasci I, Comert B, Isik AT, Yener N. Acinar cell ultrastructure after taurine treatment in rat acute necrotizing pancreatitis. Saudi Med J. 2006;27:446-452. |
| 12. | Mas N, Isik AT, Mas MR, Comert B, Tasci I, Deveci S, Ozyurt M, Ates Y, Yamanel L, Doruk H. Hyperbaric oxygen-induced changes in bacterial translocation and acinar ultrastructure in rat acute necrotizing pancreatitis. J Gastroenterol. 2005;40:980-986. |
| 13. | Gressner AM, Weiskirchen R. Modern pathogenetic concepts of liver fibrosis suggest stellate cells and TGF-beta as major players and therapeutic targets. J Cell Mol Med. 2006;10:76-99. |
| 14. | Neuman MG, Cameron RG, Haber JA, Katz GG, Blendis LM. An electron microscopic and morphometric study of ursodeoxycholic effect in primary biliary cirrhosis. Liver. 2002;22:235-244. |
| 15. | Rodrigues CM, Fan G, Ma X, Kren BT, Steer CJ. A novel role for ursodeoxycholic acid in inhibiting apoptosis by modulating mitochondrial membrane perturbation. J Clin Invest. 1998;101:2790-2799. |
| 16. | Hernandez-Munoz R, Diaz-Munoz M, Lopez V, Barrera FL, Yanez L, Vidrio S, Fraustro AA, De Sanchez VC. Balance Between Oxidative Damage and Proliferative Potential in an Experimental Rat Model of CCl4-Induced Cirrhosis: Protective Role of Adenosine Administration. Hepatology. 1997;26:1100-1110. |