Published online Feb 7, 2008. doi: 10.3748/wjg.14.776
Revised: November 15, 2007
Published online: February 7, 2008
AIM: To detect Salmonella enteritidis (S. enteritidis) in paraffin slices and antigen location in infected duck tissues.
METHODS: Rabbits were immunized with purified bacillus to obtain S. enteritidis-specific antibody, which were then extracted by the caprylic-ammonium sulphate method, purified through High-Q columns. An indirect immuno-fluorescent staining method (IFA) was established to detect the S. enteritidis antigen in paraffin slices. S. enteritidis was detected in each organ tissue of ducklings experimentally infected with S. enteritidis.
RESULTS: The gland of Garder, heart, kidney, spleen, liver, brain, ileum, jejunum, bursa of Fabricius from S. enteritidis experimentally infected ducklings were positive or strongly positive, and the S. enteritidis antigen was mainly distributed in the infected cell cytoplasm.
CONCLUSION: IFA is an intuitionist, sensitive and specific method in detecting S. enteritidis antigen in paraffin wax slices, and it is a good method in diagnosis and antigen location of S. enteritidis. We also conclude that the gland of Garder, heart, kidney, spleen, liver, ileum, jejunum are target organs in S. enteritidis infections of duck, and S. enteritidis is an intracellular parasitic bacterium.
-
Citation: Yan B, Cheng AC, Wang MS, Deng SX, Zhang ZH, Yin NC, Cao P, Cao SY. Application of an indirect immunofluorescent staining method for detection of
Salmonella enteritidis in paraffin slices and antigen location in infected duck tissues. World J Gastroenterol 2008; 14(5): 776-781 - URL: https://www.wjgnet.com/1007-9327/full/v14/i5/776.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.776
Salmonella enteritidis (S. enteritidis) is the infectious disease causing Zoonoses. S. enteritidis is one of the primary causes of human food poisoning throughout the world, and has become a pointed public health problem[1–4]. China is the biggest country in the raising and consumption of duck in the world, but the S. enteritidis bacillus infection is a severely important infectious disease in the duck industry[5]. S. enteritidis outbreaks have been found to be associated with the consumption of contaminated and undercooked poultry products, such as eggs and egg-containing products, and have become a serious economic and public health problem[6]. Conventional methods for isolation of S. enteritidis are too laborious, not sufficiently sensitive or correct[78]. To our knowledge, a serum method for detection and antigen location of S. enteritidis has not been reported. Thus, to establish a rapid, sensitive and highly specific method for detection of S. enteritidis is necessary. We established the Indirect Immunofluorescent (IFA) method to detect and antigen locate Salmonella enteritidis to offer a clinical means of diagnosis and apathogenic mechanism of ecology.
S. enteritidis MY1 strain (obtained from Avain Disease Research Center of Sichuan Agriculture University).
Briefly, 5 mL of S. enteritidis, an overnight culture grown in LB broth, was harvested by centrifugation after 18 h at 37°C. Then diluted at 4 × 109 cfu and inactivated by formaldehyde and equaled to complete Freund’s adjuvant (CFA) made the antigen. Rabbits were immunized with 1 mL antigen to obtain S. enteritidis-specific antibody, which was then extracted by the caprylic-ammonium sulphate method[9], purified through High-Q columns.
According to references[10], the following factors were considered to establish the method: (1) antigen recovery, to partly recover the antigens based on a microwave using 0.01 mol/L pH 6.0 citrate buffer solution for 20 min, 200 &mgr;g/mL parenzyme for 20min and no recovery; (2) rinsing water: 0.01 mol/L pH 7.4 PBS, 0.01 mol/L pH 7.4 PBS (containing 0.05% Tween-20) and rinsing water separately; (3) confining liquid: using 10% horse serum and 10% BSA to confine; (4) the diluted content of anti-rabbit-SE IgG: 1:25; 1:50; 1:100 and 1:200, incubate time: 4°C overnight and 37°C for 40 min; (5) the diluted content of the FITC labeled goat anti-rabbit IgG: 1:25; 1:50; 1:10 and 1:200; (6) foiled liquid: partly used 0.01% Evans Blue and unfoiled.
Experimental infections were performed with a S. enteritidis MY1 strain. In brief, a group of 24 (specific antibody of SE negative) Bei Jing duck were inoculated with 0.2 mL (2 × 109 cfu) of S. enteritidis at the age of 7 d in the crop. Twenty-four uninoculated 7-d-old ducks were used as control animals.
After 24 h post-inoculation some of the ducks began to die resulting in organ harvesting. The organs were detected by IFA and conventional isolation respectively.
Treatment included first, using a non-selective pre-enrichment medium of buffered Peptone Water, incubated at 37°C for 18 h. Then, using the selective enrichment media-Selenite Cystine Broth, incubating at 37°C for 18 h. And then, transferring to SS agar, two differential media, Macconkey agar and Triple sugar iron agar were streaked and incubated at 37°C for 24 h. Suspected colonies were picked up for biochemical and serological tests. The following tests were used: glucose, maltose, arabopyranose, mannitol, glycitol, hydrogen sulfide, MR, lysine decarboxylase, argininedecarboxylase, ornithinedecarboxylase, etc. Serotypes were identified by multivalence serum of OA-F and O1, 9, 12 factor serum. The results were referred to[1112].
The other organs were submerged in 4% formaldehyde, after 24 h; the organs were then embedded in paraffin. Then the tissue sections were made at 4 &mgr;m and stained with an indirect immunofluorescent technique.
The established method was applied to detect the livers from the dead ducks infected by S. pullorum, S. gallinarum, E. coli Riemerella anatipestifer, P. multocida and Duck plague virus (obtained from the Avian Disease Research Center of Sichuan Agriculture University).
The standard of the results are according to the presence, quantity, the depth and lighting of the coloured cells under the fluorescent microscope. There are no flavoviren cells judged negative, infra 5% weakly positive, 5%-50% positive, super 50% strong positive.
The purified anti-rabbit-SE IgG has an Antigen Jade Enlarge (AGE) potency to 1:32, through the SDS-PAGE, there are duplicate bands, and the molecular weight equals to the l-chain and H-chain of IgG. This suggests the IgG is highly pure.
The optimum conditions of this IFA were as follows: Ten minutes antigen retrieval by microwave with 0.01 mmol/L citrate buffer solution (pH 6.0); 10 min antigen retrieval by pancreatin; incubate in 10% bovine serum albumin at 37°C for 30 min; dilute the primary antibody (1:25) and incubate for 40 min at 37°C; and then incubated at 37°C for 30 min with diluted FITC-labeled-secondary antibody (1:100) which contains 0.01% Evans.
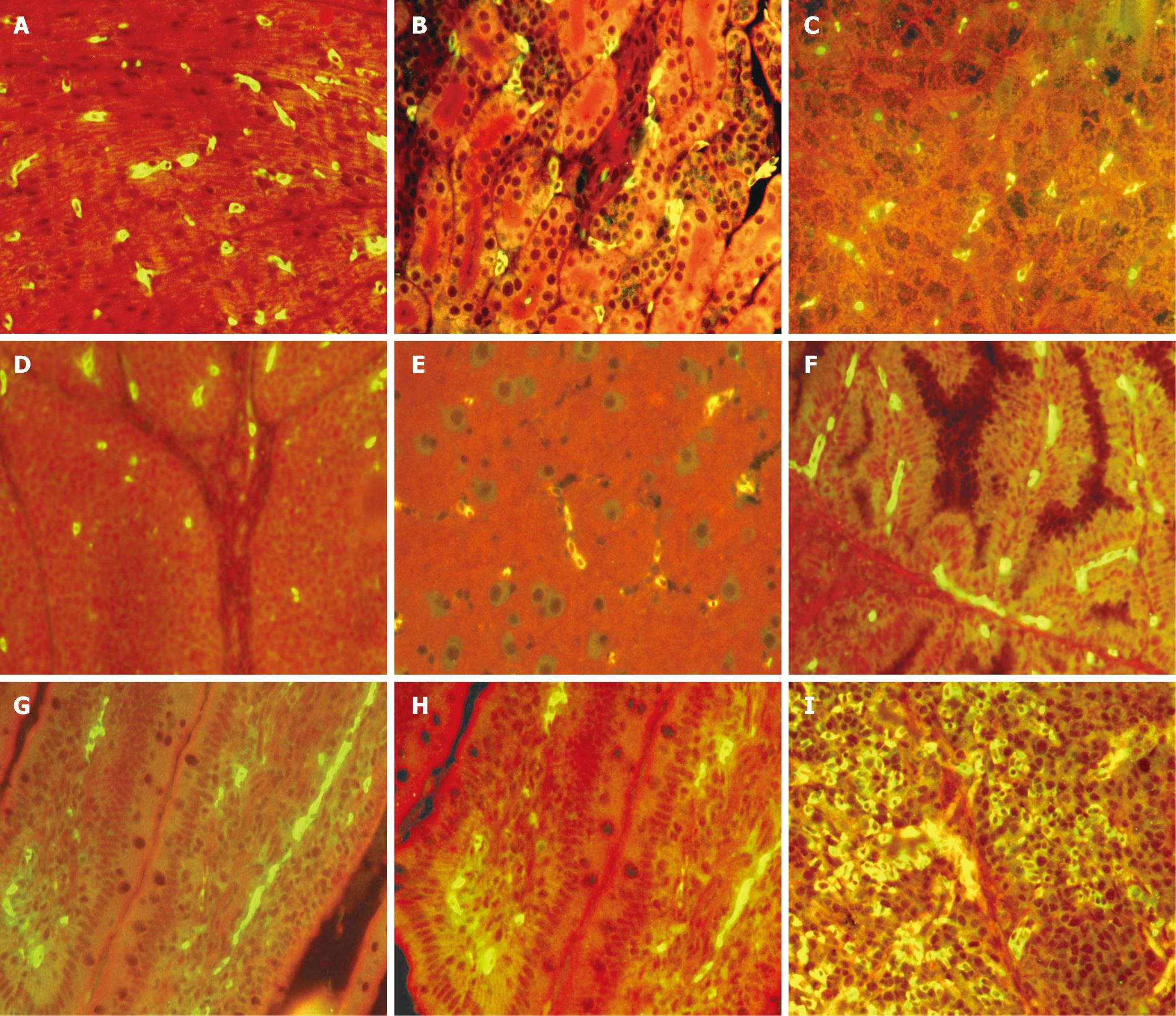
We detected the organs by IFA after 24 h, a large part of the organs had positive results, and the results were as follows (Figure 1): Heart: Presented as a strong positive, Cardiac muscle fiber had positive cells, and the positive signal distributing the cytoplasm (Figure 1A). Kidney: Presented positive, the mesenchyme between the tube of the Kidney had positive cells, and the positive signal distributing the cytoplasm (Figure 1B). Liver: Presented positive, the mesenchyme between the hepatic cord had positive cells, and the positive signal distributing the cytoplasm (Figure 1C). Bursa of Fabricius: presented positive, the area of the medulla of the follicle had positive cells, and the positive signal distributing the cytoplasm (Figure 1D). Brain: Presented positive, positive cells presented in capillary vessels, and the positive signal distributing the cytoplasm (Figure 1E). Gland of Garder: Presented as a strong positive, the mesenchyme of the tube of the gland had positive cells, and the positive signal distributing the cytoplasm (Figure 1F). Small intestine: Numerous S. enteritidis organs were observed lying free within the interstitial tissue of the lamina propria in the villi, and the strong positive signals observed in the cytoplasm (Figure 1G, H). Spleen: Presented strong positive, a number of positive cells with particle immunofluorescent antigen distributed in the red medulla of the spleen (Figure 1I).
All the positive samples detected by the isolation method can be detected by this IFA (Table 1).
| Detection | Results of different organs (faeces) by different methods (positive/total) | ||||||||
| methods | Heart | Liver | Spleen | Lung | Kidney | Intestine | Brain | Thymus | BF |
| Isolation | 2/24 | 24/24 | 24/24 | 2/24 | 24/24 | 24/24 | 3/24 | 0/24 | 8/24 |
| IFA | 24/24 | 24/24 | 22/24 | 0/24 | 24/24 | 20/24 | 2/24 | 0/24 | 5/24 |
We detected the livers from the dead ducks infected by S. pullorum, S. gallinarum, E. coli Riemerella anatipestifer, P.multocida and Duck plague virus, S. enteritidis. The result showed only the S. enteritidis infected, present positive reaction; the others have negative reaction. This suggested good establishment for specificity of the IFA (Table 2).
| Strains | S. pullorum | S. gallinarum | E. coli | R.A | P.multocida | DPV | SE |
| Results | - | - | - | - | - | - | + |
In the present study, there is little to report about the clinical diagnosis beside conventional methods of isolation for S. enteritidis, but there is much about other species. Gast et al[6] collected various organs of the infected birds and isolated the bacteria, but it is too laborious, not sufficiently sensitive and correct and can not be used with the rapid diagnoses. Kim et al[13] detected the S. enteritidis by enzyme immunoassay and had a good result. Desmidt et al[14] observed the S. enteritidis in the ceca of chickens by immunohistochemistry, which can be used to locate sub-cells of organ tissues of S. enteritidis infected chickens. Coope[15] detected the S. enteritidis by Dot-Blot and Western-Blot, which is rapid and specific, but could not detect the location in sub-cells of organ tissues. Compared to the above method, the IFA method established has the ability to detect and locate cells and organ tissues of S. enteritidis infection, thus offering a clinical means of diagnosis and a pathogenic mechanism of ecology.
We checked all the ducks when they began to die. At 24 h post-inoculation, the positive signals were observed in the small intestine and spleen; at 36 h, in the heart, kidney, livers, bursa of Fabricius, and Gland of Garder; at 72 h, the positive signals were at the peak; at 6 d post-inoculation, the signals began to reduce. From the result (Figure 1) of the study, we know that the IFA can detect the antigens in almost all organ tissues. The small intestine (ileum, jejunum), heart, liver, kidney, spleen, the Gland of Garder have strong positive signals (Figure 1A-C, I), and presented in the cytoplasm of phagocytic cells of the macrophile system. This may be caused by phagocytic cells after entering the host. Meanwhile, we can detect a positive signal in the brain, a positive signal presented in the vascular endothelial cell, which may be caused by S. enteritidis invading the organs and S. enteritidis in blood. At the anaphase of infection, the infection appeared bacteremic, the S. enteritidis needed to transit the vascular endothelial cells, and uniformly distributed in the cytoplasm of the vascular endothelial cells. We also detected small positive signals in the bursa of Fabricius.
Immuno-fluorescent labeled bacilli were associated with the epithelial surface of the intestinal villi at 4 h. At 24 a strong positive signal was detected intracellularly in the small intestine (Figure 1G, H). This resulted in a positive signal which only presented in the lamina propria of the villi. This is a big difference from Duck plague virus (DPV)[16] and Duck Hepatitis Virus (DHV)[17], which was located in the lamina propria. This variation might be related to a different microenvironment[18] in different parts of the gut, dividing into a microcolony at that site. The microenvironment has an important effect in the colonization of the gut, primarly as follows. Gastric acid is the first defense of the intestinal infection, the bacterium can hardly survive (pH < 1.5). The slime layer of the intestinal mucosa has a barrier function to the S. enteritidis; moreover pankrin, bile salt and small intestinal juice can depress the bacterium. Some studies suggest that the gene encoding the invasive protein is depressed because of the bile[1920]. Meanwhile, the involvement of mucosal IgA in the protection against S. enteritidis involving the epithelial surface has been reported[2122]. The cathodolyte antibacterial peptide secreted by pit cells in the small intestine belongs to the Toalexin family, which can increase the permeability of the cytomembrane of the bacterium, and not profit to survive[23].
The intestine is an important barrier for prevention against invasion by bacteria. The lymphoid tissue called Peyer macular under the muscoa[24] consists of T cells, B cells, macrophages and dendritic cells, following the swallowing of the pathogenic bacterium.
The immune reaction also played an important role in preventing S. enteritidis lying free in the endothelial cells of the intestine. After swallowing the S. enteritidis by macrophage and heterophil granulocyte, both happened to outbreak by breathing, which produced oxygen free radical, for instance, O2-, H2O2, OH-, and O2- and so on. The oxygen free radical can kill and wound bacteria; the cell factors (IL-1, IL-6, TNF-α[25]) composed and released have a pathopoiesis effect and a bactericidal effect; when the S. enteritidis invades the enterocyte, it can induce the secretion of IL-8[26], which can kill and wound bacteria. CD4+ T cells also have an effect on the immune defense[27].
Infection with bacteria is usually started by oral ingestion of the pathogen and is followed by bacterial colonization of the gut and invasion of internal tissues. Intracellular replication is essential for the virulence.
Attachment to host tissues is the first important step to establish a bacterial infection. The attachment is primarily referred to as the inv gene[28]. Once attached to the host cells, the bacterium manifests athletic phenomenon of invasion of the cells, phagocytosis by cell invagination. After fusion of macrophage lysosomes with phagosomes, the S. enteritidis appears in the phagocytotic vesicle of the cytoplasm[29], and may destroy the cell invagination. In the present study, there are different reports about the S. enteritidis entering the cell, the bulk of the investigations support the view that S. enteritidis enters the cell by a virulence factor. Higashide and McGhie suggest that when S. enteritidis enter the cell, firstly SspC excreted by TTSS insert into the plasma membrane, then Sip and SipA bind to the actin of the cytoskeleton and rearrange the frame, which enhances the entrance of the bacterium[3031]. Scientists report recently that the dendritic cell (DC) residing in the mucosa of the small intestine can use the dendrite to open the tight junction of the cells, and directly ingest the bacteria from the mucosa[32]. From the result (Figure 1), the positive signal distributed in the cytoplasm of infected cells of the organ tissues, we conclude that S. enteritidis is an intracellular parasitic bacterium.
Salmonella enteritidis (S. enteritidis) is the infectious disease causing Zoonoses. S. enteritidis is one of the primary causes of human food poisoning throughout the world. China is the biggest country in growing and consumption in the world, but the S. enteritidis bacillus infection is the severely important infectious disease in raising duck’s industry. But the pathogenic mechanism of ecology is unknown.
The S. enteritidis can be isolated from various organs of the infected birds. It is possible that the S. enteritidis invades the tissues of these organs; electron microscopic studies of chicks have shown passage of Salmonella through ileocecal mucosa. The observation of attachment and invasion of the ceca of infected chicks have been reported. Also, in mammalian animal models, the colonization of S. enteritidis has been studied further. However, there are few studies about ducks.
The present study was to establish a serum method for detection and antigen location of S. enteritidis which has not been reported.
The results can offer a clinic means of diagnosis and pathogenic mechanism of ecology.
This is a methodology manuscript. The author of this manuscript establishes a method of IFA and detected the organs of the S. enteritidis infected ducks. The author conclude that IFA is a sensitive and specific method in detecting S. enteritidis antigen in paraffin slice; it’s a good method in diagnosis and antigen location of S. enteritidis; the Gland of Garder, heart, kidney, spleen, liver, and small intestine are target organs in S. enteritidis infections of duck; S. enteritidis is an intracellular parasitic bacterium.
| 1. | Chiu CH, Su LH, Chu C. Salmonella enterica serotype Choleraesuis: epidemiology, pathogenesis, clinical disease, and treatment. Clin Microbiol Rev. 2004;17:311-322. |
| 2. | Baskerville A, Humphrey TJ, Fitzgeorge RB, Cook RW, Chart H, Rowe B, Whitehead A. Airborne infection of laying hens with Salmonella enteritidis phage type 4. Vet Rec. 1992;130:395-398. |
| 3. | Fantasia M, Filetici E. Salmonella enteritidis in Italy. Int J Food Microbiol. 1994;21:7-13. |
| 4. | Lee WC, Sakai T, Lee MJ, Hamakawa M, Lee SM, Lee IM. An epidemiological study of food poisoning in Korea and Japan. Int J Food Microbiol. 1996;29:141-148. |
| 5. | Angulo FJ, Swerdlow DL. Epidemiology of human. Salmonella enterica serovar Enteritidis infections in the United States. 7th ed. Ames: Iowa State University Press. 1999;33–41. |
| 6. | Braden CR. Salmonella enterica serotype Enteritidis and eggs: a national epidemic in the United States. Clin Infect Dis. 2006;43:512-517. |
| 7. | Gast RK, Beard CW. Isolation of Salmonella enteritidis from internal organs of experimentally infected hens. Avian Dis. 1990;34:991-993. |
| 8. | Keller LH, Schifferli DM, Benson CE, Aslam S, Eckroade RJ. Invasion of chicken reproductive tissues and forming eggs is not unique to Salmonella enteritidis. Avian Dis. 1997;41:535-539. |
| 9. | Kuper CF, Koornstra PJ, Hameleers DM, Biewenga J, Spit BJ, Duijvestijn AM, van Breda Vriesman PJ, Sminia T. The role of nasopharyngeal lymphoid tissue. Immunol Today. 1992;13:219-224. |
| 10. | Homburger HA, Cahen YD, Griffiths J, Jacob GL. Detection of antinuclear antibodies: comparative evaluation of enzyme immunoassay and indirect immunofluorescence methods. Arch Pathol Lab Med. 1998;122:993-999. |
| 11. | Gast RK, Beard CW. Serological detection of experimental Salmonella enteritidis infections in laying hens. Avian Dis. 1990;34:721-728. |
| 12. | Chart H, Rowe B, Baskerville A, Humphrey TJ. Serological analysis of chicken flocks for antibodies to Salmonella enteritidis. Vet Rec. 1990;127:501-502. |
| 13. | Kim CJ, Nagaraja KV, Pomeroy BS. Enzyme-linked immunosorbent assay for the detection of Salmonella enteritidis infection in chickens. Am J Vet Res. 1991;52:1069-1074. |
| 14. | Desmidt M, Ducatelle R, Haesebrouck F. Research notes: Immunohistochemical observations in the ceca of chickens infected with Salmonella enteritidis phage type four. Poult Sci. 1998;77:73-74. |
| 15. | Cooper GL, Thorns CJ. Evaluation of SEF14 fimbrial dot blot and flagellar western blot tests as indicators of Salmonella enteritidis infection in chickens. Vet Rec. 1996;138:149-153. |
| 16. | Yuan GP, Cheng AC, Wang MS, Liu F, Han XY, Liao YH, Xu C. Electron microscopic studies of the morphogenesis of duck enteritis virus. Avian Dis. 2005;49:50-55. |
| 17. | Zhao XL, Phillips RM, Li GD, Zhong AQ. Studies on the detection of antibody to duck hepatitis virus by enzyme-linked immunosorbent assay. Avian Dis. 1991;35:778-782. |
| 18. | Popiel I, Turnbull PC. Passage of Salmonella enteritidis and Salmonella thompson through chick ileocecal mucosa. Infect Immun. 1985;47:786-792. |
| 19. | Prouty AM, Brodsky IE, Manos J, Belas R, Falkow S, Gunn JS. Transcriptional regulation of Salmonella enterica serovar Typhimurium genes by bile. FEMS Immunol Med Microbiol. 2004;41:177-185. |
| 20. | Cunliffe RN. Alpha-defensins in the gastrointestinal tract. Mol Immunol. 2003;40:463-467. |
| 21. | Sheela RR, Babu U, Mu J, Elankumaran S, Bautista DA, Raybourne RB, Heckert RA, Song W. Immune responses against Salmonella enterica serovar enteritidis infection in virally immunosuppressed chickens. Clin Diagn Lab Immunol. 2003;10:670-679. |
| 22. | Hassan JO, Porter SB, Curtiss R 3rd. Effect of infective dose on humoral immune responses and colonization in chickens experimentally infected with Salmonella typhimurium. Avian Dis. 1993;37:19-26. |
| 23. | Acheson DW, Luccioli S. Microbial-gut interactions in health and disease. Mucosal immune responses. Best Pract Res Clin Gastroenterol. 2004;18:387-404. |
| 24. | Mahida YR, Cunliffe RN. Defensins and mucosal protection. Novartis Found Symp. 2004;263:71-77; discussion 77-84, 211-218. |
| 25. | Chowers Y, Cahalon L, Lahav M, Schor H, Tal R, Bar-Meir S, Levite M. Somatostatin through its specific receptor inhibits spontaneous and TNF-alpha- and bacteria-induced IL-8 and IL-1 beta secretion from intestinal epithelial cells. J Immunol. 2000;165:2955-2961. |
| 26. | Salerno-Goncalves R, Wyant TL, Pasetti MF, Fernandez-Vina M, Tacket CO, Levine MM, Sztein MB. Concomitant induction of CD4+ and CD8+ T cell responses in volunteers immunized with Salmonella enterica serovar typhi strain CVD 908-htrA. J Immunol. 2003;170:2734-2741. |
| 27. | Leung KY, Finlay BB. Intracellular replication is essential for the virulence of Salmonella typhimurium. Proc Natl Acad Sci USA. 1991;88:11470-11474. |
| 28. | Amin II, Douce GR, Osborne MP, Stephen J. Quantitative studies of invasion of rabbit ileal mucosa by Salmonella typhimurium strains which differ in virulence in a model of gastroenteritis. Infect Immun. 1994;62:569-578. |
| 29. | Oh YK, Alpuche-Aranda C, Berthiaume E, Jinks T, Miller SI, Swanson JA. Rapid and complete fusion of macrophage lysosomes with phagosomes containing Salmonella typhimurium. Infect Immun. 1996;64:3877-3883. |
| 30. | Higashide W, Dai S, Hombs VP, Zhou D. Involvement of SipA in modulating actin dynamics during Salmonella invasion into cultured epithelial cells. Cell Microbiol. 2002;4:357-365. |
| 31. | McGhie EJ, Hayward RD, Koronakis V. Cooperation between actin-binding proteins of invasive Salmonella: SipA potentiates SipC nucleation and bundling of actin. EMBO J. 2001;20:2131-2139. |
| 32. | Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl JP, Ricciardi-Castagnoli P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361-367. |