INTRODUCTION
The treatment of abdominal wall neoplasms, whether primary or secondary, continues to present a challenging problem to general surgeons. Extensive tumor resection of those neoplasms can result in giant abdominal wall defects. Reconstruction of the abdominal wall is still not easy to an average general surgeon, even to a plastic surgeon. Most reports described reconstructing giant abdominal wall defects by using synthetic mesh implants, autologous tissue graft, or a combination of the two methods mentioned above.
Synthetic mesh such as polypropylene (PP) and expanded polytetrafluoroethylene (ePTFE) are the mainstays of ventral hernia repair and commonly used to repair other trunk defects today. For PP, as a result of its macroporous structure, the mesh induces intense fibrovascular infiltration and incorporates into the surrounding musculofascial tissue to provide a strong repair. However, it is also associated with adhesions to intraabdominal viscera and enterocutaneous fistula formation[1–3]. ePTFE has been used in an attempt to reduce these complications and has been reported to cause fewer adhesions to the bowel because of its microporous surface structure. However, incorporation into the surrounding tissue is limited[4–6]. Furthermore, both of them contain permanent prosthetic material and are largely intolerant of infection[7–10], particularly if used in contaminated fields or in the event of exposure owing to dehiscence or breakdown of the overlying skin. However, abdominal wall tumors, particularly secondary ones, are frequently accompanied by contamination or infection. Such situations are a contradiction for implanting PP or ePTFE. Absorbable mesh, such as Vicryl can be used as a temporary support in contamination or infection situation[1112]. But the patient needs a second-stage operation for implanting an inabsorbable mesh to complete a definite repair. Additionally, some secondary tumors invade the complete abdominal wall including skin. Therefore, a single mesh is not enough without skin coverage.
The use of autologous tissue flaps, in most time musculocutaneous flaps such as tensor fasciae latae, rectus femoris, rectus abdominis and latissimus dorsi, combined with or without incorporation of synthetic meshes, has been described in the successful repair of abdominal wall defects[13–16]. It eliminates the complications associated with synthetic implant intolerance and is a good replacement of the full-thickness abdominal wall. Despite the advantages of autologous tissue, the creation of a donor site, the potential for donor site morbidity and limited size decrease this technique’s application[1718]. The preparation of a musculocutaneous flap, whatever pedicled or free, greatly increases operation time, complexity, and are generally outside the scope of practice of many general surgeons[1319].
Recently, some biologic materials were introduced to hernia repair and abdominal wall reconstruction. Human acellular dermal matrix (HADM) is a biologic material derived from human cadaveric dermis. In 1992 it was first used as a dermal replacement in burn surgery[20]. Since then it has been used successfully in the repair of oral defects and replacement of soft tissues in plastic and pelvic procedures[21–25]. The advantages of HADM is its resistance to infection,the absence of a permanent foreign body at the repair site and its excellent mechanical properties (strength to failure, plasticity and flexibility)[2326–28]. These properties make it an attractive alternative to prosthetic mesh in abdominal wall replacement. Animal models and some case reports using HADM have shown successful hernia repair, abdominal wall reconstruction and resistance to infection in contaminated cases[129–39]. Therefore, HADM is an ideal alternative to synthetic mesh for abdominal wall restoration after tumor resection, especially in the situation of infection or contamination.
The omentum serves a multipurpose in the medical field. The pedicled omentum flap is a special autologous tissue flap, but its preparation is much easier than musculocutaneous flaps. It has been reported to reconstruct abdominal wall defects successfully[40–43]. The omentum consists of abundant blood vessels, fat, and lymphatic tissue and is known for its unique immunologic and angiogenic properties[44]. We suppose, in the reconstruction of abdominal wall after tumor resection, it is a good alternative to the musculocutaneous flap and favorable for HADM remodeling after its implantation.
The purpose of this article is to present our approach of using a combination of the HADM implant and an interpositional omentum flap to repair giant abdominal wall defects after extensive tumor resection.
MATERIALS AND METHODS
Patients
Between February and October of 2007, three patients (two men and one woman) with giant defects of the abdominal wall after extensive secondary tumor resection underwent reconstruction with HADM (8 mm thickness, Renov, China) and omentum flap. The first patient was a 51-year-old woman with secondary gallbladder carcinoma, the second one was a 66-year-old man with secondary renal carcinosarcoma, and the third was a 62-year-old man with secondary sigmoid carcinoma. Defect size ranged from 10 cm × 8 cm to 22 cm × 16 cm. The first patient had an overlying skin defect that was covered with a free thigh skin graft and the later two had enough skin and subcutaneous tissues coverage.
Surgical technique
Operations are performed by a team consisting of general and plastic surgeons. Initially, the tumor was widely resected at least 3-5 cm beyond the margin of the tumor and completeness of clearance was ascertained by frozen sections. The abdominal cavity was entered and any bowel that was adherent to the parietal peritoneum along the fascial margin was freed to create space for the underlying portion of the HADM patch.
Then, the greater omentum was evaluated with regard to its size, vascular pattern, and reach for a given defect. The omentum was mobilized fully and pedicalized on either the right or left gastroepiploic arteries. The exit point was a 3-4 cm slit, located on the side ipsilateral to the defect. The right subcostal site is preferred because the liver protects against herniation of bowel.
Next, the HADM patch was applied using an underlay technique. The number of HADM sheets used depended on the size of the defect. After rehydration, several sheets of HADM were sewn together and inserted as an underlay patch with interrupted horizontal mattress sutures. An allowance of 2-3 cm circumferentially was given for the underlaid portion. The patch should be taut but not excessively tight to achieve a tension-free repair. The omentum flap was then gently spread over the HADM and loosely interrupted sutured to the muscle and fascia margin with 0 Prolene. Closed suction drains were placed above and below the omentum flap.
Finally the subcutaneous tissues and skin or graft skin was closed above the omentum. All patients were given abdominal binders postoperatively for additional support for at least 6 mo while the repair matured.
RESULTS
Case 1
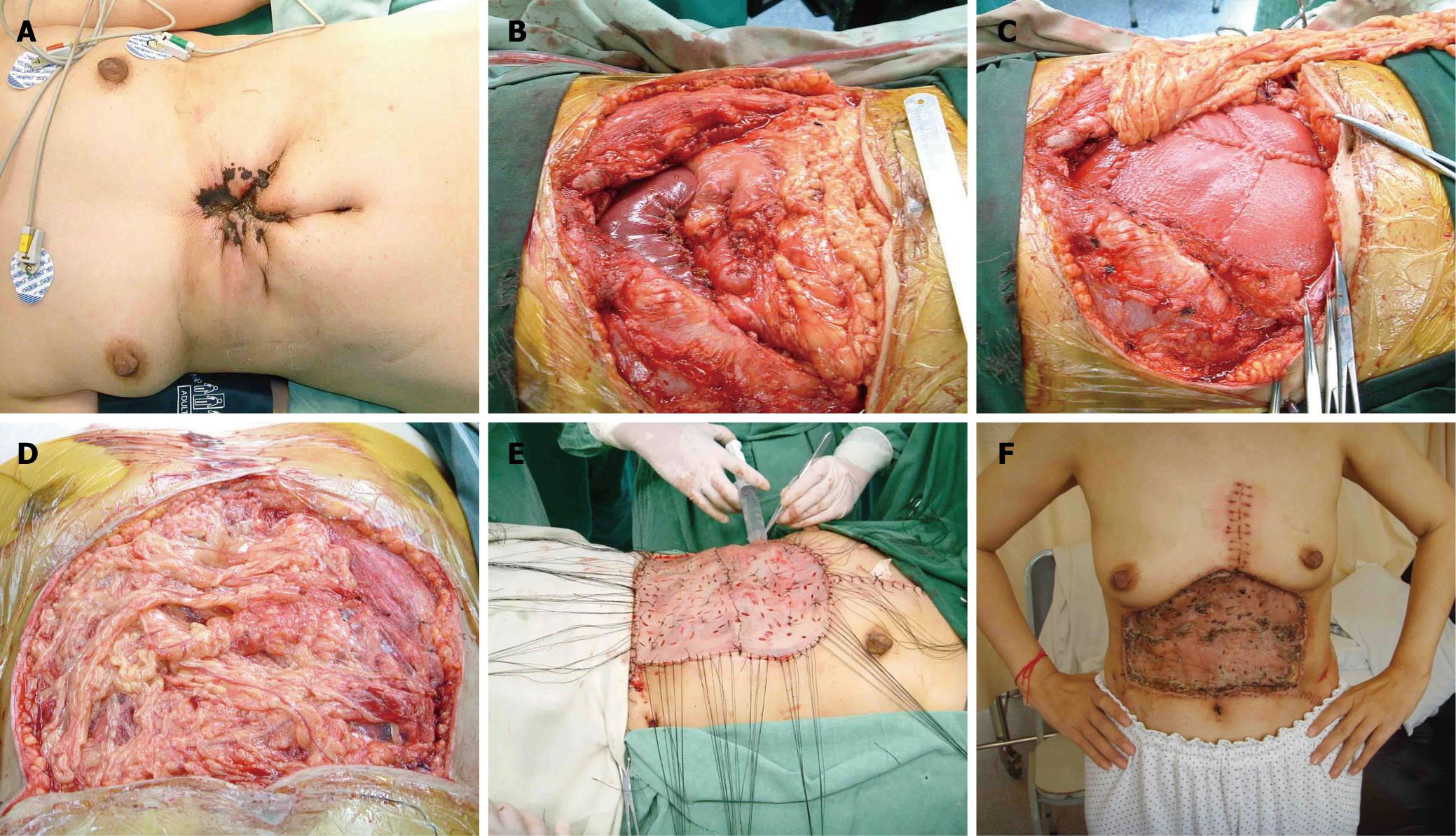
A 51-year-old woman presented with secondary gallbladder carcinoma over her upper abdominal wall. She had a history of radical operation of gallbladder carcinoma three years before and was diagnosed with abdominal wall metastasis two years postoperation. Radiotherapy and systemic chemotherapy proved not to be effective and an ulcer with infection developed one year later (Figure 1A). Intraoperatively, the tumor was noted to involve the entire thickness of the abdominal wall and adhered to the left lobe of the liver and pylorus. Wide excision resulted in a full-thickness upper abdominal wall defect measuring 22 cm ×16 cm (Figure 1B). The greater omentum was mobilized fully and pedicalized on the left gastroepiploic artery. The exit point was a 4 cm slit, located on the left subcostal site. Reconstruction was performed using the HADM patch described above. Five sheets of HADM (four 10 cm × 8 cm and one 10 cm × 5.5 cm) were sewn together and inserted as an underlay patch with 0 Prolene interrupted horizontal mattress sutures (Figure 1C and D). The overlying skin defect was covered with a free thigh skin graft (Figure 1E). She recovered uneventfully (Figure 1F). At 7-mo follow-up, the repair was noted to be intact and there was no sign of herniation. Several metastatic lesions were found in her left lung at the sixth month post-operation, but the patient is still alive.
Figure 1 Case 1.
A: The view of the abdominal wall before operation; B: The defect after extensive tumor resection; C: Reconstruction with HADM which was placed in an underlay fashion. The pedicled omentum flap was brought into the subcutaneous plane by means of a 4-cm slit at the left subcostal site; D: The HADM patch was completely covered by the omentum flap; E: A left free thigh skin graft was raised to cover the skin defect; F: The view of abdominal wall 1 mo postoperatively.
Case 2
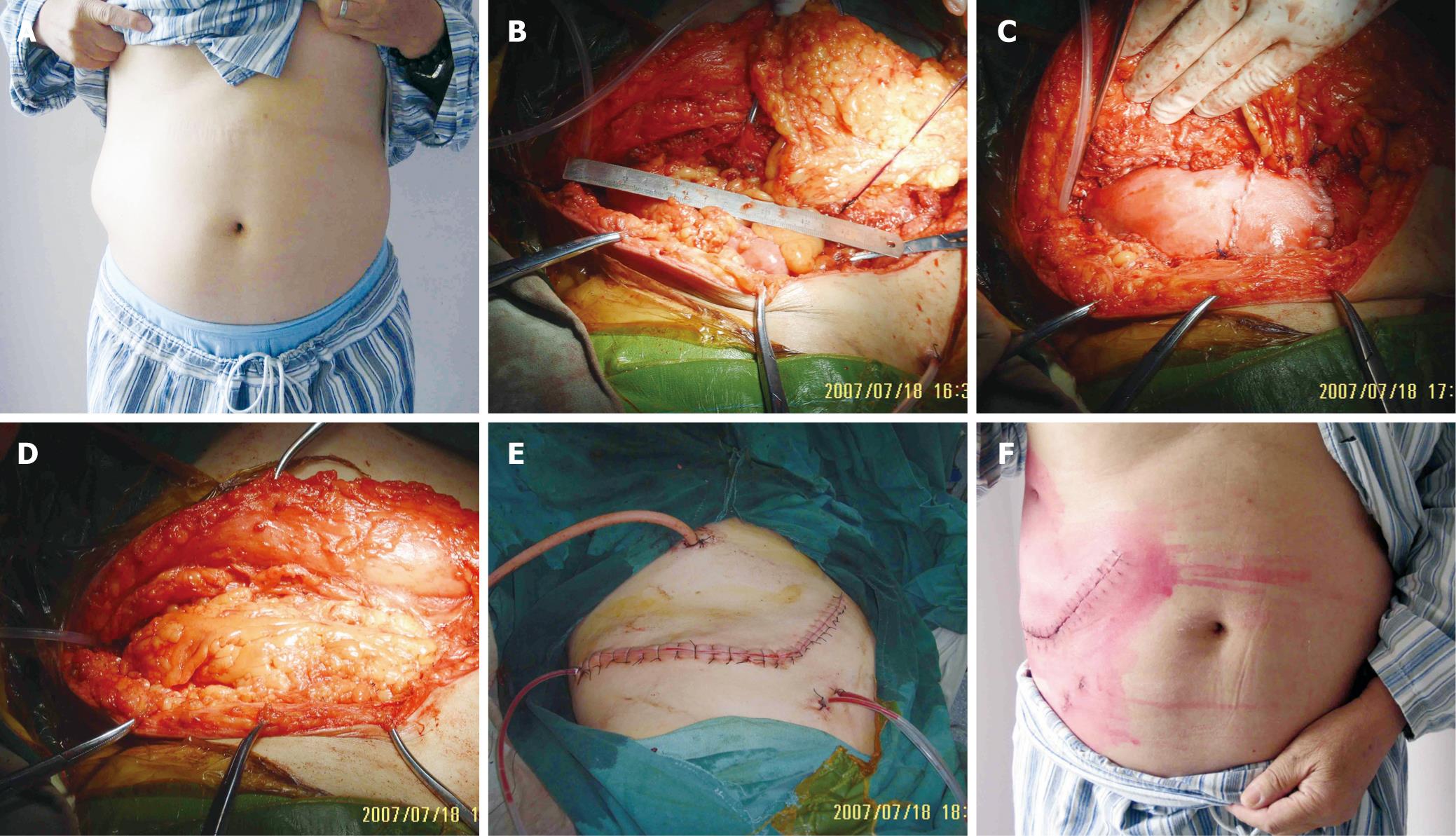
A 66-year-old man presented with recurrent renal carcinosarcoma over his right upper lateral abdominal wall (Figure 2A). He had a history of renal carcinosarcoma excised half a year previously. Intraoperatively, the tumor was found between the peritoneal membrane and muscle. There was no invasion of the subcutaneous tissue. Wide excision resulted in a right lateral abdominal wall defect measuring 15 cm × 11 cm (Figure 2B), but the excision was not complete in the original renal zone because some tumor tissue was located beside the spinal column and circled around the inferior caval vein which was only leaving for radiotherapy and chemotherapy. Reconstruction was performed as similar as in case 1. One sheet of 10 cm × 8 cm and one of 10 cm × 5.5 cm HADM were sewn together and inserted as an underlay patch. The upper part was fixed on the diaphragm. The omentum was transposed extraperitoneally through a 4-cm opening on the right subcostal site (Figure 2C and D). The subcutaneous tissue and skin were closed above the omentum flap (Figure 2E). He recovered uneventfully (Figure 2F). At 4-mo follow-up, the reconstruction remains stable and he is able to perform normal daily activities.
Figure 2 Case 2.
A: The view of the abdominal wall before operation; B: The defect after extensive tumor resection; C: Reconstruction with HADM which was placed in an underlay fashion. The pedicled omentum flap was brought into the subcutaneous plane by means of a 4-cm slit at the right subcostal site; D: The HADM patch was completely covered by the omentum flap; E: The subcutaneous tissue and skin were closed above the omentum flap; F: The view of the abdominal wall 2 wk after operation.
Case 3
A 62-year-old man presented with an advanced sigmoid colon carcinoma, which perforated chronically into the lower part of the left musculus rectus abdominis and infiltrated to the bladder. Computed tomography demonstrated infiltration through the peritoneum to the abdominal wall muscles. Excision of the abdominal lesion was performed with a 2 cm lateral margin, removing the parietal peritoneum and the full thickness of the muscle. Intraoperative frozen section revealed clear resection margins. This resulted in a defect measuring 10 cm × 8 cm. Reconstruction was performed similar to case 2. The omentum was mobilized and pedicled on the left gastroepiploic artery. The exit point was located on the inner side of the defect. The patient’s post-operative recovery was uneventful and there was no infection of his abdominal wall. The patient was followed up for only 1 mo and there is no herniation up to now.
DISCUSSION
Extensive resection of abdominal wall tumors frequently leads to a giant defect. Additionally accompanying with infection or contamination, repairing such a giant defect is still a challenging task to general surgeons. The current techniques of abdominal wall reconstruction all have their disadvantages: the transferring of a musculocutaneous flap creates a donor site where it can create potential donor site morbidities and is time-consuming. Inabsorbable synthetic mesh is intolerant to infection and sometimes results in an excessive foreign body reaction, absorbable mesh is sometimes used only as a temporary support of abdominal wall in a two stage operation. Therefore, new alternatives are called on to replace the old techniques. Here, we report our trial in three secondary abdominal wall tumor patients by combining HADM with an interpositional omentum flap as a technique to successfully repair giant abdominal wall defects after extensive tumor resection.
HADM is a biologic material derived from human cadaveric dermis. Chemical and physical processing removes all cellular components of the dermis while preserving the extracellular matrix and basement membrane components. This results in a sheet consisting of extracellular material that acts as a signal for fibroblast incorporation, collagen deposition, and collagen maturation. These changes occur when the material is implanted into the human body[26–28]. The extracellular matrix stays intact and is gradually revascularized and remodeled into autologous tissue while maintaining its structural integrity. Animal models proved that early revascularization of the graft is thought to enhance resistance to infection and contamination[29]. So its biggest benefit is its tolerance of overlying infection without breakdown or loss of strength. Additionally, the advantages of HADM also include the absence of a permanent foreign body at the repair site and excellent mechanical properties, such as strength to failure, plasticity and flexibility[2326–28]. Based on these facts, we believed that HADM is an ideal alternative of synthetic mesh for abdominal wall restoration after tumor resection, especially in the infected or contaminated situation.
A pedicled omentum flap is easy to prepare, and in most times it can reach defects over all quadrants of the abdominal wall. The omentum, consisting of abundant blood vessels, fat, and lymphatic tissue, is known for its unique immunologic and angiogenic properties. Therefore, it can be used in infected or contaminated situations. By using it as an interpositional flap, we harness such properties to facilitate wound healing[4445] and promote remodeling of HADM after implantation. We have demonstrated that the omentum, with its size, vascularity and bulk, can be used successfully as a local flap in combination with the HADM in abdominal wall restoration. Although an omentum flap is not stronger than a musculocutaneous flap, the HADM sheet plays a role of resisting pressure from the abdominal cavity, and an additional free skin graft could easily be taken for the patient who has a full-thickness abdominal wall defect. An omentum flap is somewhat easier to prepare compared to a musculocutaneous flap which avoids a donor site and its associated morbidities and additionally shortens the operation time. Sometimes it also avoids microsurgery that is needed for a free musculocutaneous flap, which represents an advantage in patients with significant comorbidities such as diabetes. In our cases, an omentum flap combined with HADM allowed us to repair a giant abdominal wall defect after an extensive tumor resection in a relatively short operation with no severe morbidity. To our knowledge, this technique has never been described before.
Up to now, no herniation was observed in these three patients. But we know that the number of our cases is too small and the follow-up period is too short. Because of the theorized structural remodeling of HADM, the long-term integrity of the abdominal wall after reconstruction remains in question. But this technique may potentially be used in the restoration of abdominal wall after extensive tumor resection, especially in patients presenting with a secondary tumor with infection or contamination.
In conclusion, the combination of HADM and omentum flap offers a safe and effective alternative to traditional forms in the repair of giant abdominal wall defects. The HADM appears to have the ability to survive in contaminated or infected surgical fields, the omentum flap is easy to prepare and avoids a donor site defects and morbidities that may result from a free or pedicled musculocutaneous flap transferring. The combination offers the opportunity for a single-staged reconstruction after extensive tumor resection. That also means it provides an option when neither synthetic prosthetic mesh nor musculocutaneous flap can be used to achieve closure. Further analysis of the long-term outcome and more cases also are needed to assess the reliability of this technique; nonetheless favorable short-term results are a welcome change for the surgeon called on to deal with such challenging dilemma.
COMMENTS
Background
Extensive resection of abdominal wall tumor frequently leads to a giant defect, which is difficult to repair for general surgeons. The current techniques of abdominal wall reconstruction, such as transferring a musculocutaneous flap and implanting a synthetic mesh have their disadvantages. Here, we report our trial in three secondary abdominal wall tumor patients by combining human acellular dermal matrix (HADM) with an interpositional omentum flap as a technique to successfully repair giant abdominal wall defects after extensive tumor resection.
Innovations and breakthroughs
The technique of combining the HADM implant and an interpositional omentum flap is to be used for the first time to repair giant abdominal wall defects after extensive tumor resection.
Applications
The combination of HADM and omentum flap offers a safe and effective alternative to traditional forms in the repair of giant abdominal wall defects after extensive tumor resection, especially in secondary tumor patients with infection or contamination.
Terminology
HADM: a biologic material derived from human cadaveric dermis, which has been used successfully in the repair of oral, abdominal and other tissue defects. Pedicled omentum flap: a special autologous pedicled flap which serves multipurpose in medical field.
Peer review
This manuscript presents a new way to reconstruct giant abdominal wall defects using the combination of a HADM implant and an interpositional omentum flap which appears to provide a safe and reliable alternative in this area.