INTRODUCTION
Kupffer cells[1], resident macrophages in the liver, constitute the largest population of macrophages in the body and are critical effector cells for the defense against invading micro-organisms and foreign molecules. On the other hand, Kupffer cells in the pathogenesis of different types of liver injury[2–4] have been demonstrated to be involved in several models of liver damage induced by chemicals including carbon tetrachloride, alcohol and allyl alcohol[56]. The pathologic role of Kupffer cells has been confirmed by the attenuation of hepatotoxicity in terms of steatosis, inflammation, and necrosis in animals. It is hypothesized that Kupffer cells are activated in response to chemical administration and promote tissue damage by releasing biologically active mediators such as prostaglandins, TNFα, oxygen free radicals, and other proinflammatory mediators[7–9]. Platelet-activating factor (PAF) is the trivial name for a phospholipid called 1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine that has unique phospholipid autacoid exhibiting diverse biological activities in various tissues and organs such as isolated lung, liver and brain, and different types of cells (platelet, neutrophils, endothelial cells, neural cells)[10–15]. These activities include platelet secretion and aggregation, broncho-constriction, increased vascular permeability, and systemic hypotension[16–18]. Previous studies demonstrate that PAF could raise portal vein pressure and stimulate glycogenolysis[1920], the infusion of low doses of synthetic PAF into the systemic circulation of normal rats caused hyperdynamic status, and PAF production may be dangerous in patients with portal hypertension[2021]. PAF acts as both a multifunctional soluble proinflammatory agent and a specific membrance-bound adhesion molecule[22–24]. Since the biological effects of PAF are mediated by its specific receptor[2526], almost all actions of PAF can be prevented by blocking its receptor with antagonists. PAF binding to its receptor induces release of arachidonic acid and produces cycloxygenase-derived eicosanoids[27], glycogenolysis, etc. which may mediate vasoconstriction and affect portal vein pressure and hemodynamics. Recent studies have shown that Kupffer cells possess a large number of high affinity PAF receptors which, when stimulated, synthesize and release several active lipid mediators such as eicosanoides and PAF[28]. Houssert et al showed that Kupffer cells contain mRNA for the endothelin B (ETB) receptor. However, they did not measure ET binding in Kupffer cells. PAF synthesis/release and its receptor expression in activated Kupffer cells are far from clear in carbon tetrachloride induced cirrhosis. The aim of this study was to elucidate the characteristics of PAF synthesis/release and its receptor expression of the Kupffer cells in rat carbon tetrachloride-induced cirrhosis.
MATERIALS AND METHODS
Establishment of animal model of liver cirrhosis
The experimental protocols were approved by the Animal Care and Use Committee of the Institute of Infectious Diseases of PLA. Cirrhosis was induced in male Sprague-Dawley rats (230-250 g) by intraperitoneal injection of CCl4 (0.15 mL/kg twice a week for 8 wk). Control rats received injection of the carrier (peanut oil). Rats were allowed access to water containing phenobarbital (0.4 g/L) as the only drink source throughout the induction four days before the CCl4 treatment. This treatment caused complete distortion of the hepatic architecture due to excessive deposition of the extracellular materia (ECM), portal hypertension, hyperdynamic state and ascites in more than 70% of rats.
Preparation of Kupffer cells
Kupffer cells were prepared from carbon tetrachloride induced male Sprague-Dawley rats . Briefly, after the collagenase (type IV from clostridium histoticum, 0.025%)/protease (type XIV, from streptomyces griseus, 0.05%) digestion of liver and removal of the hepatocytes and cells debri by low speed centrifugation, Kupffer cells and endothelial cells were purified from other nonparenchymal cells by density gradient centrifugation using 30%(W/V) metrizamide. Kupffer cells were then isolated with a modified centrifugal elutriation procedure. The viability of the Kupffer cells was greater than 95% as determined by trypan blue exclusion. Finally, Kupffer cells were suspended in Williams' medium E (Sigma) containing 10% heat-inactivated fetal calf serum, 5000 U/mL penicillin and 5000 &mgr;g/mL streptomycin and plated onto 12- well culture plates at a density of 0.5 × 106 cells/cm2, and incubated in an atmosphere of 95% air/5% CO2 at 37°C. The medium was renewed after incubated for 3 h and the cells were used for experiments after an overnight incubation. Purity of the cells determined was greater than 95% by immunostaining with ED2 (Kupffer cells), desmin (stellate cells) and factor VIII related antigen (endothelial cells).
Determination of PAF level in associated Kupffer cells and medium
Lipid extraction: Lipid in associated Kupffer cells and medium was extracted according to the method of Bligh & Dyer. In brief, 1 mL medium was added with methanol (2.5 mL) and chloroform (1.25 mL) was prepared (methanol: chloroform:medium is 2:1:0.8 v:v:v). The mixture was kept for 1 h at room temperature, and added with chloroform (1.25 mL) and water (1 mL), (methanol:chloroform:water is 1:1:0.9 v:v:v). The new mixture was kept for 1 h at room temperature and centrifuged at 1200 ×g for 15 min. The chloroform layer was dried under a stream of nitrogen at 35°C. In associated Kupffer cells, methanol (2.5 mL), chloroform (1.25 mL) and water (1 mL) (methanol:chloroform:water is 2:1:0.8 v:v:v) were added into the medium.
Purification of PAF: Amprep silica mini-column was used according to the instructions of its manufacturer. In brief, residue was dissolved in 200 &mgr;L chloroform and applied to a Bond Elut SI column. The column was washed with 3 mL chloroform, 2 mL chloroform-methanol (3:2, v:v) and 3 mL chloroform-methanol-28% aqueous ammonia (70:85:7, v:v:v), and eluted with 2 mL chloroform-methanol-28% aqueous ammonia (50:50:7, v:v:v). Eluate was evaporated till dried, and the residue was dissolved in 200 &mgr;L saline containing 0.1% Triton X-100.
Determination of PAF: Platelet activating factor [3H] scintillation proximity assay (SPA) system kit (Amersham Pharmacis Biotech, USA) was used according to the instructions of the manufacturer.
Morphometric analysis: The activated Kupffer cells were incubated for a day and stained by the method of immunohistochemistry to recognize membrane-bound PAF within activated Kupffer cells. The primary antibody used was a sheep anti-PAF polyclonal preparation [platelet activating factor [3H] scintillation proximity assay (SPA) system kit, Amersham Pharmacis Biotech, USA] The primary antibody was incubated overnight and followed by 0.5 h with secondary antibody in streptavidin-horseradish peroxidase at 1:2500 dilution (DAKO LSAB+ kit, peroxidase, KO679).
Determination of mRNA expression of PAF receptor in activated Kupffer cells
The levels of the low abundance mRNA transcripts of PAF receptor were determined by semiquantitative reverse transcriptase polymerase chain reaction (RT-PCR) using the following procedure. β-actin mRNA expression was determined using the same amount of cDNA as β-actin mRNA. RNA was isolated from the livers using an RNA isolation kit (ToTALLY RNATM, Ambion, Austin, TX) as per the instructions of the manufacturer. The concentration of RNA was determined by measuring A260, and the purity was checked by the A260/A280 ratio (greater than 1.8). Two &mgr;g total RNA obtained from Kupffer cells was used for the preparation of cDNA by reverse transcriptase-PCR. The PCR primers for PAF cDNA and β-actin cDNA are: 5’-GCCACAACACAGAGGCTTGA-3’ (F) and 5’-TCCATTGCTCTGGGCAGGAA-3’ (R) (121 bp); 5’-TTCTACAATGAGCTGCGTGTG-3’ (F) and 5’-TTCATGGATGCCACAGGATTC-3’ (R) (561 bp), respectively. DNA equivalent of 5 ng original RNA was used in PCR. The reaction mixture (50 &mgr;L) contained 10 mmol/L Tris-HCl (pH 8.3), 50 mmol/L KCl, 1.5 mmol/L MgCl2, 0.2 mmol/L dNTPs, 20 pmol PCR primer and 2 units of Platinum Taq DNA polymerase (GIBCO-Invitrogen, Carlsbad, CA). The reaction for PAF receptor was run for 35 cycles as follows: denaturation at 94°C for 1 min, annealing at 60°C for 30 s, and extension at 72°C for 30 s. The PCR products were resolved in a 2.5% agarose gel and stained with 1 × SYBR Green I (FMC Biproduct, Rockland, ME). The gels were scanned under blue fluorescence light using a phosphorimager and the band intensity was quantified using ImageQuant software (Molecular Dynamics, Sunnyvale, CA, USA).
Determination of PAF receptor in activated Kupffer cells
After an overnight incubation, Kupffer cells were washed and incubated in 50 mmol/L Tri-HCl , pH 7.2 (5 mmol/L MgCl2, 125 mmol/L choline chloride, 0.25% BSA) containing 0.125-32 nmol/L 3H -PAF (151 Ci/mmol, UK) ± 10 &mgr;mol/L unlabelled PAF (C16) (saturation binding) at 22°C for 3 h. The reaction was terminated with the addition of 5 mL ice-cold assay buffer, the cells were washed in assay buffer and digested with 5% SDS at 60°C for 30 min. Radioactivity was detected with 5 mL β-scintillation solution. The results were expressed as CPM of 3H -PAF bound per &mgr;g protein.
Determination of mRNA expression of preproET-1 and ET-1 receptors in Kupffer cells
Semiquantitative RT-PCR assay was employed to assess the relative levels of preproET-1, ET-1 receptors in Kupffer cells. β-actin mRNA expression was determined using the same amount of cDNA. The PCR primers specific for prproET-1 cDNA are: 5’-CCAACTCTGGGCTCTCCATGCTGG-3’ (F) and 5’-GAATGGCACTGTGTCTCTGCTCTC-3’ (R) [241 bp]; for ETA cDNA: 5’-CCCTTCGAATACAAGGGCGA-3’ (F) and 5'-GAAGAGGGAACCAGCACAGTTTCTACTTCTGC-3' (R) [291 bp]; and for ETB cDNA: 5’-GGCTGTTCAGTTTTCTACTTCTGC-3’ (F) and 5’-AGAATCCTGCTGAGGTGAAGG-3’ (R) [210 bp]. PCR amplification reaction was performed as follows. PCR products were resolved in a 1.2% agarose gel and stained with 1 × SYBR Green I (FMC Biproduct, Rockland, ME, USA). The other procedures were the same for the PAF receptor mentioned above.
Determination of ET-1 receptor in Kupffer cells
After an overnight incubation, activated Kupffer cells were washed with HBSS containing 10 mmol/L HEPES, pH 7.4, and 0.1% bovine serum albumin (HBSS/BSA) and then placed in the medium containing 6.25-800 pmol/L [125I]-ET-1 ± 1 &mgr;mol/L unlabelled ET-1 (saturation binding). In the competition binding assay, cells were incubated with 50 pmol/L [125I]-ET-1 ± 1 &mgr;mol/L unlabelled ET-1, 5 &mgr;mol/L ETA antagonist BQ-123 or 5 &mgr;mol/L ET B antagonist BQ-788. After incubation at 22°C for 3 h, the cells were washed with HBSS/BSA and digested with 0.75N NaOH for determination of radioactivity. Specific binding of [125I]-ET-1 was different in cell-associated radioactivity in the presence and absence of 1 &mgr;mol/L unlabelled ET-1.
Statistical analysis
The values are presented as averages of triplicate determinations ± SEM. Each experiment was repeated at least three times using cells from different animals. Student t test was employed for statistical comparison of the paired samples. A P value of < 0.05 was considered statistically significant.
RESULTS
Effect of ET-1 on PAF synthesis in activated Kupffer cells
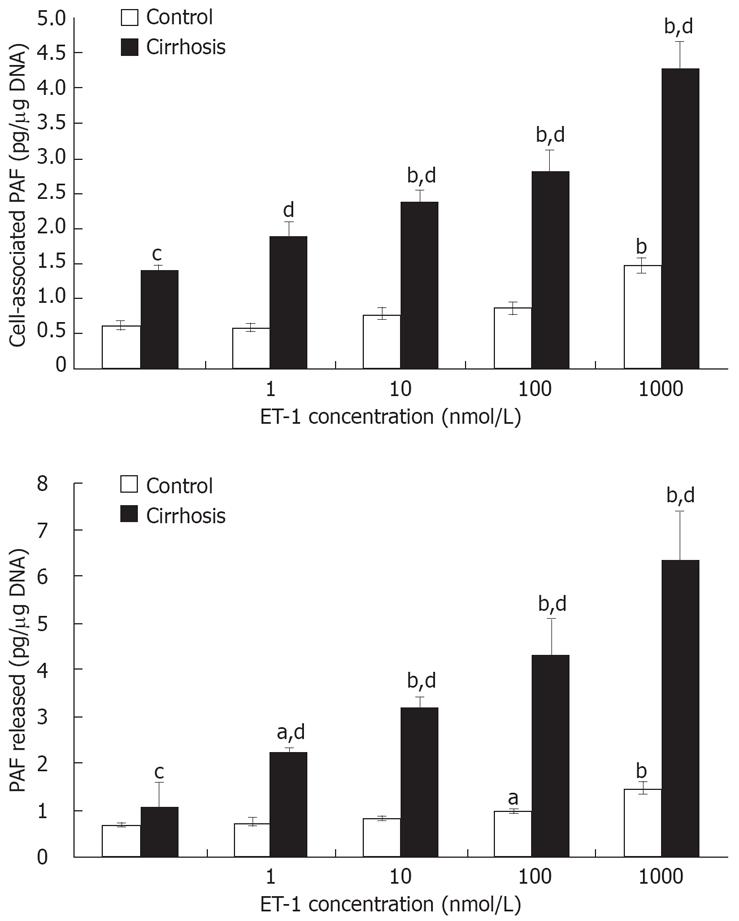
Basal release of PAF was increased by about two-fold in cirrhotic rat Kupffer cells as compared with the control (1.42 ± 0.14 vs 0.66 ± 0.04 pg/&mgr;g DNA, P < 0.01), while basal release of membrane-bound platelet-activating factor was only increased by about 1.48-fold (1.02 ± 0.06 vs 0.69 ± 0.07 pg/&mgr;g DNA, P < 0.01) (Figure 1). Interestingly, activated Kupffer cells appeared to release more PAF than membrane-bound platelet-activating factors. Application of ET-1 to rat Kupffer cells induced the PAF synthesis in both control and activated Kupffer cells (Figure 1). However, the effect was more pronounced in the latter. Significantly induced PAF synthesis occurred at 100 nmol/L ET-1 in control and cirrhotic Kupffer cells, and therefore, statistical significance was observed at 1-1000 nmol/L ET-1. Immunochemical detection of ET-1 induced PAF synthesis in cirrhotic and normal Kupffer cells showed that cytoplasm PAF was stained, strongly and increased in a concentration-dependent manner (B, D pulsed ET-1 100 nmol/L), and the cytoplasm of PAF staining in activated Kuppffer cells increased more significantly than that in the controls.
Figure 1 Effect of ET-1 on PAF synthesis in cultured activated Kupffer cells.
Kupffer cells from control and cirrhotic rats were cultured and stimulated with increasing concentrations of ET-1 for 15 min in serum-free medium. Cell-associated and released PAF levels were determined as explained in the Methods section. Each point represents an average of triplicate values ± SEM from a representative of two experiments. aP < 0.05 and bP < 0.01 vs vehicle; cP < 0.05 and dP < 0.01 vs control.
PAF receptor expression in cirrhotic Kupffer cells
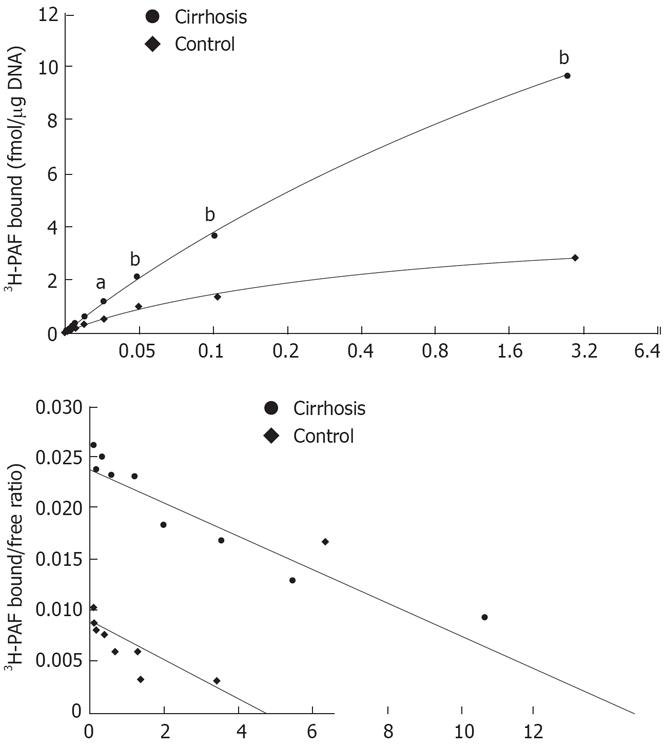
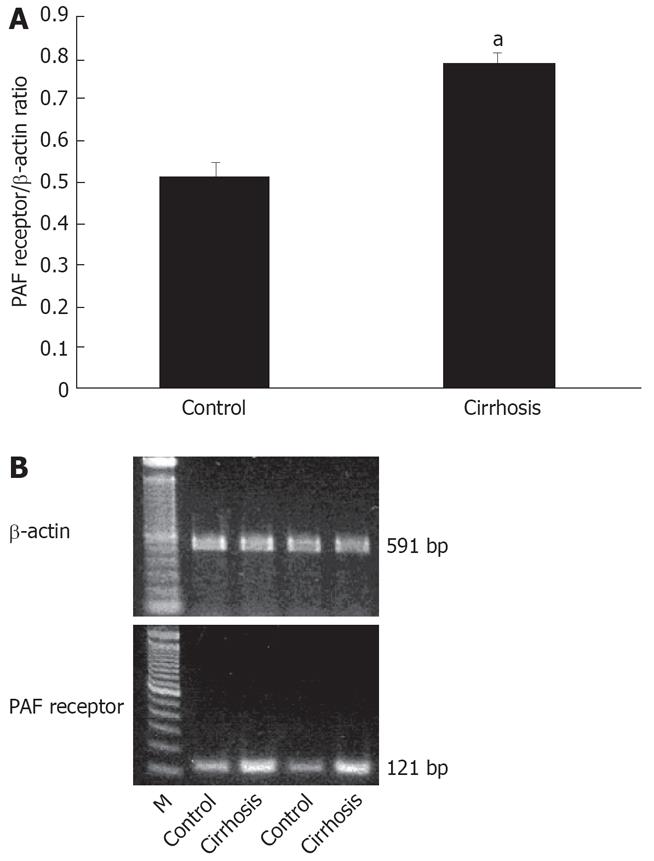
Scattered blot analysis of the saturation binding data revealed about a six-fold increase in 3H -PAF binding capacity in cirrhotic Kupffer cells as compared with the controls (Bmax of 27.19 ± 2.09 vs 4.40 ± 0.32 fmol/&mgr;g. DNA). The affinity of the receptors was not altered in the cirrhosis (Kd of 2.08 ± 0.22 nmol/L in the cirrhosis and 1.57 ± 0.17 nmol/L in the control) (Figure 2A and B). In cirrhosis, PAF receptor expression of activated Kupffer cells increased 1.5-fold as agaist the control (0.78 ± 0.03 vs 0.52 ± 0.03) (Figure 3A and B), which was consistent with PAF receptor saturation binding data.
Figure 2 A: Saturation curve binding to cirrhotic and normal rat Kupffer cells.
Cells were used after overnight culture for receptor binding assay as described in the Method section. 3H-PAF at a concentration between 0.125 and 3.2 nmol/L was incubated with Kupffer cells in presence or absence of 5 &mgr;mol/L unlabeled PAF at 22°C for 3 h. aP < 0.05, bP < 0.01 vs control; B: Scattered blot analysis of 3H-PAF binding to cirrhotic and normal rat Kupffer cells. The results were analyzed by scatchard plot. Cirrhosis: R = 0.99, Kd = 2.08 nmol/L, Bax = 27.1882 ± 2.0885 fmol/mg. DNA; Control: R = 0.96, Kd = 1.57 nmol/L, Bax = 4.4024 ± 0.3155 fmol/mg. DNA.
Figure 3 A: The mRNA expression of cirrhotic rat Kupffer cell PAF receptor.
Expression of β-actin mRNA (β-actin control: 90 311.14 ± 3882.67; cirrhosis: 110 958.2 ± 1484.58). aP < 0.05 vs control; B: Character of the PAF receptor expression in cirrhotic Kupffer cells. Total RNA was extracted from the cells for determination of PAF receptor mRNA expression by semiquantitative reverse transcriptase polymerase chain reaction assays as described in the Method section.
Preproendothelin-1 and ET-1 receptor expression in cirrhotic Kupffer cells
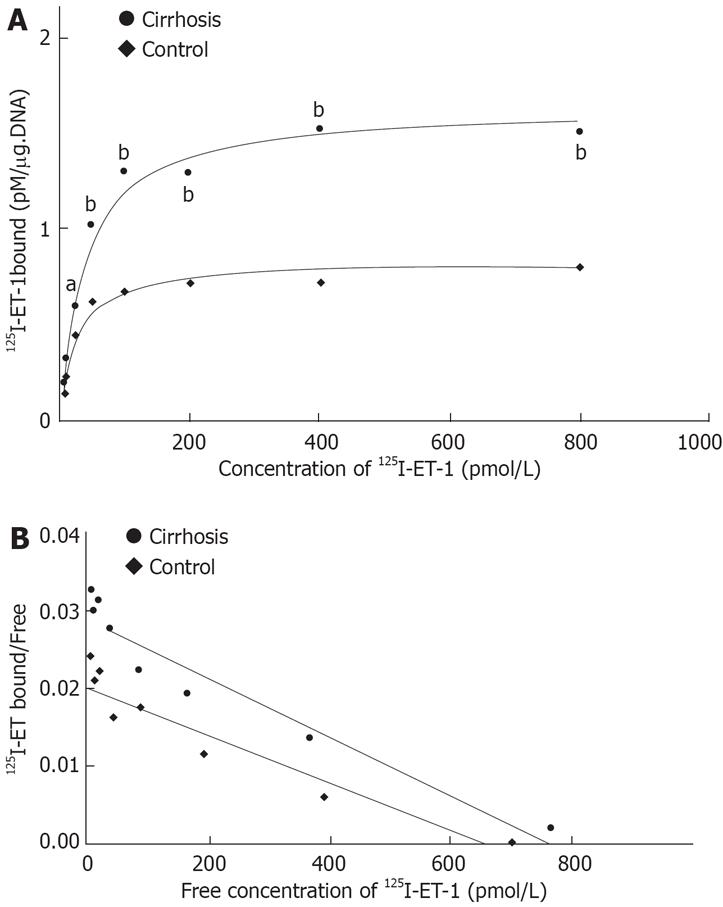
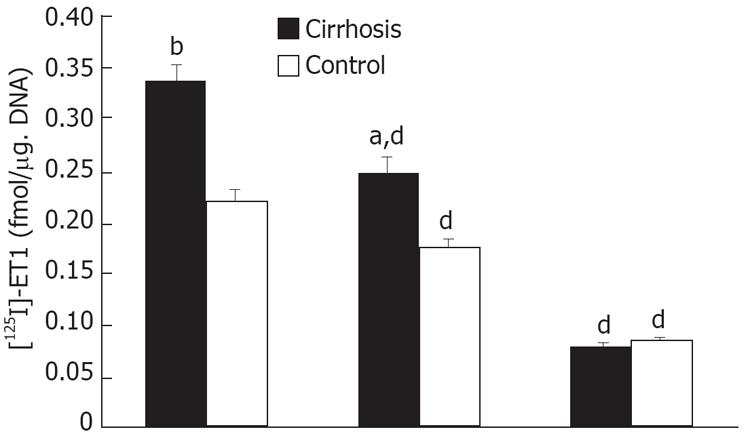
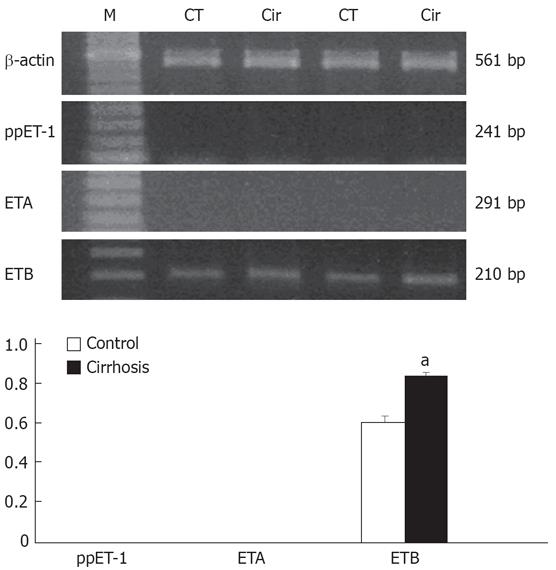
Figure 4 illustrates the scattered blot of the results of [125I]-ET-1 saturation binding assays in cirrhotic Kupffer cells. Activated Kupffer cells increased their binding capacity by about two-fold as compared with the control (Bmax of 1639 ± 27.71 vs 820.4 ± 28.75 fmol/&mgr;g DNA). No change was observed in the affinity of the receptors in the cirrhosis (Kd of 37.72 ± 6.08 pmol/L in cirrhosis and 22.79 ± 3.46 pmol/L in the control). As shown in Figure 5, in 50 pmol/L [125I]-ET-1, the [125I]-ET-1 specific binding capacity increased by about 1.7-fold (Bmax of 0.34 ± 0.02 vs 0.20 ± 0.01 fmol/&mgr;g DNA). In the cirrhotic Kuppfer cells, the number of ET-1 receptor was larger than that of the control. ETA receptor antagonist BQ-123 did not affect [125I]-ET-1 specific binding (specific binding: 0.34 ± 0.02 fmol/&mgr;g DNA; BQ-123: 0.25 ± 0.02 fmol/&mgr;g DNA in cirrhosis; specific binding: 0.22 ± 0.01 fmol/&mgr;g DNA, BQ-123: 0.18 ± 0.01 fmol/&mgr;g DNA in control), but ETB receptor antagonist BQ-788 significantly blocked [125I]-ET-1 specific binding (cirrhosis: 0.08 ± 0.003 fmol/&mgr;g DNA; control: 0.09 ± 0.002 fmol/&mgr;g DNA). This revealed that activated Kupffer cells only increased the number of ETB receptor, but never changed the ET-1 receptor expression. Figure 6 shows the characteristics of the expression of preproendothelin-1 and ET-1 receptor subtypes in the Kupffer cells of cirrhotic rats. The activated Kupffer cells neither expressed preproendothelin-1 nor changed the expression of ET-1 receptor subtypes, they only expressed ETB receptor which was increased by 166.07% as compared with the control (0.34 ± 0.01 vs 0.21 ± 0.01).
Figure 4 A: Saturation curve of 125I-ET-1 binding to cirrhotic and normal rat Kupffer cells.
125I-ET-1 at a concentration between 6.25-800 pmol/L was incubated with Kupffer cells (1 × 106), in presence or absence of 2 &mgr;mol/L unlabeled ET-1 at 20°C for 3 h; B: Scattered blot analysis of 125I-ET-1 binding to cirrhotic and normal rat Kupffer cells. The result was analyzed by scatchard plot. Cirrhosis: R = 0.98, Kd = 37.72 pmol/L, Bax = 1639 ± 67.7 fmol/mg. DNA; Control: R = 0.94, Kd = 22.79 pmol/L, Bax = 820.4 ± 28.7 fmol/mg. DNA.
Figure 5 Competition binding assay (50 pmol/L 125I-ET-1 ± 1 &mgr;mol/L unlabelled ET-1, 5 &mgr;mol/L ETA antagonist BQ-123 or 5 &mgr;mol/L ETB antagonist BQ-788).
Specific binding of [125I]-ET-1 was different in cell-associated radioactivity in the presence and absence of 1 &mgr;mol/L unlabelled ET-1. aP < 0.05, bP < 0.01 vs control; dP < 0.01 vs [125I]-ET-1.
Figure 6 Characteristics of the preproendothelin-1 and ET-1 receptor subtype expression in the cirrhotic rat Kupffer cells.
RNA was extracted from the cells for determination of preproET-1 and ET-1 receptor subtypes mRNA expression by semi-quantitative reverse transcriptase polymerase chain reaction assay. Graphical presentation of data as standardized relative to expression of β-actin mRNA (β-actin control: 90 311.14 ± 3882.67; cirrhosis: 11 095.82 ± 1484.58). aP < 0.05 vs control.
DISCUSSION
Elucidation of the interactions between activated Kupffer cells and ET-1 as a method for detecting hepatic cirrhosis and attendant hemodynamic abnormalities in pathophysiology, has been a topic of intensive investigations in the recent years. Previous studies have demonstrated that ET activates both phosphoinositide metabolism and prostaglandin synthesis in rat Kupffer cells[30] and ET-1 stimulates PAF synthesis in Kupffer cells[29], while the current study has demonstrated that ET results in biosynthesis and release of PAF[31] in cultured rat Kupffer cells. The diversity of ET actions in Kupffer cells might be explained by the existence of more than one receptor subtype or activation of more than one signal transduction pathway after endothelin binding. Houssert[32] found that Kupffer cells contain mRNA for the ETB receptor, however they did not measure ET binding in Kupffer cells. Based on the growing body of evidence regarding hepatic cirrhosis, it is evident that Kupffer cells act as critical effector cells in the pathogenesis of cirrhosis and hemodynamic abnormalities[33]. Our study has demonstrated that PAF could lower the systemic arterial pressure and raise the portal pressure[3435], so PAF is a principal mediator of hyperdynamic circulation and portal hypertension. As illustrated in Figure 1, activated Kupffer cells enhanced the synthesis of PAF, and appeared to secrete more PAF than membrane-bound platelet-activating factor. PAF synthesis was increased in a concentration-dependent manner in both cirrhotic and normal rat Kupffer cells. In the present study, enhanced synthesis of PAF by activated Kupffer cells may account for the increased hepatic concentration and PAF level. The increase (Figures 2 and 3) in activated Kupffer cells demonstrated that PAF had an autocrine function on activated Kupffer cells. PAF increase in activated Kupffer cells has been implicated to be an important mediator of hyperdynamic circulation associated with liver cirrhosis. In agreement with the previous reports in cirrhotic patients, increased blood PAF levels were observed in CCl4-induced cirrhotic rats[36]. The results of the present study suggest that the enhanced synthesis and release of PAF by activated Kupffer cells may contribute to its elevated circulating concentration in cirrhotic rats. The basal levels of cell-associated and secreted PAF were significantly higher in activated Kupffer cells than in the controls. Because the biological effects of PAF are mediated by its specific receptor, almost all actions of PAF can be prevented by blocking its receptor antagonists. The binding of PAF to its receptor may induce the release of arachidonic acid and PAF, and the vasoconstriction, portal vein pressure and hyperdynamic circulation may be mediated and influenced by eicosanoids.
ET-1 induced PAF synthesis in activated Kupffer cells increased in a concentration-dependent manner, revealing that endogenously produced ET-1 can increase the hepatic concentration and PAF levels in cirrhosis. The activated Kupffer cells did not express preproendothelin-1, either, indicating that ET-1 stimulates PAF synthesis in activated Kupffer cells by paracrine. Clearly, Kupffer cells are major contributors to the increased hepatic concentration and PAF level in cirrhosis.
It has been known that Kupffer cells contain only ETB receptor. The ET-1 saturation and competition binding assay show that ET-1-induced increase in PAF synthesis was mediated via ETB receptor. Activated Kupffer cells enhanced the binding capacity, but did not change the affinity of the receptors (Figures 4 and 5), although the number of PAF receptors was increased. Activated Kupffer cells did not express ETA receptor (Figure 6). As a result of hepatic injury, ETA receptor did not appear in activated Kupffer cells. In cirrhosis, activated Kupffer cells did not change the character of ET-1 receptor expression.
In conclusion, activated Kupffer cells in the course of CCl4-induced cirrhosis are the main source of increased PAF; endogenously, ET-1 can stimulate PAF synthesis in activated Kupffer cells via ETB receptor by paracrine; and as a result of hepatic injury, ETA receptor did not appear in activated Kupffer cells. The PAF receptor expression in activated Kupffer cells and PAF binding capacity are markedly increased in cirrhosis, which may exacerbate the hepatic and extrahepatic complications of cirrhosis.
COMMENTS
Background
Kupffer cells in the pathogenesis of different types of liver injury have been demonstrated to be involved in several models of liver damage induced by chemicals including carbon tetrachloride, alcohol and allyl alcohol. The high affinity platelet-activating factor (PAF) receptors, when stimulated, synthesize and release several active lipid mediators including eicosanoides and PAF. Kupffer cells have been shown to contain mRNA for the ETB receptor. However, no ET binding was measured in Kupffer cells. PAF synthesis/release and its receptor expression in activated Kupffer cells is far from clear in the carbon tetrachloride induced cirrhosis. Kupffer cells are activated in response to chemical administration and promote tissue damage by releasing biologically active mediators such as prostaglandins, TNFα, oxygen free radicals, and other proinflammatory mediators. PAF raised portal vein pressure and stimulated glycogenolysis, and PAF production may be dangerous in patients with portal hypertension as reported previously. The biological effects of PAF are mediated by its specific receptor, therefore, almost all actions of PAF can be prevented by blocking its receptor with antagonists. PAF binding its receptor induces release of arachidonic acid and production of cycloxygenase-derived eicosanoids, glycogenolysis, etc. which may mediate vasoconstriction and affect portal vein pressure and hemodynamics.
Research frontiers
Elucidation of the interactions between activated Kupffer cells and ET-1 as the mechanism detecting hepatic cirrhosis and attendant hemodynamic abnormalities in pathophysiology, has been a topic of intensive investigations in the recent years.
Innovations and breakthroughs
This paper has confirmed that expression of PAF receptor in activated Kupffer cells and PAF binding capacity are markedly increased in cirrhosis, which may exacerbate the hepatic and extrahepatic complications of cirrhosis.
Applications
Based on the result of this research, we can decrease the synthesis of PLT in Kupffer cells by suppressing the secretion of ET-1, or blocking its receptor, which may lighten or defer the hepatic and extrahepatic complications of cirrhosis.
Terminology
Kuppfer cells, resident macrophages in the liver, constitute the largest population of macrophages in the body and are critical effector cells for the defense against invading micro-organisms and foreign molecules. Platelet-activating factor (PAF) is the trivial name for a phospholipid called 1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine that has unique phospholipid autacoid exhibiting diverse biological activities on various tissues and organs such as isolated lung, liver and brain, and different types of cells (platelet, neutrophils, endothelial cells, neural cells). ET-1 stimulates PAF synthesis in Kuffer cells.
Peer review
The data presented by the authors strongly supports the hypothesis that Kupffer cells in cirrhotic livers are a main source of PAF, which contribute significantly to secondary signs of cirrhotic liver like portal hypertension. The topic is very interesting, and the experiments performed mainly prove the conclusions of the authors.