INTRODUCTION
HIV infection, which frequently involves the gastro-intestinal tract, may be associated with diarrhea, abdominal pain, esophageal symptoms, malabsorption, and weight loss[12]. The gastrointestinal tract is the major target for pathogens in HIV-infected patients and as many as a third of patients with AIDS are initially seen by a gastroenterologist[3]. In those instances, diarrhea is the most frequent manifestation in AIDS and HIV-positive patients. Diarrhea has been reported to be present at the same time during their illness in as many as 80% of North American patients with AIDS, and the prevalence may approach to 100% in developing countries, causing a significant morbidity and mortality[4–6]. Those suffering from chronic diarrhea tend to have a low quality of life and seem to have higher annual health costs. Typical infectious diseases affecting the intestinal tract in HIV-infected patients are caused by protozoa (Microsporidium, Cryptosporidium), bacteria (Clostridium difficile, Salmonella, Shigella, Campylobacter, and MAC), viruses (Cytomegalovirus, Herpes simplex) and fungi (Candida species). Before the HIV epidemic, NTM was an unusual cause of disease in humans. Nowadays, however, NTM especially MAC, is one of the most common causes of intestinal bacterial infection in AIDS patients[27]. But non-MAC NTMs, including Mycobacterium ulcerans, have not been reported as a pathogen in the gastrointestinal tract.
We report herein a case of colonic infection with an unusual NTM, Mycobacterium ulcerans, which caused chronic diarrhea in a patient with AIDS.
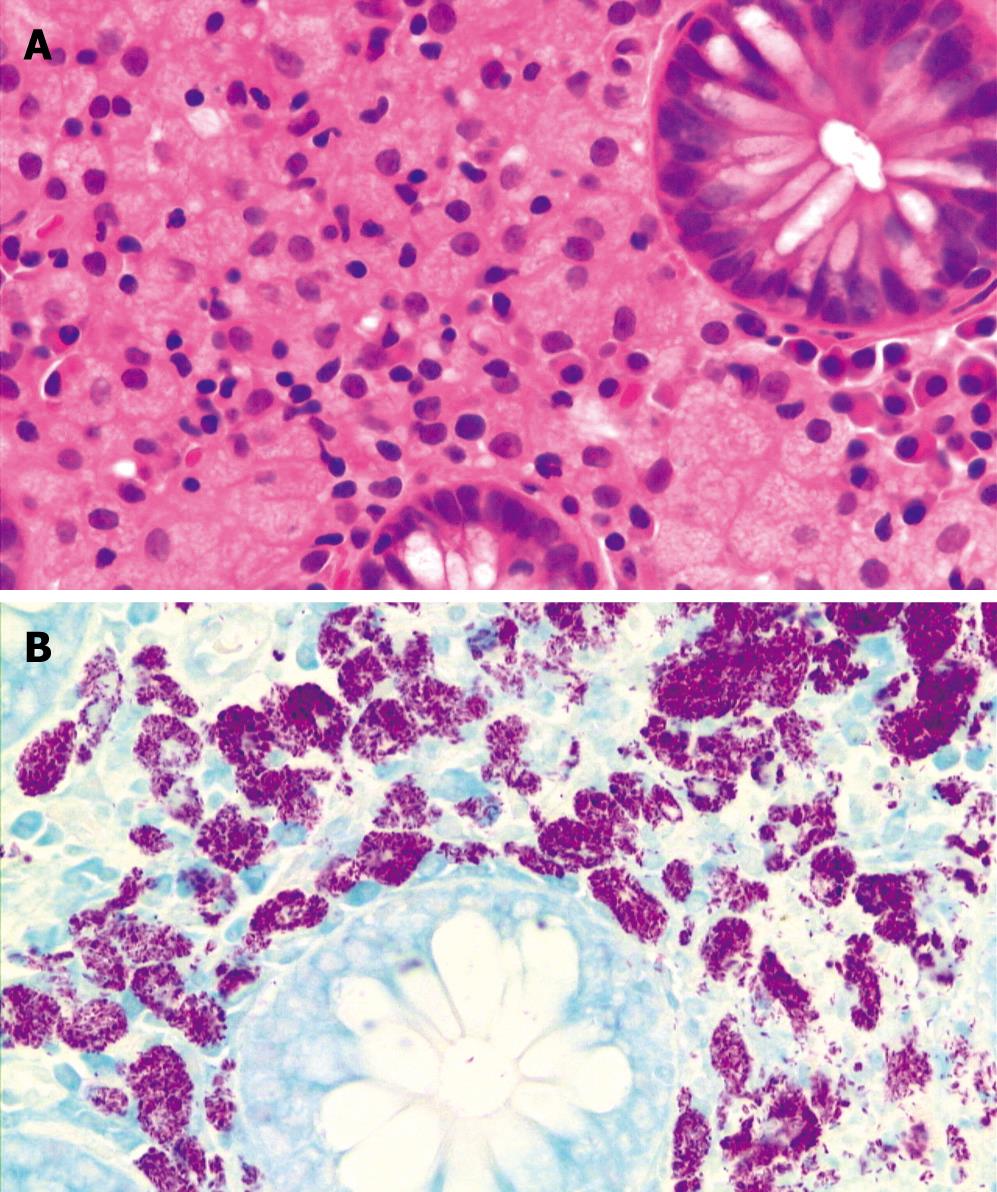
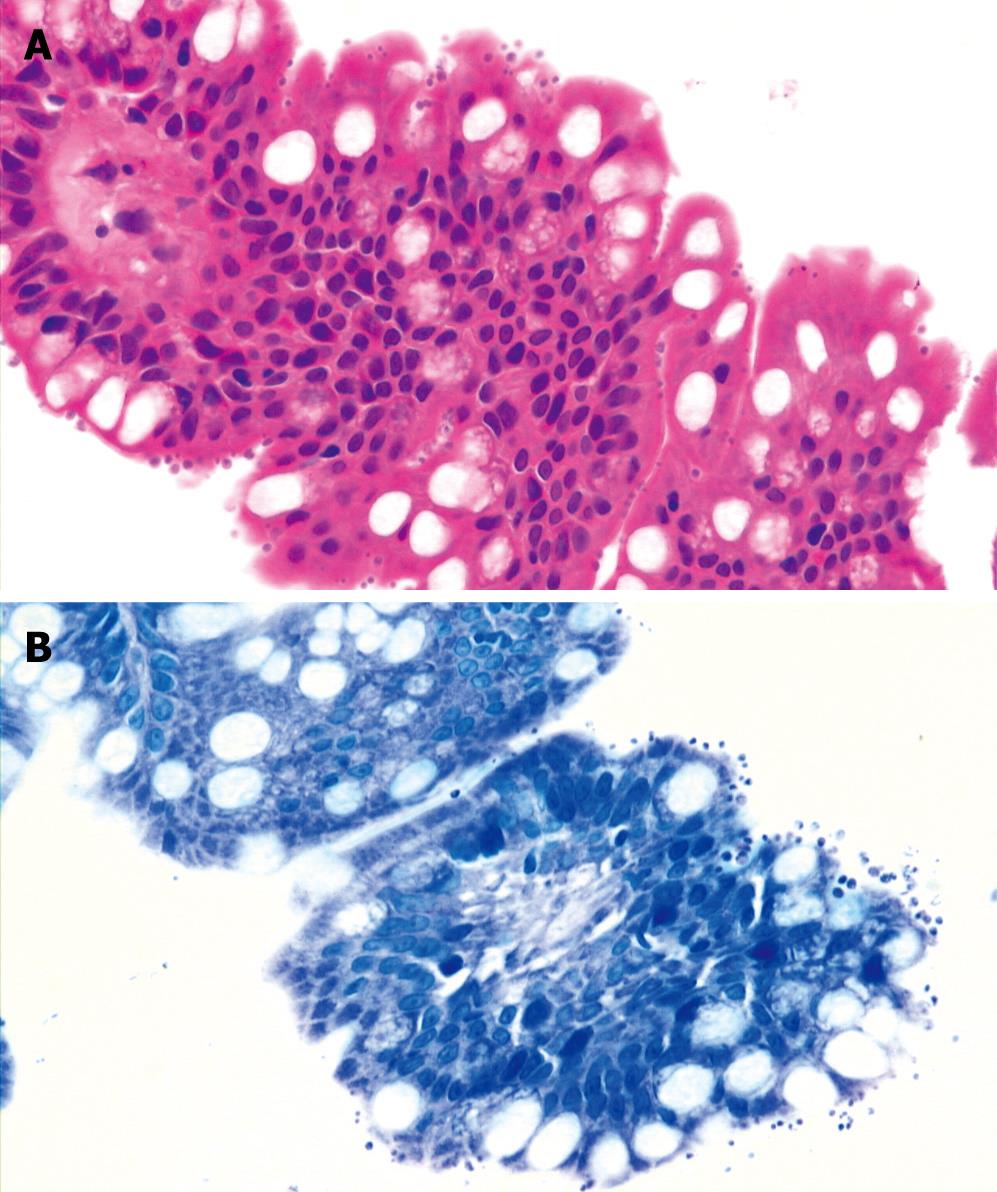
CASE REPORT
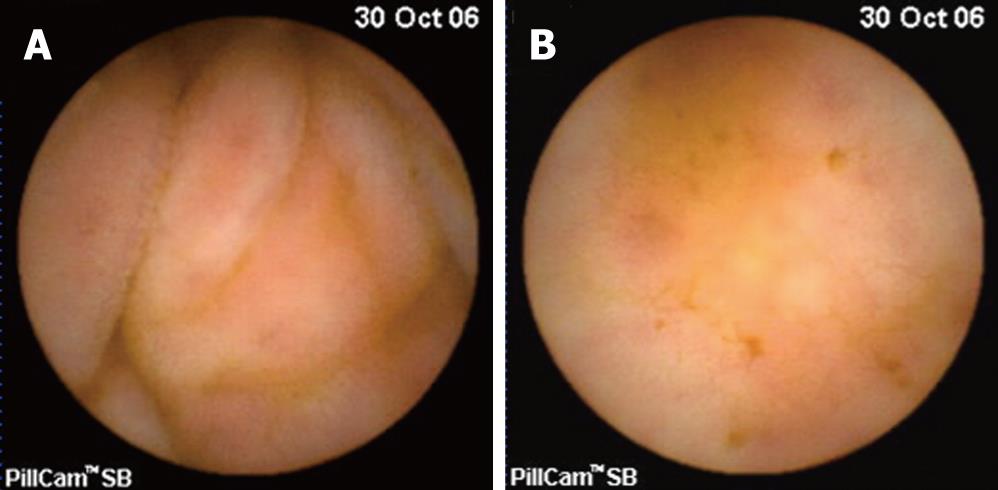
A 40-year-old man complained of persistent diarrhea associated with a 9 kg weight loss during the last 3 mo. He described his diarrhea as watery stools that occurred five to six times per day, especially at night. There was no previous history of travel, food, medication, or any medical or familial disease. He denied melena or hematochezia. On physical examination, the patient had a chronic ill-looking appearance but there were no remarkable findings except for a slightly dehydrated tongue. His blood pressure was 110/70 mmHg, pulse was 70 beats per minute, respiratory rate was 20 breaths per minute, and temperature was 36.2°C. His height was 167 cm and weight was 54 kg. The abdomen was soft and nontender without hepatosplenomegaly. The bowel sound was slightly increased. Rectal examination revealed no palpable masses or blood clots. The hemoglobin level was 14.4 g/dL, white blood cell count was 4200/mm3, and platelet count was 159 000/mm3. The serum electrolyte levels, urinalysis, and tests of renal, liver, and thyroid function were normal. The C-reactive protein level was 0.1 mg/dL. Stool examinations for ova, parasites, bacterial pathogens, and Clostridium difficile toxin were negative. Colonoscopy showed normal findings but a blind mucosal biopsy was done at the terminal ileum and sigmoid colon to evaluate the chronic diarrhea. Also, anti-HIV antibody was checked to rule out AIDS, which is an important cause of chronic diarrhea. The result of anti-HIV antibody was positive. CD4 T cell count was 168/mm3. We asked the patient about his medical history again and he admitted to an HIV positive history to us. He was diagnosed with AIDS at another hospital in 2002 and treated with highly active antiretroviral therapy (HAART). However, he voluntarily discontinued his treatment in 2004. The biopsy specimen from the sigmoid colon demonstrated foamy histiocytic collections in lamina propria by hematoxylin and eosin stain and revealed innumerable acid-fast bacilli collections by Ziehl-Neelsen stain (Figure 1). The possibility of NTM on colonic biopsy would have to be considered instead of Mycobacterium tuberculosis because of innumerable acid-fast bacilli collections. The biopsy specimen from the terminal ileum demonstrated mild chronic inflammation with protozoa infection consistent with cryptosporidiosis (Figure 2). To further evaluate the small bowel, a capsule endoscopy was performed. The whole small bowel showed mild edematous and hyperemic mucosal change (Figure 3).
Figure 1 Pathologic examination of biopsy specimen from sigmoid colon revealing foamy histiocytic collections in lamina propria by hematoxylin and eosion stain (A × 400) and innumerable acid-fast bacilli collections by Ziehl-Neelsen stain (B × 400).
Figure 2 Pathologic examination of biopsy specimen from terminal ileum showing many tiny organisms of the Cryptosporidium species with mild chronic inflammation on the surface of the luminal border of the crypt epithelial cells by hematoxylin and eosion stain (A × 400) and Giemsa stain (B × 400).
Figure 3 Capsule endoscopic findings of small bowel showing mild edematous (A) and hyperemic (B) mucosal change.
PCR-restriction fragment length polymorphism analysis (PRA)
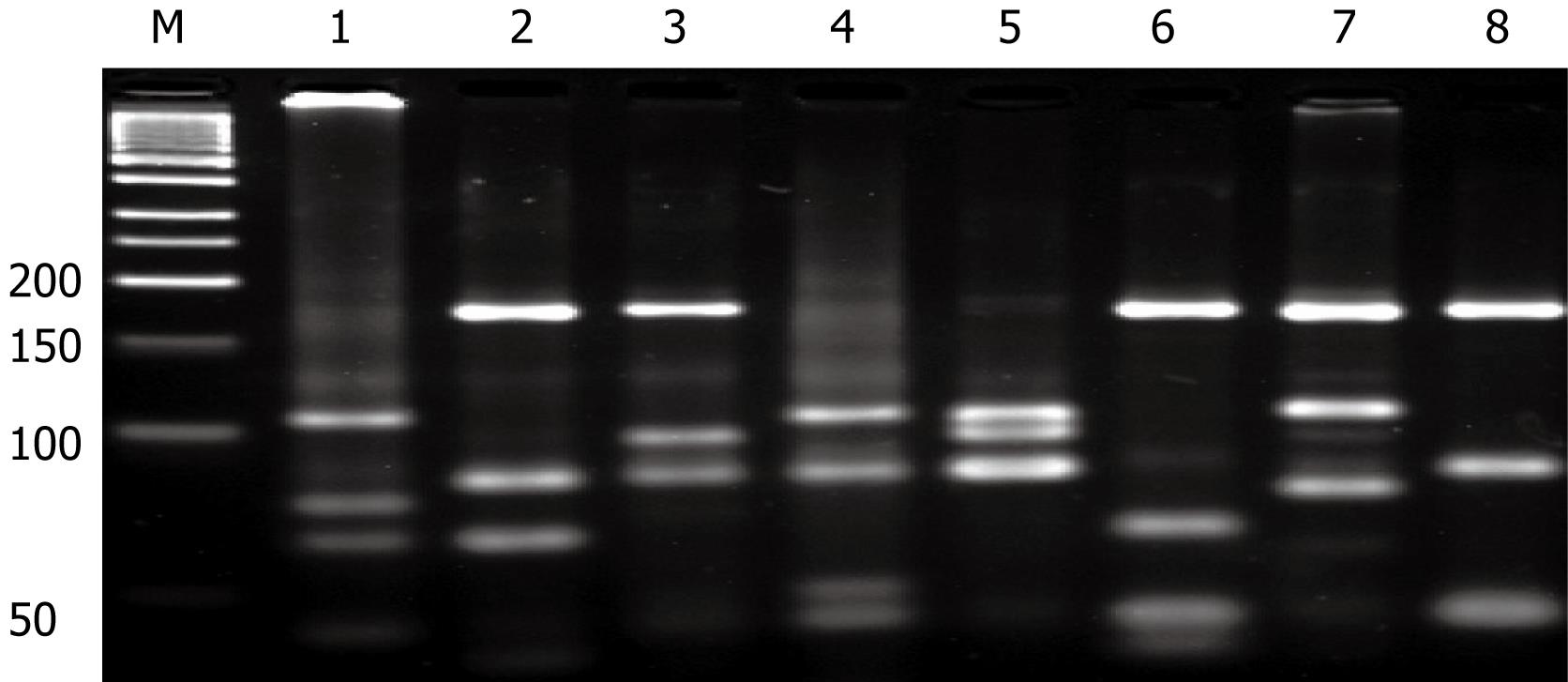
To identify the species of NTM, we used PCR-restriction fragment length polymorphism of the rpoB gene. PRA offers an easy, rapid, and inexpensive way to identify Mycobacterium species in a single experiment. Recently, Lee et al[8] chose the rpoB gene as a target and the restriction analysis of a 360-bp fragment within the rpoB gene after single MspIdigestion showed that it was highly effective for differentiating most Mycobacterium. They suggested that PRA using the novel region of the rpoB gene is more simple, rapid, and accurate versus other conventional methods such as chromatography or DNA sequence analysis. Briefly, we got the genomic DNA from paraffin-embedded colonic specimen. PCR was carried out with 10 ng genomic DNA, 10 pmol of each primer, 5’-TCAAGGAGAAGCGCTACGA-3’ (RPO5’) and 5’-GGATGTTGATCAGGGTCTGC-3’ (RPO3’) and DyNAzyme II DNA polymerase (FINNZYMESY, Espoo, Finland) resulted in a 360-bp PCR product. After successful amplification, the PCR products were subjected to restriction enzyme digestion with 5U of MspI(Boehringer Mannheim Biochemicals, Mannheim, Germany). For interpreting the PRA profile generated by each species, a 50-bp ladder DNA size marker (Boehringer Mannheim) was used. From the PRA, we discovered that the NTM species was Mycobacterium ulcerans (Figure 4).
Figure 4 Identification of the species of NTM by PCR-restriction fragment length polymorphism of the rpoB gene.
Lane: M, DNA size marker; lane1, M. ulcerans; lane 2, M. tuberculosis; lane 3, M. intracellulare; lane 4, M. avium; lane 5, M. abscessus; lame 6, M. kansasii; lane 7, M. fortuitum; lane 8, M. gordonae.
The patient was diagnosed with Mycobacterium ulcerans and cryptosporidium co-infection. He was treated with clarithromycin and ethambutol to target the NTM and with HAART for underlying AIDS. After three months of treatment for NTM, the patient said that his diarrhea was much improved.
DISCUSSION
Since the discovery of AIDS in 1981, HIV infection has spread quickly and become a very serious health problem for humans, not only in Western world, but also in Asian area. Since the first case of HIV infection was reported in Korea at 1985, the number of those infected with HIV has steadily increased. Despite the introduction of HAART, diarrhea remains a common disease manifestation related to HIV infection and is a diagnostic problem. The incidence of chronic diarrhea in AIDS patients does not change after HAART[9]. In other words, the incidence of opportunistic infections is still too high to ignore them. Opportunistic infections are responsible for 68%-85% of diarrhea in HIV-infected patients[710].
Initially, we did not know the exact medical history of the patient. However, HIV-related diarrhea must be included in evaluating chronic diarrhea, especially when it is associated with weight loss. Therefore, we performed a routine serologic test for HIV infection. Patients with chronic diarrhea in HIV-infected persons often cause a difficult diagnostic problem. There are proved pathogens or causes of drugs including protease inhibitors, but no causes of diarrhea are commonly encountered. The usefulness of endoscope in evaluating diarrhea in HIV-infected patients has been well investigated[11–13]. Because of the common involvement of the small bowel, colonoscopy is preferred to flexible sigmoidoscopy in HIV-infected patients with chronic, unexplained diarrhea[13]. Although colonoscopy is frequently performed in these patients, the usefulness of biopsy of normal mucosa is uncertain. Shah et al[14] suggested that colonoscopy with biopsy is a useful tool in the investigation of patients with chronic diarrhea, which yields a histological diagnosis in 31% of patients. In our case, we performed a blind biopsy from normal mucosa, because the serology for HIV was positive, and we thought that the histological evaluation would give us good information for the proper diagnosis. With the histological diagnosis, we had a clue as to how to properly treat the patient. This is in accordance with the suggestion of Shah et al[14] that colonoscopy with biopsy is a useful tool in the investigation of patients with chronic diarrhea, not only from the abnormal mucosal lesion but also from the normal one.
NTMs are ubiquitous organisms that are different from tuberculosis and represented by more than 65 different species[15]. The most frequent forms of NTM include Mycobacterium avium complex (MAC, 61%), Mycobacterium fortuitum (19%) and Mycobacterium kansasii (10%), with smaller percentages of Mycobacterium gordonae and Mycobacterium chelonae[1516]. Mycobacterial involvement of the bowel either by tuberculosis or MAC may lead to diarrhea. Antony et al[17] reported that MAC is the most commonly identified organism in patients with chronic diarrhea and low CD4 lymphocyte counts. Although tuberculosis appears to be symptomatic in all cases, a large number of patients with MAC have an asymptomatic infection of the gut. Endoscopic findings of MAC infection may be nonspecific. Sun et al[18] reviewed 55 reported cases of MAC infection with an endoscopic description which involved the gastrointestinal tract in AIDS patients, and found that the most common endoscopic finding is multiple raised nodules (38%) in the involved sites. The other endoscopic findings of MAC infection include normal findings (36%), erythemas (13%), ulcerations (11%), edemas (11%), friabilities (11%), reduced mucosal vascular patterns (9%), erosions (4%), confluent nodules (4%), strictures (2%), and aphthous erosions (2%). Therefore, for a definitive diagnosis in symptomatic patients, especially those without typical endoscopic findings, it is essential to obtain tissue specimens for histopathologic examination and microbiologic culture. Isolates of Mycobacterium from tissue biopsies or blood are almost always indicative of the disease[19]. The organism is readily seen on biopsy specimens with acid-fast staining, and the number of organisms is often striking. Rarely, a NTM other than a MAC has been isolated upon a colonic biopsy in a HIV patient[19]. In our case, a very rare NTM, Mycobacterium ulcerans, was isolated in colonic mucosa, and it is presumed to be the cause for the patient’s chronic diarrhea. Many clinical syndromes are similar to those seen with MAC and only can be differentiated by identification of the organism, like PRA or DNA sequence analysis[20].
Mycobacterium ulcerans is known to cause skin diseases, such as Buruli ulcer, an important health problem in several West African countries. Although the disease is known to be associated with contaminated water, the mode of transmission has not yet been understood[2122]. Mycobacterium ulcerans produces the toxin mycolactone, which induces necrosis and ulceration by its own cytotoxic and immunosuppressive properties. HIV infection has not been reported as a risk factor for Buruli ulcer[23]. However, severe clinical forms of the disease have been described in HIV-positive patients[24]. To our knowledge, this is the first case of colonic infection with Mycobacterium ulcerans, which caused chronic diarrhea in a patient with AIDS.
In conclusion, Mycobacterium ulcerans and crypto-sporidium co-infection is the cause of chronic diarrhea in AIDS patients. Serologic test of HIV must be performed when evaluating chronic diarrhea, especially when it is associated with significant weight loss. We think that colonoscopy with routine biopsy is a useful tool for accurate diagnosis of chronic diarrhea.