Published online Feb 7, 2008. doi: 10.3748/wjg.14.725
Revised: December 6, 2007
Published online: February 7, 2008
AIM: To assess the outcome of patients with acute necrotizing pancreatitis treated by percutaneous drainage with special focus on the influence of drainage size and number.
METHODS: We performed a retrospective analysis of 80 patients with acute pancreatitis requiring percutaneous drainage therapy for infected necroses. Endpoints were mortality and length of hospital stay. The influence of drainage characteristics such as the median drainage size, the largest drainage size per patient and the total drainage plane per patient on patient outcome was evaluated.
RESULTS: Total hospital survival was 66%. Thirty-four patients out of all 80 patients (43%) survived acute necrotizing pancreatitis with percutaneous drainage therapy only. Eighteen patients out of all 80 patients needed additional percutaneous necrosectomy (23%). Ten out of these patients required surgical necrosectomy in addition, 6 patients received open necrosectomy without prior percutaneous necrosectomy. Elective surgery was performed in 3 patients receiving cholecystectomy and one patient receiving resection of the parathyroid gland. The number of drainages ranged from one to fourteen per patient. The drainage diameter ranged from 8 French catheters to 24 French catheters. The median drainage size as well as the largest drainage size used per patient and the total drainage area used per patient did not show statistically significant influence on mortality.
CONCLUSION: Percutaneous drainage therapy is an effective tool for treatment of necrotizing pancreatitis. Large bore drainages did not prove to be more effective in controlling the septic focus.
- Citation: Bruennler T, Langgartner J, Lang S, Wrede C, Klebl F, Zierhut S, Siebig S, Mandraka F, Rockmann F, Salzberger B, Feuerbach S, Schoelmerich J, Hamer O. Outcome of patients with acute, necrotizing pancreatitis requiring drainage-does drainage size matter? World J Gastroenterol 2008; 14(5): 725-730
- URL: https://www.wjgnet.com/1007-9327/full/v14/i5/725.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.725
The course of acute pancreatitis ranges from a mild transitory edematous to a severe necrotizing form. Necrotizing pancreatitis occurs in about 20% of all patients suffering from acute pancreatitis[1]. If infection of the necrotic tissue occurs mortality rates of up to 50% are reported with sepsis and multiorgan failure as most frequent causes[2–5]. It is generally accepted that in infected necrotizing pancreatitis the infected non-vital solid tissue has to be removed in order to control the sepsis. The standard treatment has traditionally been surgery[56]. By using modern surgical techniques like open packing, repeated laparatomies, closed packing or closed continuous lavage mortality rates could be decreased to 20%-40%[7–12]. However, these techniques are associated with a considerable surgical trauma which often causes escalation of multiorgan failure and sepsis[713]. Moreover, total anaesthesia is mandatory. Thus, in the last decade minimal invasive treatment regimes and in particular percutaneous drainage therapy were included in the management of infected necrotizing pancreatitis.
Ultrasound (US) or computed tomography (CT) guided placement of drainages is reported to be effective in up to 90% for drainage of fluid collections or abscesses with purely liquid content[14]. However, the success rates for drainage of contained fluid collections in necrotizing pancreatitis are less convincing ranging between 26% and 50%[1516], with a subsequent frequent need for further surgery. The presumed reason is that infected fluid collections/abscesses in necrotizing pancreatitis are often not completely liquid but contain solid debris. The value of drainage therapy for removal of solid debris is equivocal. Usage of large bore catheters and placement of several drainages is generally considered to be more effective. This hypothesis, however, has not yet been evaluated systematically.
The aim of this retrospective study was to evaluate if patient outcome in infected necrotizing pancreatitis correlates with the number and size of percutaneously placed drainages.
This is a retrospective study performed at a university hospital. By searching the radiological, surgical and internal medicine databases all patients with the diagnosis acute pancreatitis treated with drainages between 1992 and 2004 were identified. Demographic (age, sex), clinical (length of primary hospital stay, eventual second hospital stay, admittance to intensive care unit, etiology of pancreatitis, diagnostic and conservative therapeutic approaches), laboratory data and characteristics of surgical procedures were collected by reviewing patient charts and surgical reports.
Number of CTs performed per patient and characteristics of percutaneous interventional therapy were collected by reviewing all radiological reports. In particular, number of drainages per patient, date of drainage placement, size of each drainage in French, largest drainage per patient according to French number, estimate of draining lumen of each drainage (by calculating the axial plane of each drainage), and length of drainage placement in days were determined. Moreover, the cumulative draining area (by adding the draining lumina of all drainages per patient), the average draining area (by calculating the average of the draining lumina of all drainages per patient), the cumulative diameter (by adding the diameter according to French number of all drainages per patient), and the average diameter (by calculating the average of the diameter according to French number of all drainages per patient) were calculated.
All drainages had been placed under CT guidance using Seldinger technique. The number and the size of catheters were determined individually for each patient by the intensive care unit (ICU) team and the interventional radiologist. Clinical considerations and the size and location of necroses as well as the suspected amount of fluid and debris (based on CT findings) guided this decision.
For patients with ongoing sepsis and solid tissue within the fluid collections, active necrosectomy was performed. This technique has been described in detail elsewhere[16–18]. Briefly, under local anaesthesia the drainage was replaced by a 24F-30F peel away sheath. Under fluoroscopic and/or endoscopic guidance the solid tissue was fragmented and extracted through the sheath. For this, snare catheters, dormia baskets, forceps and brisk flushing/aspiration were applied.
For patients receiving intensive care treatment, the APACHE II score, SAPS II, and Ranson score were determined by reviewing the laboratory data and the ICU flow charts.
The Balthazar score and computed tomography severity index (CTSI) were determined by retrospectively reviewing the CT examination at the time of first drainage placement[19]. Radiological analysis was done by a board certified radiologist with 6 years of experience.
Endpoints for patient outcome during hospital stay were defined as patient death and length of hospital and/or ICU stay. All patients were observed until d 250 or censored at the last day of observation.
A telephone interview was performed to investigate long term outcome parameters. The questionnaire included life quality parameters such as ongoing symptoms of pancreatitis after hospital discharge (abdominal pain, diarrhea, intolerance of fat, weight loss), development of exocrine or endocrine dysfunctions, repeated hospital stays and need for further surgery.
Parameters assessing drainage characteristics and clinical scores (for ICU patients) were correlated with mortality using logistic regression. Drainage characteristics and clinical scores (for ICU patients) were correlated with length of hospital stay and ICU stay using a Cox proportional hazard model in a univariate model. Variables with a significant influence on the hazard ratio were then included in a multivariate model. The statistical analysis was made with the SPSS 12.0 statistical software (SPSS inc., Chicago, IL). Values are given as total numbers, median with range or as percentages where necessary. A P-value ≤ 0.05 was considered statistically significant.
Eighty patients fulfilled the inclusion criteria and were included in the study. The demographic, clinical, laboratory and interventional/surgical data are shown in Table 1.
| Patient, n = 80 | n/median (%/range) |
| Demographics | |
| Age | 57 (17-79) |
| Sex, female | 26 (32.5) |
| Days of hospitalisation | 51 (3-241) |
| Required second hospitalisation | 5 (6.3) |
| Aetiology | |
| Biliary | 26 (32.5) |
| Alcoholic | 32 (40.0) |
| Post-ERCP | 3 (3.8) |
| Toxic | 1 (1.3) |
| Hyperlipidemia | 1 (1.3) |
| Hyperparathyroidism | 1 (1.3) |
| Posttraumatic | 1 (1.3) |
| Idiopathic/unknown | 15 (18.8) |
| Ranson score | 2 (0-4) |
| Diagnostic procedures | |
| Ultrasound | 74 (92.5) |
| ERCP | 32 (40.0) |
| CT | 80 (100) |
| Balthazar score | 4 (3-4) |
| CTSI | 6 (4-10) |
| Inflammatory markers | |
| Leucocyte count d 1 of our hospital admission (/nL) | 13 (3.5-55.0) |
| Leucocyte count on day of first drainage placement (/nL) | 14.4 (3.5-46.6) |
| C-reactive protein day 1 (mg/L) of our hospital admission | 208 (10-477) |
| C-reactive protein on day of first drainage placement (mg/L) | 191 (20-477) |
| Therapy | |
| Surgical | 20 (25.0) |
| Minimal-invasive | |
| Number of drainages, range | 2 (1-14) |
| Active necrosectomy | 18 (22.5) |
| Intensive care treatment | 65 (81.3) |
| length of ICU stay, range | 22 (2-104) |
| Required second ICU stays | 9 (11.3) |
| APACHE II, range | 18 (1-38) |
| SAPS II, range | 61 (15-94) |
| Non-invasive positive pressure ventilation | 2 (2.5) |
| Mechanical ventilation | 50 (62.5) |
| Renal replacement therapy | 23 (28.8) |
| Plasma exchange therapy | 7 (8.8) |
| Mortality | 27 (33.8) |
| Cause of Death | |
| Septic multi-organe failure | 24 (88.9) |
| Pulmonary embolism | 1 (3.7) |
| Intracerebral bleeding | 1 (3.7) |
| Due to polytrauma | 1 (3.7) |
All 80 patients received antibiotic treatment over a median of 44 d with a range from 2 d to 205 d. Antimycotic treatment was established in 43 patients (54%). The diagnosis of infection of the necrotic tissue was based on the examination of aspirated material with Gram's stain, culture or both. Fine needle aspiration was not routinely performed. Thus, the material which was obtained during the placement of the first drainage was evaluated. Fifty-two patients (65%) showed positive microbiological results. The other 35% might have shown false negative results due to prior initiation of antibiotic therapy.
Intensive care treatment was necessary in 65 out of 80 patients (81%); nine patients required a second ICU stay later on. Mechanical ventilation was necessary in 50 patients (63%), renal failure with the need of renal replacement therapy occurred in 23 patients (29%).
Each patient received a median of six CTs ranging from 1 to 23, which were all contrast enhanced. The CTSI of all 80 patients was in median 6 ranging from 4 to 10, the Balthazar score was in median 4, ranging from 3 to 4.
Necrosis of the pancreas was diagnosed in 73 patients (91%) on the basis of contrast enhanced CT findings. The location of necrosis was only in the head of the pancreas in 13 patients, in the head and the body in five patients, in the head and the tail in four patients, in the body in three patients, in the body and tail in 10 patients, only in the tail in six patients and involving the whole pancreas in 32 patients. Seven patients did not show necrosis of the pancreas itself, however, extensive peripancreatic fluid collections with presumed necrosis of the peripancreatic fat was present.
Different catheter types were used (Thal-Quick, Cook, Europe; van Sonnenberg, Peter Pflugbeil, Zorneding, Germany; APD and Flexima, Boston Scientific, Natick, USA). Up to 14 catheters per patient were placed, in median two catheters per patient. Percutaneous catheter drainage was performed on median d 3.5 after admission (range 1 d to 40 d), but with 71 patients being transferred from peripheral hospitals and median hospitalisation of 7 d before (complete data of prior hospital stays was available in 54 out of 71 patients). Drainages remained in situ for a median of 36.5 d (range 1 d to 260 d). The drainage size ranged from 8 French to 24 French catheters. The median drainage plane was 16.9 mm2, the median cumulative drainage plane was 37.0 mm2 per patient. The median diameter was 14.0 mm, the median cumulative diameter was 30.0 mm per patient.
No acute complication (like bleeding, bowel perforation, accidental puncture of liver, spleen or kidney) related to catheter placement occurred. However, in 23 patients (29%) the development of fistulas in the pathway was observed later on.
Catheters were exchanged in median two times per placed catheter (range from 1 to 9). Catheter exchange was either done because of drainage blockage or to insert a larger drainage for presumed better drainage of necrotic tissue.
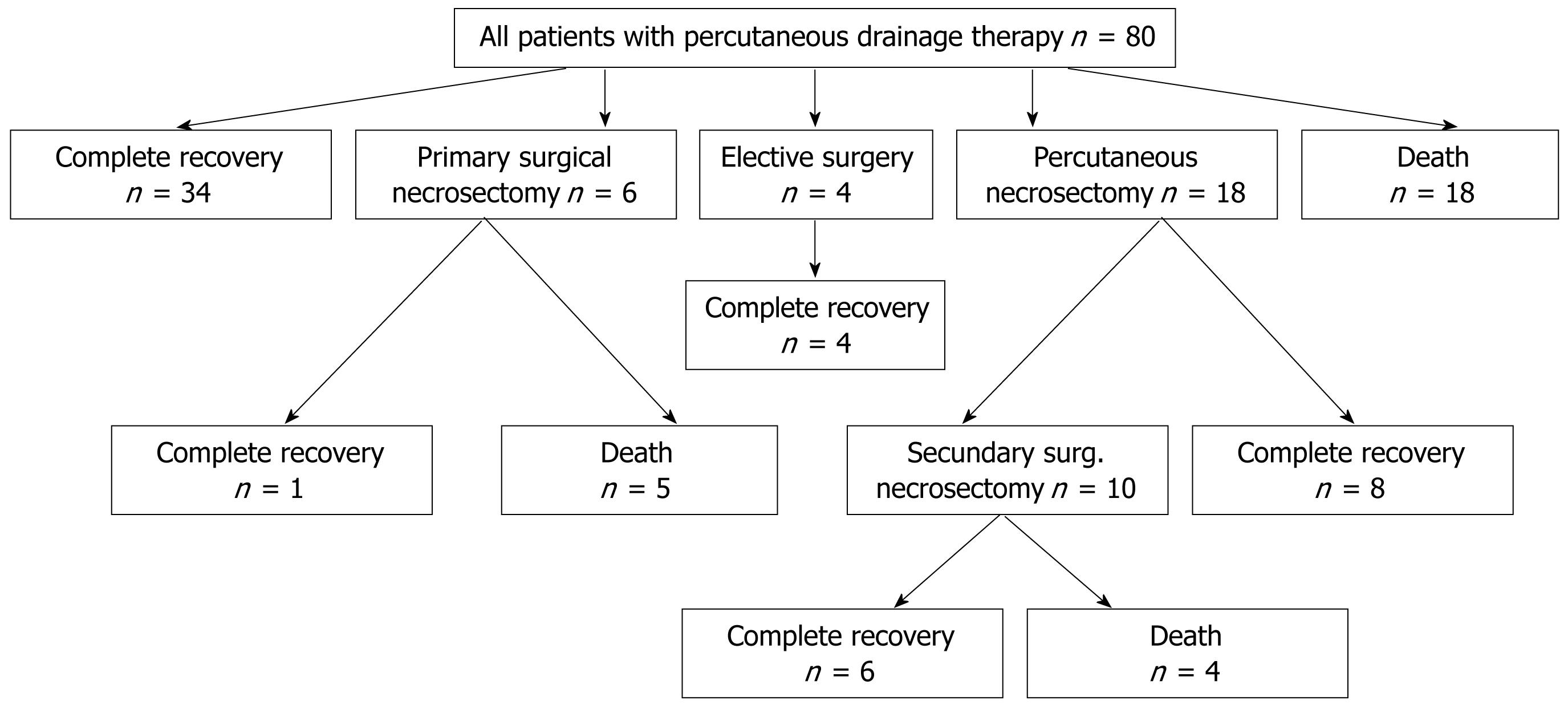
In 18 of 80 patients (23%) an active percutaneous necrosectomy was performed. Ten patients out of these required surgical necrosectomy later on. Six patients underwent surgical necrosectomy without prior percutaneous necrosectomy. Elective surgery was performed in 4 patients (cholecystectomy in three patients and adenomectomy of the parathyroid gland for primary hyperparathyreoidism in 1 patient) during the same hospital stay (Figure 1).
Length of hospital stay was in median 51 d with a range from 3 d to 241 d. A second hospital stay was necessary in 5 patients (6%). The length of stay in the ICU ranged from 2 d to 104 d, in median 22 d. The overall mortality was 34% (27 of 80 patients).
The correlations of the radiological and clinical scores (CTSI, APACHE II, SAPS II), clinical treatment modalities (invasive ventilation and renal replacement therapy) as well as interventional treatment modalities (number of drainages, median drainage size, largest drainage size and total drainage lumen) with mortality are given in a cox proportional hazard model for overall mortality using uni- and multivariate analysis (Table 2). Significant risk factors for mortality in univariate analysis were the need for mechanical ventilation, renal replacement therapy, the APACHE II score and the SAPS II score. The total drainage plane showed borderline significance with a P-value of 0.052. The median drainage size, the largest drainage size used per patient and the CTSI were not significantly correlated with mortality. In the multivariate model the CTSI was as the only not significant variable included adjusting for the extent of necrosis. Renal replacement therapy and the need for ventilation support were the only parameters which remained significant in the multivariate model.
| Variable | Mono-HR | P | Multi-HR | P |
| Age | 0.985 | 0.098 | - | |
| Sex | 0.926 | 0.793 | - | |
| CTSI | 0.933 | 0.281 | 0.982 | 0.819 |
| Apache II Score | 0.961 | 0.016 | 0.989 | 0.716 |
| SAPS II Score | 0.976 | 0.002 | 1.004 | 0.809 |
| Clinical treatment modalities | ||||
| Invasive ventilation | 0.175 | 0.000 | 5.618 | 0.001 |
| Renal replacement therapy | 0.329 | 0.005 | 2.347 | 0.046 |
| Interventional treatment modalities | ||||
| Number of drainages | 0.892 | 0.077 | - | |
| Median drainage size | 0.989 | 0.830 | - | |
| Largest drainage size | 0.954 | 0.307 | - | |
| Total drainage plane | 0.994 | 0.052 | 0.993 | 0.084 |
In 29 out of the 53 surviving patients (55%) long-term outcome parameters could be collected. Data are presented in Table 3. Hereby endocrine pancreatic dysfunction is the most frequent factor affecting life quality (35%), followed by diarrhea, weight loss and exocrine pancreatic dysfunction with the need of enzyme-substitution in 24%; 10% reported on repeated pancreatitis episodes; 28% on further hospital stays for related symptoms.
| Outcome parameters from 29 patients | n | % |
| Life quality parameters | ||
| Abdominal Pain | 5 | 17.2 |
| Diarrhea | 7 | 24.1 |
| Intolerance of fat | 4 | 13.8 |
| Weight loss | 7 | 24.1 |
| Exocrine pancreatic dysfunction (with substitution of pancreatic enzymes) | 7 | 24.1 |
| Endocrine pancreatic dysfunction | 10 | 34.5 |
| Repeated development of pancreatitis | 3 | 10.3 |
| Further hospitalisations | 8 | 27.6 |
| Surgery after discharge | 7 | 24.1 |
| Death after discharge | 4 | 13.8 |
Percutaneous drainage is the therapy of choice for treating fluid collections. The success rates for drainage of fluid collections, pseudocysts or simple abscesses in variable sites are reported to be around 90%[20–24]. In contrast, reports on percutaneous drainage in patients with acute necrotizing pancreatitis were overall disappointing showing drainage therapy to be often inefficient with subsequent need for open surgery[1525]. The presumed reason for this discrepancy is that pancreatic/peripancreatic necroses and pancreatic abscesses contain solid, avital debris. However, some studies reported significantly better success rates treating necrotizing pancreatitis by drainage therapy only[152627]. A common feature of these studies was that large bore catheters (up to 28F) were used. The rationale for this strategy was the hypothesis that large bore catheters might be more effective for mobilizing solid tissue. However, the drawback of using large catheters is the more traumatic approach with the risk of bleeding and organ injury. In the present study, we evaluated the effect of drainage characteristics on the outcome of patients with necrotizing pancreatitis.
The 80 patients included in this study all suffered from acute, necrotizing pancreatitis. The diagnosis was based on the results of contrast-enhanced CT as well as clinical and laboratory findings. Seventy-three patients presented with necrosis of the pancreatic parenchyma itself, 7 patients demonstrated presumed necrosis of peripancreatic tissue. No patients with pseudocysts were included. Fifty-two patients (65%) had microbiologically proven infection of the necrotic tissue. Although for 28 patients (35%) culture was negative, interventional therapy was initiated because the clinical, laboratory and radiological findings strongly suggested infection.
The gold standard for the treatment of infected, necrotizing pancreatitis still is open surgery[28]. However, minimal-invasive interventional approaches continue to gain importance. In the present study, 43% of patients were cured by percutaneous drainage and conservative treatment only. When including those patients who additionally were treated with active, percutaneous necrosectomy without the need for open surgery the cure rate increased to 53%. Six surviving patients (8%) required surgical necrosectomy because percutaneous therapy failed in controlling sepsis. The mortality rate in this study was 34% which is within the expected range of 20%-40%[10–12] reported in the literature. Considering that the study population represented a seriously ill subset of patients as evidenced by high clinical and radiological scores, a mortality rate of 34% is an acceptable outcome.
The time of placement of the first drainage after hospital admission with a median of 3.5 d seems quite short because infection of necroses occur usually within 7 d to 10 d after the onset of symptoms[228]. However, it has to be considered, that 71 out of 80 patients (89%) were transferred from other hospitals with median hospitals stays of 7 d before the transfer to the university hospital. Due to the retrospective nature of this study we were able to assess the time of hospital admission, which naturally was not always identical with the time of symptom onset.
As opposed to the APACHE II and SAPS II scores the CTSI score did not show a good correlation with patient outcome in our study. This is discrepant to results published by Balthazar et al who found the mortality and morbidity to be closely correlated to the CTSI[19]. However, Balthazar et al studied a patient population covering the whole range of possible CTSI values whereas our population was seriously ill with CTSI values of at least 4. Presumably, the CTSI is not a good parameter to predict outcome in this subset of patients.
No significant correlation between drainage size/number and outcome parameters was found in this study. Rather than abolishing the concept of using large bore drainages we think that our results reflect the fact that the outcome of patients suffering from infected, necrotizing pancreatitis is influenced by a multitude of factors. 81% of the patients in this study required ICU admission with the need for mechanical ventilation and renal replacement therapy in many cases. It seems to be difficult to isolate one single parameter which might modify overall prognosis in such a complex study group. An aggravating factor was the retrospective nature of this study. Different lavage schemes and various types of drainages were used over the years and the size of drainages used is influenced by a multitude of factors. Especially the latter fact is important since the inner diameter of the adapter and of the drainage itself is different for different companies despite the identical outer diameter given in French. Moreover, the length of the drainage is inversely correlated with the flow. A prospective study seems to be necessary to evaluate the impact of drainage characteristics. However, it is questionable if such a study is feasible because large study groups are necessary to provide adequate power.
In conclusion, percutaneous drainage and minimal invasive necrosectomy are efficient tools for treatment of necrotizing pancreatitis. Large bore drainages did not prove to be more effective in controlling the septic focus. However, it seems to be difficult to determine a single prognostic parameter in these multimorbid patients.
Percutaneous, interventional treatment modalities are safe and efficient tools in the therapy of patients with necrotizing pancreatitis. Special therapy details, like the optimal drainage size, have not yet been evaluated.
Whereas surgical debridement is often associated with a considerable trauma and may cause escalation of multiorgan failure and sepsis, minimal invasive methods are a safe and effective tool to bridge time to surgery or avoid it at all.
In this article we analyze if drainage size affects outcome in 80 patients with necrotizing pancreatitis.
Large bore drainages (≥ 16F) did not prove to be more effective in controlling the septic focus than smaller ones (< 16F).
Interventional methods in our article include percutaneous, radiologically assisted drainage therapy.
This is a well written manuscript. Publication is recommended.
| 1. | Banks PA. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 1997;92:377-386. |
| 2. | Beger HG, Bittner R, Block S, Blchler M. Bacterial contamination of pancreatic necrosis. A prospective clinical study. Gastroenterology. 1986;91:433-438. |
| 3. | Beger HG, Rau B, Mayer J, Pralle U. Natural course of acute pancreatitis. World J Surg. 1997;21:130-135. |
| 4. | Bradley EL 3rd. A fifteen year experience with open drainage for infected pancreatic necrosis. Surg Gynecol Obstet. 1993;177:215-222. |
| 5. | Rau B, Uhl W, Buchler MW, Beger HG. Surgical treatment of infected necrosis. World J Surg. 1997;21:155-161. |
| 6. | Frey CF, Bradley EL 3rd, Beger HG. Progress in acute pancreatitis. Surg Gynecol Obstet. 1988;167:282-286. |
| 7. | Carter R. Management of infected necrosis secondary to acute pancreatitis: a balanced role for minimal access techniques. Pancreatology. 2003;3:133-138. |
| 8. | Connor S, Alexakis N, Raraty MG, Ghaneh P, Evans J, Hughes M, Garvey CJ, Sutton R, Neoptolemos JP. Early and late complications after pancreatic necrosectomy. Surgery. 2005;137:499-505. |
| 9. | Connor S, Ghaneh P, Raraty M, Rosso E, Hartley MN, Garvey C, Hughes M, McWilliams R, Evans J, Rowlands P. Increasing age and APACHE II scores are the main determinants of outcome from pancreatic necrosectomy. Br J Surg. 2003;90:1542-1548. |
| 10. | Branum G, Galloway J, Hirchowitz W, Fendley M, Hunter J. Pancreatic necrosis: results of necrosectomy, packing, and ultimate closure over drains. Ann Surg. 1998;227:870-877. |
| 11. | Buchler MW, Gloor B, Muller CA, Friess H, Seiler CA, Uhl W. Acute necrotizing pancreatitis: treatment strategy according to the status of infection. Ann Surg. 2000;232:619-626. |
| 12. | Werner J, Uhl W, Hartwig W, Hackert T, Muller C, Strobel O, Buchler MW. Modern phase-specific management of acute pancreatitis. Dig Dis. 2003;21:38-45. |
| 13. | Connor S, Raraty MG, Howes N, Evans J, Ghaneh P, Sutton R, Neoptolemos JP. Surgery in the treatment of acute pancreatitis--minimal access pancreatic necrosectomy. Scand J Surg. 2005;94:135-142. |
| 14. | van Sonnenberg E, Wittich GR, Goodacre BW, Casola G, D’Agostino HB. Percutaneous abscess drainage: update. World J Surg. 2001;25:362-369; discussion 370-372. |
| 15. | Freeny PC, Hauptmann E, Althaus SJ, Traverso LW, Sinanan M. Percutaneous CT-guided catheter drainage of infected acute necrotizing pancreatitis: techniques and results. AJR Am J Roentgenol. 1998;170:969-975. |
| 16. | Gmeinwieser J, Feuerbach S, Zirngibl H, Agha E, Holstege A, Jauch K, Schölmerich J. Percutaneus treatment of infected necrotizing pancreatitis. Bologna: Mandozzi Editori 1997; 575-578. |
| 17. | Gmeinwieser J, Holstege A, Zirngibl H, Palitzsch KD, Hugl S, Strotzer M, Feuerbach S, Scholmerich J. Successful percutaneous treatment of infected necrosis of the body of the pancreas associated with segmental disruption of the main pancreatic duct. Gastrointest Endosc. 2000;52:413-415. |
| 18. | Zorger N, Hamer OW, Feuerbach S, Borisch I. Percutaneous treatment of a patient with infected necrotizing pancreatitis. Nat Clin Pract Gastroenterol Hepatol. 2005;2:54-57; quiz 58. |
| 19. | Balthazar EJ, Robinson DL, Megibow AJ, Ranson JH. Acute pancreatitis: value of CT in establishing prognosis. Radiology. 1990;174:331-336. |
| 20. | Karlson KB, Martin EC, Fankuchen EI, Mattern RF, Schultz RW, Casarella WJ. Percutaneous drainage of pancreatic pseudocysts and abscesses. Radiology. 1982;142:619-624. |
| 21. | Srikanth G, Sikora SS, Baijal SS, Ayyagiri A, Kumar A, Saxena R, Kapoor VK. Pancreatic abscess: 10 years experience. ANZ J Surg. 2002;72:881-886. |
| 22. | Lee MJ, Rattner DW, Legemate DA, Saini S, Dawson SL, Hahn PF, Warshaw AL, Mueller PR. Acute complicated pancreatitis: redefining the role of interventional radiology. Radiology. 1992;183:171-174. |
| 23. | Freeny PC. Radiology of the pancreas: two decades of progress in imaging and intervention. AJR Am J Roentgenol. 1988;150:975-981. |
| 24. | van Sonnenberg E, Wittich GR, Casola G, Stauffer AE, Polansky AD, Coons HG, Cabrera OA, Gerver PS. Complicated pancreatic inflammatory disease: diagnostic and therapeutic role of interventional radiology. Radiology. 1985;155:335-340. |
| 25. | Gouzi JL, Bloom E, Julio C, Labbe F, Sans N, el Rassi Z, Carrere N, Pradere B. Percutaneous drainage of infected pancreatic necrosis: an alternative to surgery. Chirurgie. 1999;124:31-37. |
| 26. | Cheung MT, Ho CN, Siu KW, Kwok PC. Percutaneous drainage and necrosectomy in the management of pancreatic necrosis. ANZ J Surg. 2005;75:204-207. |
| 27. | Echenique AM, Sleeman D, Yrizarry J, Scagnelli T, Guerra JJ Jr, Casillas VJ, Huson H, Russell E. Percutaneous catheter-directed debridement of infected pancreatic necrosis: results in 20 patients. J Vasc Interv Radiol. 1998;9:565-571. |