INTRODUCTION
Visceral hypersensitivity, which refers to a lowered threshold for visceral pain/discomfort elicited by intraluminal mechanical/chemical stimuli, is a hallmark symptom of irritable bowel syndrome (IBS)[12]. IBS is a common functional gastrointestinal disorder that has a great impact on quality of life. It is thought that previous episodes of intestinal inflammation predispose to IBS as intestinal inflammation induces profound changes that persist even after the inflammation subsides[3]. Indeed, patients with ulcerative colitis in remission often express IBS-like symptoms[4], and animals that have recovered from experimental enteritis show visceral hypersensitivity to luminal distension[56]. Nevertheless, it has still not been completely elucidated what causes this IBS-like, post-inflammatory visceral hypersensitivity.
Increasing evidence suggests that peripheral corticotropin releasing hormone (CRH) plays an important role in intestinal inflammation. Kawahito et al[7] have reported a significant increase in the number of CRH immunoreactive macrophages and enterochromaffin cells in the colonic mucosa of patients with inflammatory bowel disease. In a rat model of chronic granulomatous enterocolitis, both mRNA and protein expression of CRH was found to be increased in cecal inflammatory cells, mesenchymal cells, and myenteric plexi[8]. More recently, an increase in CRH and its receptors was reported in a mouse ileitis model injected with Clostridium difficile toxin A[9]. In this model, peripheral injection of CRH receptor antagonists was able to significantly reduce intestinal secretion and inflammation. In accordance with this, genetically CRH-deficient mice showed diminished intestinal responses to C. difficile toxin A[10], and RNA interference to silence CRH expression in the ileum ablated the toxin A-induced inflammatory responses, such as epithelial damage, mucosal edema, and neutrophil infiltration[11]. Collectively, these results indicate that peripheral CRH acts as a pro-inflammatory mediator in intestinal inflammation.
Considering the involvement of peripheral CRH in intestinal inflammation, together with the putative role of intestinal inflammation in the development of visceral hypersensitivity, we hypothesized that increased peripheral CRH might be involved in post-inflammatory visceral hypersensitivity. We tested this hypothesis using a rat model that shows elevated nociceptive responses to luminal distension 7 d after acetic acid-induced colitis without histological and biochemical signs of overt gut inflammation[12]. In this rat model, we found that high levels of peripheral CRH were related to post-inflammatory visceral hypersensitivity.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats, weighing 270-310 g, were housed in a colony room maintained under a 12-h light/dark cycle with a room temperature of 22 ± 1°C and humidity of 65%-70%. Water and food were available ad libitum. Before the intracolonic instillation of chemicals, rats were fasted for 18 h. Colitis was induced by instillation of 1 mL 4% acetic acid into the colon. After 30 s, saline (1 mL) was instilled to flush the lumen. Control animals received saline only (1 + 1 mL). All experimental protocols in this study were reviewed and approved by the Animal Care and Use Committee of Seoul National University.
Myeloperoxidase (MPO) activity
A segment of distal colon (for MPO activity assay) and trunk blood (for plasma CRH level assay) were collected from rats sacrificed by cervical dislocation, following anesthesia with tiletamine/zolazepam (50 mg/kg) between 2:00 and 4:00 pm. The animals had not undergone any treatment (e.g. colorectal distension) other than intracolonic saline or acetic acid instillation. The mucosal layer of the distal colon was stripped off from the segment and minced in 1 mL 50 mmol/L potassium phosphate buffer (pH 6.0) that contained 14 mmol/L hexadecyltrimethylammonium bromide (Sigma-Aldrich, St. Louis, MO, USA). After homogenization and sonication, the lysates were frozen and thawed three times, then centrifuged for 2 min under 4°C at 15 000 ×g. Aliquots of the supernatants were mixed with o-dianisidine-HCl (Sigma-Aldrich) and 0.0005% H2O2. MPO activity was calculated from the rate of absorbance change, during 1 min, at 460 nm using the extinction coefficient for oxidized o-dianisidine (1.13 × 104/mol/L/cm) and expressed as units/mg of tissue protein quantified by the Bradford method. The enzyme unit was defined as the conversion of 1 &mgr;mol of H2O2 per min at 25°C.
Plasma CRH level
Blood samples collected in ice-cold EDTA-coated tubes were centrifuged at 1600 ×g for 15 min at 4°C. Plasma obtained after centrifugation was transferred into sterile microcentrifuge tubes and stored at -70°C until analyzed. As it is known that the CRH-binding protein interferes with plasma CRH level estimation, we extracted CRH from plasma using ice-cold methanol[13]. Specifically, plasma samples were mixed and incubated with methanol for 15 min at 4°C, and then centrifuged at 2000 ×g for 20 min at 4°C. The supernatants were transferred to another set of tubes. The pellets were washed with 0.2 mL ice-cold methanol and centrifuged at 2000 ×g for 15 min at 4°C. The supernatants were pooled and dried by blowing heated air. The extracts were then reconstituted with assay buffer (one-third of original plasma volume) included in the CRH enzyme immunoassay kit (Phoenix Pharmaceuticals, Belmont, CA, USA). Each sample was processed in duplicate and the final data were expressed as pg CRH/mL plasma.
Visceral nociceptive behavior to colorectal distension
Eighteen hour-fasted rats were lightly anesthetized with ether, and a disposable silicon balloon urethral catheter was carefully inserted intra-anally until the tip of the balloon was 4 cm proximal to the anus. While animals were fully awake, ascending-limits phasic distensions (0.2, 0.4, 0.6, 0.8 and 1 mL) were applied for 30 s every 4 min, and the distension-induced abdominal withdrawal reflex (AWR) was scored by an experimenter who was blind to the treatments (0, no behavioral response to distension; 1, brief head movements followed by immobility; 2, contraction of abdominal muscle without lifting of abdomen; 3, lifting of abdomen; and 4, body arching and lifting of pelvic structure)[14]. The overall difference in the visceral nociceptive responses between groups was determined by taking the area under the curve (AUC) calculated as the sum of the responses plotted against the distension volume using the trapezoidal rule.
Chemicals
Astressin (Bachem, Budendorf, Switzerland) and rat/human CRH (American Peptide, Sunnyvale, CA, USA) were dissolved in sterilized saline. Stock aliquots of these peptides were kept at -70°C, and diluted to a final concentration before use.
Statistical analysis
Data are presented as mean ± SE, with n the number of animals. The difference between two groups was statistically analyzed using the Mann-Whitney U test (MWU test) at the P < 0.05 significance level. Comparisons between three or more groups were performed with Kruskal-Wallis test (KW test), followed by Dunnett’s procedure.
RESULTS
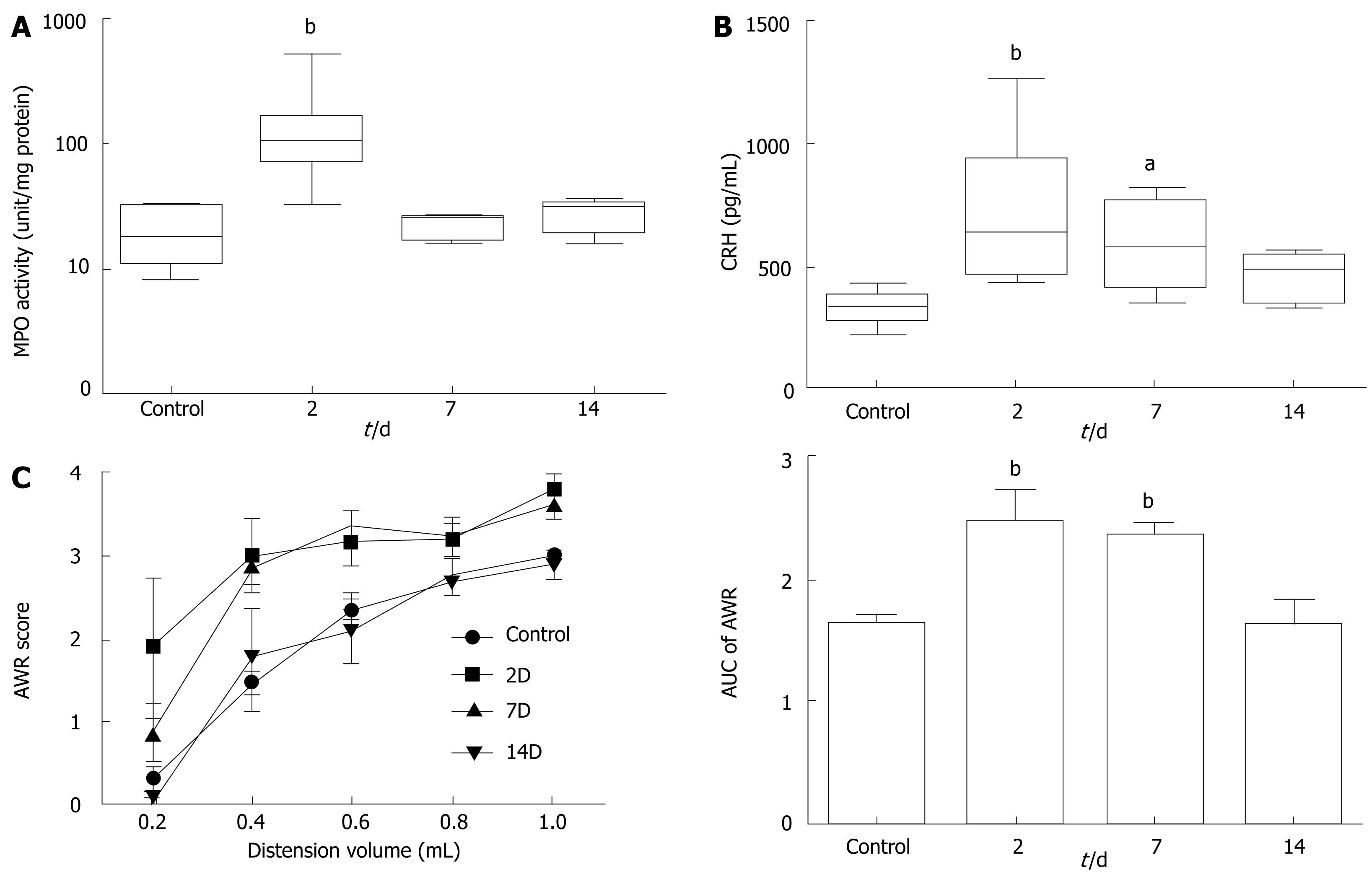
To quantify the extent of inflammation, we measured the MPO activity from colonic mucosa. As shown in Figure 1A, MPO activity was dramatically increased in rats at 2 d after induction of colitis (21.0 ± 3.4 vs 146.0 ± 45.5 U/mg, n = 10 in each group, P < 0.01). After 7 d, MPO activity returned to normal (22.0 ± 1.2 U/mg, n = 10), indicating a substantial resolution of inflammation. Plasma CRH level was also elevated about twofold in rats at 2 d (333.5 ± 19.7 vs 740.1 ± 88.8 pg/mL, n = 10 in each group, P < 0.01). Unlike MPO activity, however, the increase in plasma CRH level was maintained in rats at 7 d (587.6 ± 61.1 pg/mL, n = 8, P < 0.05 from control). It was decreased down to the control level 14 d after induction of colitis (Figure 1B).
Figure 1 Colitis-induced changes in colonic mucosal MPO activity (A), plasma level of CRH (B), and visceral nociceptive AWR (C).
In panel A and B, the upper and lower whiskers indicate the maximum and minimum values, respectively. Box lines represent the 25th (bottom), 50th (middle) and 75th (top) percentile value; (C) The area under the curve (AUC) calculated from the stimulus-response plot is illustrated as a bar graph on the right side. aP < 0.05, bP < 0.01 vs control by MWU test after KW test (P < 0.01).
Figure 1C demonstrates the response pattern of each experimental group to colorectal distension. We could not detect any difference between the day-matched control rats, thus we pooled the data obtained from control animals. At 2 d after induction of colitis, the animals showed enhanced AWR response, which resulted in a significant increase in the AUC of the stimulus-response plots (1.6 ± 0.1, n = 15 vs 2.5 ± 0.3, n = 5, P < 0.01). Rats at 7 d also clearly showed visceral hypersensitivity (2.4 ± 0.1, n = 8, P < 0.01 from control). Fourteen days after colitis induction, the animals were found to have recovered from the post-inflammatory visceral hypersensitivity.
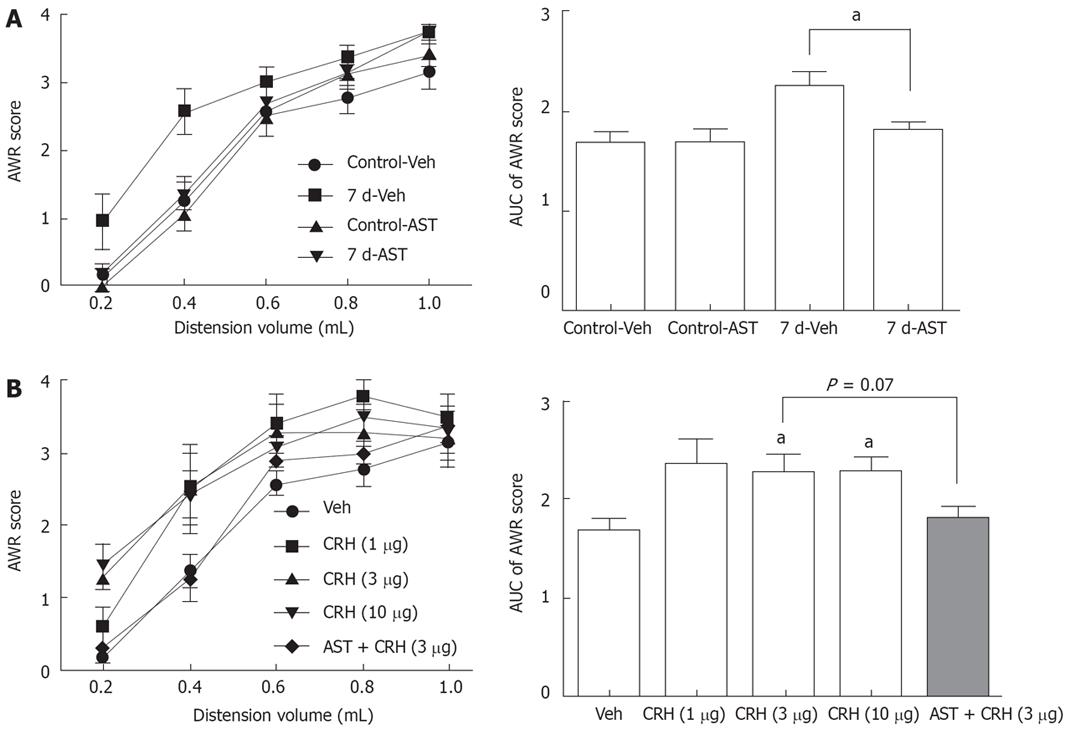
A similar time course for increased plasma CRH level and enhanced visceral nociceptive behavior, i.e., AWR to colorectal distension, raised the possibility that the sustained increase in peripheral CRH might account for the post-inflammatory visceral hypersensitivity. We further tested this possibility by intraperitoneal administration of CRH receptor antagonist astressin (30 &mgr;g/kg), 30 min before colorectal distension. The dose of astressin was chosen based on its ability to prevent defecation and c-Fos expression, which was induced by peripherally administered CRH in other studies[1516]. Astressin did not change the AWR behavior in control rats (1.7 ± 0.1 vs 1.7 ± 0.1, n = 7 in each group). However, it significantly decreased the AUC of AWR score in rats at 7 d (2.3 ± 0.2, n = 7 vs 1.8 ± 0.1, n = 6, P < 0.05), which indicated that endogenous peripheral CRH mediated the post-inflammatory visceral hypersensitivity (Figure 2A).
Figure 2 Effect of CRH receptor antagonist astressin (A) and CRH (B) on the colorectal distension-induced AWR behavior.
In each panel, the bar graphs on the right side represent the AUC of the stimulus-response plot. Astressin (AST, 30 &mgr;g/kg) and CRH (1, 3 and 10 &mgr;g/kg) were administered intraperitoneally. The same amount of saline was given as a vehicle (Veh). aP < 0.05 vs the paired group in (A) or the Veh group in (B) by MWU test after KW test (P < 0.05).
We further tested this idea by peripherally injecting exogenous CRH to naïve rats (1, 3 and 10 &mgr;g/kg, i.p., n = 5 in each group) 30 min before colorectal distension. As shown in Figure 2B, rats that received 3 or 10 &mgr;g/kg CRH developed visceral hypersensitivity to colorectal distension. Astressin, administered 15 min before the injection of CRH, showed a tendency to inhibit CRH-induced visceral hypersensitivity in naïve rats (2.3 ± 0.2 vs 1.8 ± 0.1, n = 5 in each group, P = 0.07).
DISCUSSION
The present study provides evidence that suggests increased peripheral CRH underlies the post-inflammatory visceral hypersensitivity. First, visceral hypersensitivity could be observed in rats only at the experimental time points when plasma CRH level was higher than the control level. We found a significant increase in plasma CRH in rats at 2 and 7 d after colitis induction. The level of plasma CRH was back to normal after 14 d. Interestingly, rats at 2 d and 7 d, but not 14 d, showed enhanced AWR behavior in response to colorectal distension. This correlation between sustained CRH increase and visceral hypersensitivity has also been implicated in another rat colitis model. Two different studies have revealed that rats that have recovered from trinitrobenzene sulphonic acid-induced colitis show visceral hypersensitivity to colorectal distension at 28 d after colitis induction[17], and a sustained increase in CRH mRNA expression in hypothalamic paraventricular nucleus at d 30[18]. These two independent reports collectively suggest a role of central CRH in the post-inflammatory visceral hypersensitivity. Considering that the continuous CRH increase in plasma during sustained stress is derived mainly from the hypothalamus[19], it is also likely that the sustained increase in hypothalamic CRH heightens the level of plasma CRH acting peripherally after the resolution of colitis. It will be necessary in future studies to examine the source of increased plasma CRH in our model of post-inflammatory visceral hypersensitivity. Along with the paraventricular nucleus neurons in the hypothalamus, local immune cells resident in the gut may be the sources of the increased CRH in the post-colitis condition.
The second piece of evidence is that astressin, a CRH receptor antagonist that cannot cross the blood-brain barrier, alleviated the visceral hypersensitivity in rats at 7 d when administered intraperitoneally, whereas it had no effect on the AWR behavior in control rats. These results show that endogenous peripheral CRH plays a crucial role only in the hypersensitive visceral nociception in post-colitis condition, but not in normal visceral nociception upon colorectal distension. It is interesting that transgenic mice deficient in CRH1 receptor are less sensitive to colorectal distension than wild-type mice, which indicates that activation of CRH1 receptor is crucial, even in normal visceral nociception in mice[20]. The discrepancy between the results of this study and our current results might be explained by the species difference (mice vs rats) and/or the difference in the level of CRH receptor blockade, because knocking out the CRH1 receptor gene eliminates both central and peripheral targets of CRH.
Our third piece of evidence is that peripheral administration of CRH mimicked the post-inflammatory visceral hypersensitivity in naïve rats. How can peripheral CRH render animals more sensitive to colorectal distension? Inflammatory substances are known to sensitize visceral afferents, which results in a decrease in the response threshold, as well as an increase in response magnitude[21]. The sensitization of visceral afferents is regarded as a fundamental mechanism of visceral hypersensitivity[22]. Peripheral CRH has been shown to stimulate mast cells that secrete numerous inflammatory substances including histamine, cytokines, proteases and eicosanoids[2324]. Interestingly, mast cells have long been implicated to be involved in IBS and visceral hypersensitivity. An increased number of mast cells have been noted in the mucosa of IBS patients[25–28], and the correlation between mast cells and visceral hypersensitivity has been shown in experimental animals. Gue et al[29] have reported that the mast-cell stabilizer doxantrazole effectively reduces restraint stress-induced visceral hypersensitivity in rats. McLean et al[30] have also reported that, in Nippostrongylus brasiliensis-infected rats, an increase in mucosal mast cells was observed at 30 d but not at 90 d post-infection, and that the hypersensitive depressor response to jejunal distension was observed only at 30 d post-infection. In our previous study using the same animal model used in the present study, we found that the number of degranulated mucosal mast cells was higher in rats at 7 d after colitis induction, and that doxantrazole inhibited the post-inflammatory visceral hypersensitivity in these rats[31]. Therefore, it can be speculated that mast cell activation by peripheral CRH accounts for its ability to induce visceral hypersensitivity. It would be of interest to examine if doxantrazole can prevent the development of visceral hypersensitivity caused by exogenously administered CRH.
Our present findings support the idea that peripheral CRH receptor can be a target for treating IBS symptoms. Recent studies have demonstrated that administration of alpha-helical CRH (9-41), a CRH receptor antagonist, significantly improves gastrointestinal motility, and reduces abdominal pain and anxiety evoked by rectal electrical stimulation in IBS patients[32]. The same antagonist has also been reported to blunt changes in the electroencephalogram (EEG) power spectra upon colonic distention, and almost normalize EEG activity in IBS patients[33]. The effectiveness of CRH receptor antagonist on the IBS-like symptoms, especially visceral hypersensitivity, has also been reported in animal studies. Antalarmin, a CRH1 receptor antagonist, prevents visceral hypersensitivity induced by repeated colorectal distension in female rats[34]. Peripherally administered CRH1 receptor antagonist CP-154 526, which can act centrally by freely crossing the blood-brain barrier, inhibits acute stress-induced visceral hypersensitivity in maternally separated rats[35].
In summary, we found that rats with visceral hypersensitivity after resolution of colitis showed sustained increase in plasma CRH level and that a CRH receptor antagonist inhibited the symptom, which suggests that the increased peripheral CRH mediates the post-inflammatory visceral hypersensitivity, and that CRH receptor can be a target for alleviating this symptom.
COMMENTS
Background
Intestinal inflammation is thought to induce persistent changes in gut function, and indeed, visceral hypersensitivity often develops after intestinal inflammation. However, it is still not well understood what mediates the heightened sensitivity of the gut after resolution of the inflammation.
Research frontiers
IBS is a disorder whose hallmark symptom is visceral hypersensitivity, which is believed to be caused or exacerbated by stress, and corticotropin releasing hormone (CRH) is a pivotal central mediator of the stress response. Therefore, the role of CRH in this bowel disorder is gaining interest. It is also becoming clear that peripheral CRH expression is increased in intestinal inflammation, which raises the possibility that peripheral CRH might be involved in visceral hypersensitivity developed after intestinal inflammation.
Innovations and breakthroughs
Using a rat model of acute colitis as a model of post-inflammatory visceral hypersensitivity, the present study showed the correlation between the level of peripheral CRH and the development of visceral hypersensitivity after experimental colitis. Here, it was also shown that peripheral administration of CRH receptor antagonist and CRH itself could inhibit and mimic the post-inflammatory visceral hypersensitivity, respectively.
Applications
The findings from this study support the idea that peripheral CRH receptor can be a therapeutic target for treating visceral hypersensitivity in IBS.
Terminology
Visceral hypersensitivity refers to increased sensation of stimuli in the gut, which is defined by lowered threshold for visceral pain/discomfort elicited by intraluminal mechanical/chemical stimuli.
Peer review
The design of the controls was rational and reliable. The statistical methods used were appropriate. The results provided sufficient evidence to arrive at the conclusions. The discussion was well organized, and valuable conclusions are provided.
Peer reviewer: Javier San Martin, Gastroenterology and
Endoscopy, Sanatorio Cantegril, Av. Roosevelt y P 13, Punta del
Este 20100, Uruguay