Published online Feb 7, 2008. doi: 10.3748/wjg.14.693
Revised: October 26, 2007
Published online: February 7, 2008
AIM: To investigate the expression of multiple genes in Chinese jianpi herbal recipe Wei Chang An (WCA) in human gastric cancer cell line SGC-7901.
METHODS: A human gastric adenocarcinoma cell line SGC-7901 grafted onto nude mice was used as the animal model. The mice were randomly divided into 3 groups, one control and the two representing experimental conditions. Animals in the two experimental groups received either WCA over a 34-d period or 5-fluorouracil (5-FU) over 6-d period starting at 8th d after grafting. Control animals received saline on an identical schedule. Animals were killed 41 d after being grafted. The expression profiles in paired WCA treated gastric cancer samples and the N.S. control samples were studied by using a cDNA array representing 14 181 cDNA clusters. The alterations in gene expression levels were confirmed by Real-time Quantitative polymerase chain reaction (qPCR).
RESULTS: When compared with controls, the average tumor inhibitory rate in WCA group was 44.32% ± 5.67% and 5-FU 47.04% ± 11.33% (P < 0.01, respectively). The average labeling index (LI) for PCNA in WCA group and 5-FU group was significantly decreased compared with the control group. Apoptotic index (AI) was significantly increased to 9.72% ± 4.51% using the terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate fluorescence nick end labeling (TUNEL) method in WCA group compared with the controls 2.45% ± 1.37%. 5-FU group was also found to have a significantly increased AI compared with the controls. The expression of cleaved Caspase-3 in WCA group and 5-FU group was significantly increased compared with the control group respectively. There were 45 different expressed sequence tags (ESTs) among the control sample pool and WCA sample pool. There were 24 ESTs up-regulated in WCA samples and 21 ESTs down-regulated. By using qPCR, the expression level of Stat3, rap2 interacting protein x (RIPX), regulator of differentiation 1 (ROD1) and Bcl-2 was lower in WCA group than that in control group respectively. By using SP immunohistochemical method the expression of Phospho-Stat3 (Tyr705) and Bcl-2 in WCA group and 5-FU group was significantly decreased compared with the control group respectively.
CONCLUSION: WCA could inhibit gastric cancer cell SGC-7901 growth in vivo. WCA could induce gastric cancer cell apoptosis and suppress proliferation. Its mechanisms might be involved in the down-regulation of Stat3, RIPX, ROD1 and Bcl-2 gene.
- Citation: Zhao AG, Li T, You SF, Zhao HL, Gu Y, Tang LD, Yang JK. Effects of Wei Chang An on expression of multiple genes in human gastric cancer grafted onto nude mice. World J Gastroenterol 2008; 14(5): 693-700
- URL: https://www.wjgnet.com/1007-9327/full/v14/i5/693.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.693
Gastric cancer, despite its declining incidence, remains one of the most common causes of cancer-related death worldwide. Overall, 5-year survival after the diagnosis of gastric cancer is 10%-21%[1]. Surgery is the main method of treatment. Patients who undergo a potentially curative resection have a better prognosis, but even with curative resection, gastric cancer still has a high risk of relapse. Although various chemotherapeutic agents have been reported for use in patients with gastric cancer, the median prognosis for survival in patients with advanced gastric cancer remains less then 7-12 mo at present[2–5].
We developed a Chinese jianpi herbal recipe Wei Chang An (WCA) to improve survival of gastric cancer patients. Previous clinical paired comparative studies have indicated patients with gastric carcinoma will benefit from WCA treatment[67]. Clinical data have shown that WCA can increase the 3-year survival rate of advanced gastric cancer. Another clinical study of 208 patients with gastric adenocarcinoma has suggested interaction between TNM stage (P = 0.000), radical resection (P = 0.000), chemotherapy (P = 0.002) and WCA (P = 0.000) in their effect on long-term survival. Patients who have received WCA have better prognosis on multivariate analysis, independent of other prognostic factors. The odds ratios of WCA was 0.315 (95% CI 0.204-0.486)[8].
In an animal model of human gastric cancer cell line SGC-7901 subcutaneously grafted onto nude mice, we have found tumor growth is significantly inhibited by treatment with WCA. Immunohistochemical staining for Ki-67 has shown WCA inhibits cell proliferation. The terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate fluorescence nick end labeling (TUNEL) method, flow cytometry and electron microscopy have clarified that WCA also enhances apoptosis[9]. In the orthotopic implanted model, inhibition of tumor growth and metastasis has also been verified, and higher activity of NK cells has been found in WCA-treated animals compared with the control group[10–12]. Based on previous studies, we inferred that the tumor suppressive effect of WCA involves multiple biological processes and mechanisms. This study was designed to investigate the underlying mechanism of WCA in treatment of gastric cancer, using the cDNA array technique, real-time quantitative PCR and immunohistochemical techniques.
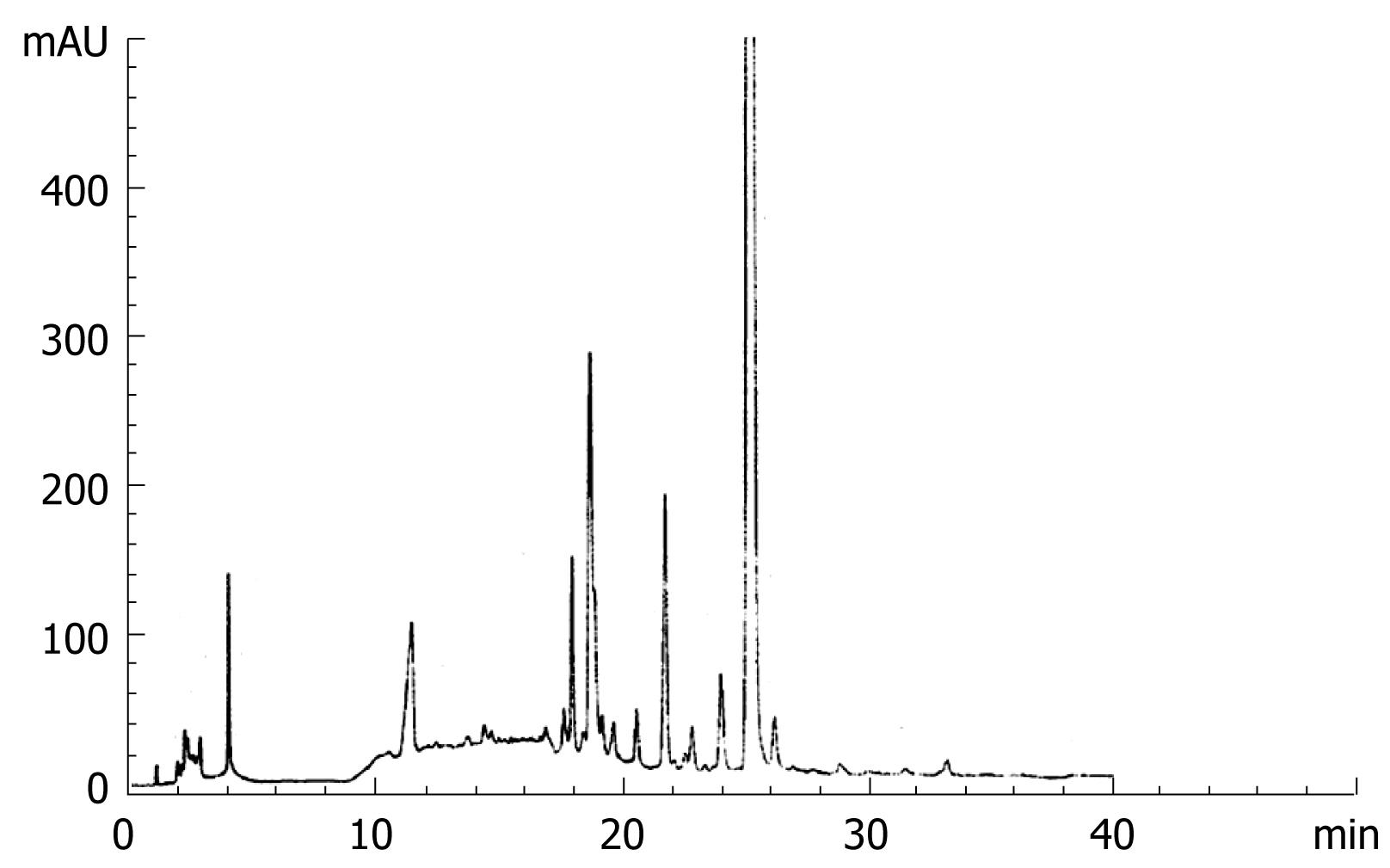
The composition of WCA includes Atractylodes macrocephala koidz., Poria cocos (Schw.) Wolf, Glycyrrhiza uralensis Fisch., Rehd. Et Wils., and Prunella vulgaris L. The preparation of WCA decoction has been described previously[89] and the concentration of the decoction was 120 g/L. High Performance Liquid Chromatography (HPLC) was used for monitoring the stability of the decoction. Lichrospher-C18 column at 25°C was used. The mobile phase consisted of methyl alcohol/water with a linear gradient as follows: methyl alcohol, 5, 5, 70, 100, 100%; water, 95, 95, 30, 0, 0%; at 0, 5, 15, 35 and 40 min, respectively, and the flow rate was 1 mL/min. The detection was performed at UV 280 nm (Figure 1). 5-fluorouracil (5-FU) was purchased from Xudong-Haipu (Shanghai) Pharma, China (Lot 030601).
A human gastric adenocarcinoma cell line SGC-7901 and 73 6-7-wk-old male BALB/C-nu/nu mice (weight 18-22 g) were obtained from Shanghai Tumor Institute [No. SCXK (Shanghai) 2002-0001; Shanghai, China]. The animal experiment was repeated three times. The mice were divided into three groups in every test, one control and two experimental. Animals in the two experimental groups received either WCA 0.5 mL/d by gastric perfusion over a 34 d period or 5-FU 20 mg/kg per day i.p., over a 6-d period starting on day 8 after grafting. Control animals received saline 0.5 mL/d by gastric perfusion on an identical schedule. Animals were killed 41 d after being grafted. Tumor weight was determined immediately by electron balance after the animals were killed. Tumor tissue was obtained within 2 min after removal from the animal. Each block was cut into three pieces, one for routine pathological diagnosis and immunohistochemical staining, and the others for molecular analysis. The latter samples were frozen immediately in liquid nitrogen and stored at -260°C.
Cell proliferation: The streptavidin peroxidase (SP) immunohistochemical method was used to detect the expression of proliferating cell nuclear antigen (PCNA) in xenografts. The dilution of PCNA mouse monoclonal antibody (Shanghai Changdao Biotech, Shanghai, China; Cat. No. M-0437, Lot 0509) was 1:150. The procedure was performed according to the manufacturer’s instructions. The positive cells were identified, counted and analyzed under the light microscope. Non-necrotic zones were selected in the tissue section. Five images (× 400) of at least 1000 cells were selected on the screen and analyzed. PCNA is predominantly localized in the nuclei.
Apoptosis: Apoptotic index (AI) was examined by TUNEL method (Roche Diagnostics, GmbH, Mannheim, Germany; Cat. No. 11 684 817 910, Lot 12 067 000). The procedure was carried out according to the kit protocol. The positive cells were identified, counted and analyzed under the light microscope. Non-necrotic zones were selected in the tissue sections. Five images of at least 1000 cells were selected on the screen and analyzed.
Expression of cleaved caspase-3: SP immunohisto-chemistry was also used to detect cleaved caspase-3. The dilution of cleaved caspase-3 (Asp175) antibody (Cell Signaling Technology, Beverly, MA, USA; Cat. No. 9661, Lot 15) was 1:200. Cleaved caspase-3 was predominantly localized in the cytoplasm and perinuclear region in apoptotic cells.
The expression profiles in paired WCA-treated gastric cancer and control samples were studied by using a cDNA array (Casarray, Shanghai, China) representing 14 181 cDNA clusters.
Membranes were exposed to a phosphor screen overnight and scanned using a FLA-3000A fluorescent image analyzer (Fuji Photo Film, Tokyo, Japan). After subtracting the background selected from an area in which no PCR product was spotted, clones with an intensity density over 10 were considered as positive signals. Hybridization data were considered invalid if there was a difference of over 1.5-fold in the intensity of any of the 12 control spots for the same control cDNA between arrays.
The hybridization intensity of corresponding dots in control and WCA groups was compared. If the difference in spot intensity in these two groups was more than twofold higher or lower, the corresponding genes were considered as being differentially expressed.
According to the result of cDNA array analysis, 19 genes related to regulation of cell proliferation, differentiation and apoptosis were selected for investigation. Quantification of gene expression levels were confirmed by real-time quantitative PCR. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal standard. Sequences of the primers used for RT-PCR analysis are described in Table 1. Primers were designed by Primer Express 2.0 and synthesized by TaKaRa Biotechnology (Dalian), China. Total cellular RNA was isolated from tumor tissues using Trizol (Gibco/BRL, Carisbad, USA). The RNA extracted from each sample was qualified by agarose gel electrophoresis and ethidium bromide staining and the A: 260/280 ratio was determined by electrophotometry. The extracted RNA was converted to first strand cDNA with AMV reverse transcriptase (Reverse Transcription System, Promega, Madison, USA). Nineteen genes and GAPDH were amplified using a sequence detection system (ABI PRISM® 7900HT; ABI, Foster City, CA, USA). Each cDNA sample was placed in three different wells (triple-offset). The PCR reaction consisted of stage 1, 50°C for 2 min; stage 2, 95°C for 15 min; stage 3, 40 cycles of 94°C for 15 s, 55°C for 30 s, and 72°C for 30 s; stage 4, 95°C for 15 s, 60°C for 15 s and 95°C for 15 s. Each reaction tube contained: 10 &mgr;mol/L primer (a pair) 0.5 &mgr;L + SYBR 2.5 &mgr;L + ROX 0.1 &mgr;L + cDNA 1 &mgr;L + ddH2O 0.9 &mgr;L (Platinum®SYBR® Green qPCR SuperMix UDG (Invitrogen, Carlsbad, CA, USA; Cat. No. 11733-038). Relative gene expression was analyzed using the 2-ΔΔCT method[13].
| Group | Test 1 | Test 2 | Test 3 | |||||||||
| Number Begin/End | Tumor weight (g) | P value | Inhibition rate (%) | Number Begin/End | Tumor weight (g) | P value | Inhibition rate (%) | Number Begin/End | Tumor weight (g) | P value | Inhibition rate (%) | |
| WCA | 8/8 | 0.99 ± 0.76 | 0.0117a | 48.7 | 8/72 | 1.02 ± 0.16 | 0.0045b | 37.91 | 8/8 | 0.88 ± 0.47 | 0.0072b | 46.35 |
| 5-FU | 10/91 | 0.77 ± 0.48 | 0.0002b | 60.1 | 7/7 | 0.97 ± 0.34 | 0.0056b | 41.29 | 7/7 | 0.98 ± 0.46 | 0.0167a | 39.74 |
| Control | 8/8 | 1.93 ± 0.58 | 8/8 | 1.65 ± 0.46 | 9/9 | 1.63 ± 0.52 | ||||||
The SP immunohistochemical method was used to detect Phospho-Stat3 and Bcl-2 expression in tumor cells. The dilution of Phospho-Stat3 (Tyr705) antibody (Cell Signaling Technology; Cat. No. 9131, Lot 5) was 1:100 and Bcl-2 (Shanghai Changdao Biotech; Cat. No. M-0025, Lot 51806) was 1:50. Phospho-Stat3 was predominantly localized in the nucleus. Bcl-2 was predominantly localized in the cytoplasm and nuclear membrane.
Statistical analysis was performed using SPSS ver. 11.0 (SPSS, Chicago, IL, USA). The results were expressed as mean ± SD and significant difference was assessed by Student’s t test. P < 0.05 was considered statistically significant.
When compared with controls, tumor growth was significantly inhibited by treatment with WCA or 5-FU (P < 0.01). The average tumor inhibition rate in the WCA group was 44.32% + 5.67% and 47.04% ± 11.33% in the 5-FU group (Table 1).
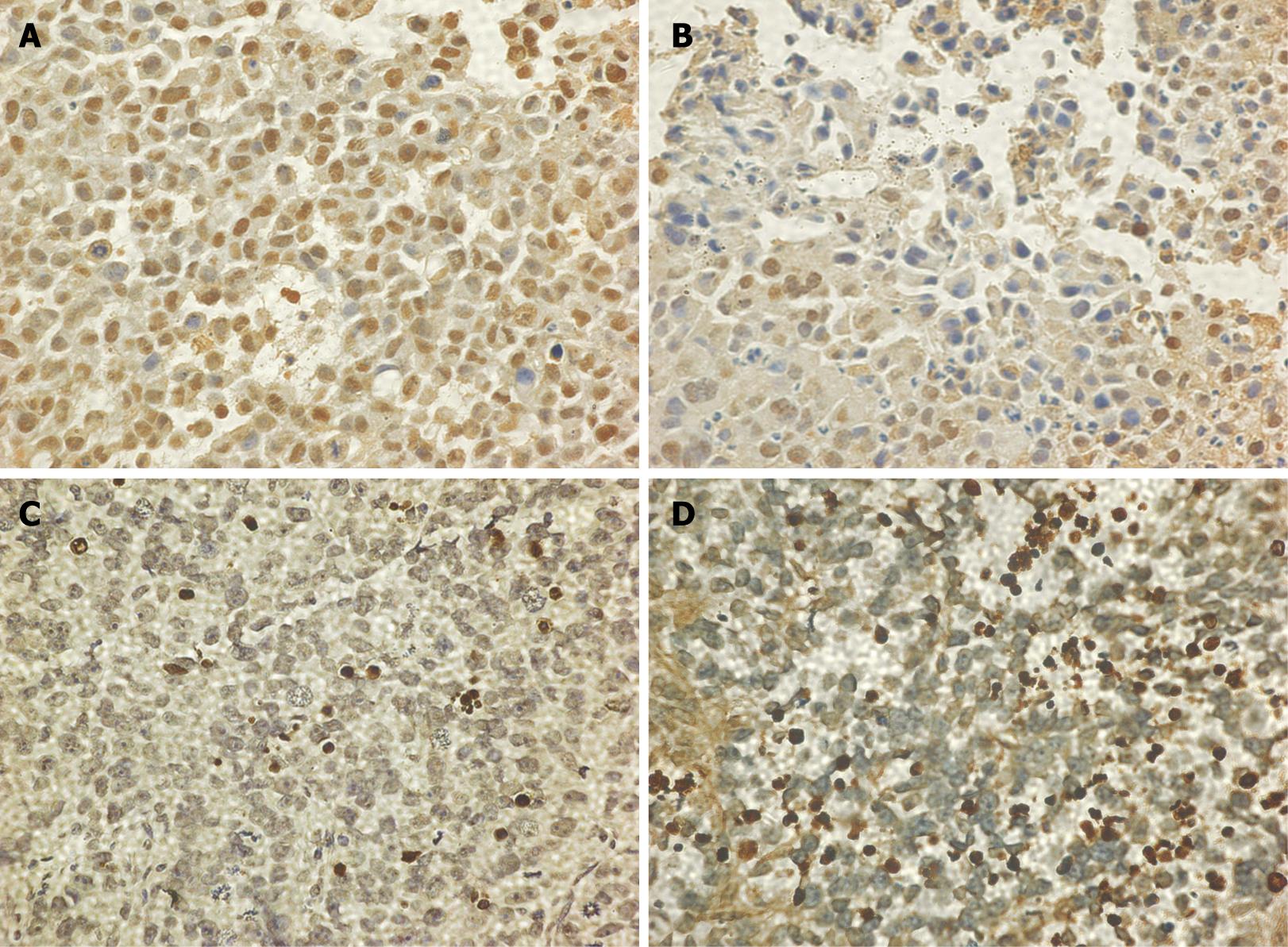
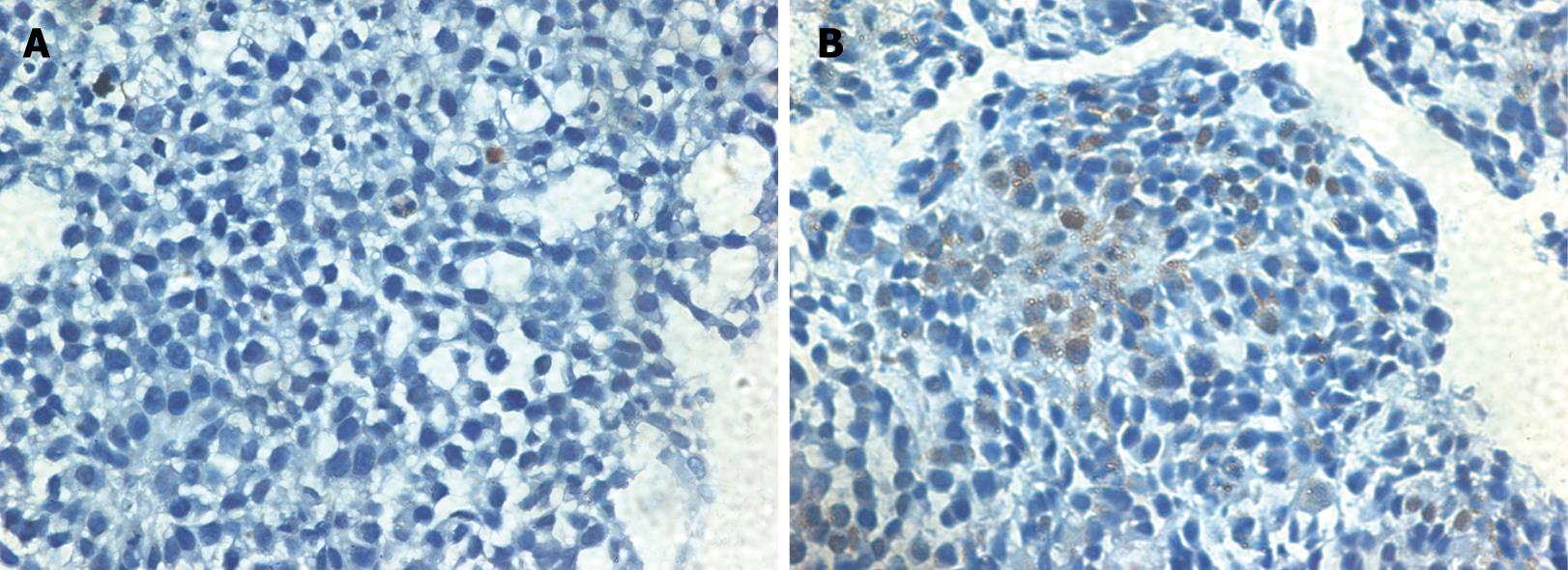
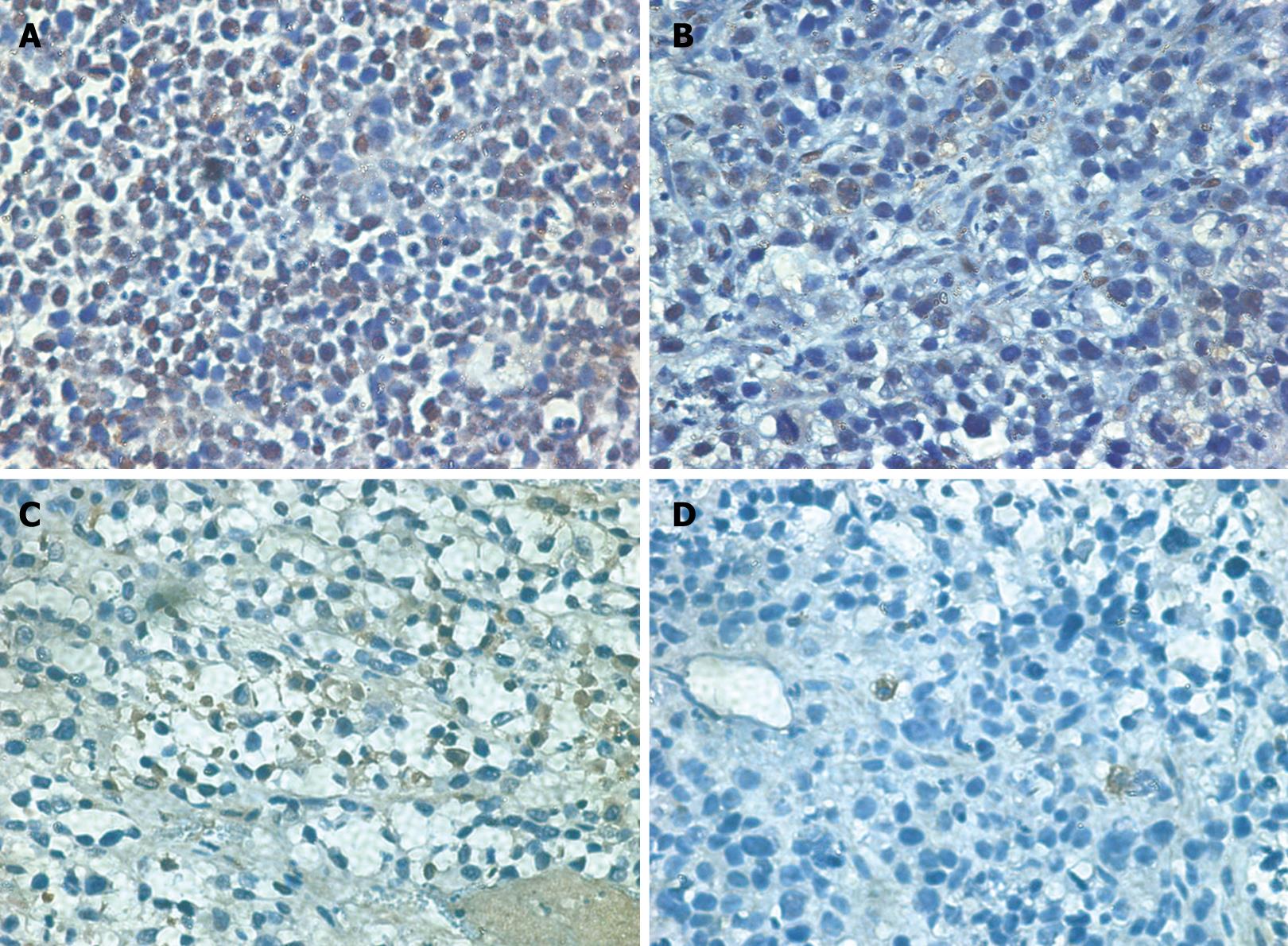
The average labeling index for PCNA in the WCA and 5-FU groups was significantly decreased compared with that in the control group (Table 2, Figure 2). AI of human gastric cancer xenografts in nude mice was significantly increased to 9.72% ± 4.51% in the WCA group compared with that in the control group (2.45% ± 1.37%). The 5-FU group also had a significantly increased AI compared with the controls (Table 2, Figure 2). The expression of cleaved caspase-3 in the WCA and 5-FU group was significantly increased compared with that in the control group (Table 2, Figure 3).
| Group | n | PCNA | TUNEL | Cleaved caspase-3 (Asp 175) (%) | |||||||
| Positive rate (%) | P value | Intense positive rate (%) | P value | Total positive rate (%) | P value | Apoptotic index (%) | P value | Positive rate (%) | P value | ||
| WCA | 8 | 35.73 ± 6.01 | 0.0005b | 3.39 ± 1.48 | 0.0155a | 39.03 ± 7.37 | 0.0009b | 9.72 ± 4.51 | 0.0007b | 5.20 ± 2.26 | 0.0367a |
| 5-FU | 9 | 37.86 ± 16.50 | 0.0325a | 5.62 ± 4.21 | 0.1972 | 43.48 ± 19.77 | 0.0406a | 5.74 ± 1.75 | 0.0007b | 4.73 ± 1.76 | 0.0451a |
| Control | 8 | 53.48 ± 9.34 | 8.78 ± 5.43 | 62.26 ± 13.80 | 2.45 ± 1.37 | 2.82 ± 1.84 | |||||
There were 45 different expressed sequence tags (ESTs) among the control and WCA samples. There were 24 ESTs up-regulated and 21 down-regulated in the WCA samples. These 45 ESTs contained 35 cloned genes and 11 unknown ESTs. According to the results of cDNA array analysis, 19 genes related to regulation of cell proliferation, differentiation and apoptosis were selected for investi-gation. Quantification of gene expression levels was confirmed by real-time quantitative PCR. The expression level of stat3 (2-ΔΔCT = 0.16), rap 2 interacting protein x (RIPX) (2-ΔΔCT = 0.18), regulator of differentiation 1 (ROD1) (2-ΔΔCT = 0.23) and bcl-2 (2-ΔΔCT = 0.10) was lower in the WCA group than that in the control group. The expression level of another 15 genes did not differ between the two groups (Table 3).
| Gene name | UniGene cluster | Primer sequences (5’-3’) | Combining sites (bp) | Amplifiers (bp) | WCA (120 mg/mL) vs NS control | ||
| ΔΔCT (n) | 2-ΔΔCT | ||||||
| stat3 | Hs.421342 | forward | CCTGGAGCAGCTCCATCAG | 254 | 58 | 2.65 (4) | 0.16 (0.11-0.24) |
| reverse | AAACTGCCGCAGCTCCATT | 311 | |||||
| RIPX | Hs.7927 | forward | GAGTGCCTTTAAGCTGCAGAGTT | 6 | 69 | 2.50 (5) | 0.18 (0.13-0.23) |
| reverse | TCCAAGCGACTGTTTAGTTCACTT | 74 | |||||
| ROD1 | Hs.269988 | forward | AACTCCTCTCTGTAAAGCATTTTGC | 525 | 64 | 2.09 (4) | 0.23 (0.12-0.23) |
| reverse | TGCACTGGGTCTTCTTTCAGAA | 588 | |||||
| bcl-2 | Hs.12677 | forward | TGTTGGCCGGATCACCAT | 2557 | 60 | 3.27 (5) | 0.10 (0.06-0.17) |
| reverse | TCCCCAATGATCAGGTCCTTT | 2616 | |||||
We performed immunohistochemistry to confirm our data at the protein level. Expression of Phospho-Stat3 (Tyr705) and Bcl-2 was significantly decreased in the WCA and 5-FU group compared with that in the control group (Table 4, Figure 4).
| Group | n | P-Stat3 | Bcl-2 | ||||||
| Positive rate (%) | P value | Intense positive rate (%) | P value | Total positive rate (%) | P value | Positive rate (%) | P value | ||
| WCA | 8 | 35.93 ± 12.67 | 0.0024b | 3.64 ± 1.72 | 0.0023b | 39.57 ± 13.31 | 0.0002b | 1.62 ± 0.82 | 0.0006b |
| 5-FU | 9 | 36.95 ± 27.21 | 0.0732 | 4.38 ± 3.62 | 0.0050b | 41.33 ± 30.22 | 0.0243a | 7.72 ± 5.31 | 0.9364 |
| Control | 8 | 56.49 ± 9.34 | 13.16 ± 7.06 | 69.65 ± 10.80 | 7.53 ± 3.73 | ||||
Gastric cancer is the second most common cancer worldwide. Its poor outcome is due to the fact that 75% of patients are considered incurable at diagnosis (advanced disease), therefore, its treatment remains a great challenge. The Chinese jianpi herbal recipe WCA has been developed to improve survival of gastric cancer patients. Previous studies have shown the tumor suppressive effect of WCA involved multiple biological processes and mechanisms, including enhanced apoptosis and inhibition of proliferation. However, further mechanisms are not fully understood[9–12].
In the present study, tumor growth was significantly inhibited by treatment with WCA at a concentration of 120 g/L in the animal model of human gastric cancer cell line SGC-7901 grafted onto nude mice (P < 0.01). The average tumor inhibitory rate in the WCA group was 44.32% ± 5.67%. The TUNEL method was used to detect apoptotic cells. AI of cancer cells was significantly increased in the WCA group compared with that in the control group. The 5-FU group also showed a significantly increased AI compared with that in the control group. Caspase-3 is one of the key executioners of apoptosis, which is either partially or totally responsible for proteolytic cleavage of many key proteins, such as the nuclear enzyme poly ADP-ribose polymerase. Activation of caspase-3 requires proteolytic processing of its inactive zymogen into activated p17 and p12 subunits. Cleavage of caspase-3 requires aspartic acid at the P1 position[1415]. Cleaved caspase-3 (Asp175) antibody detects endogenous levels of the large fragment (17/19 kDa) of activated caspase-3, which results from cleavage adjacent to Asp175. It does not recognize full-length caspase-3 or other cleaved caspases. The expression of cleaved caspase-3 in the WCA and 5-FU groups was significantly increased compared with that in the control group. This result clarifies that WCA enhances apoptosis.
Immunohistochemical staining for PCNA also showed WCA inhibited cell proliferation. These results were consistent with our previous studies[9].
Carcinogenesis and the progression of carcinoma are thought to develop from multistage activation of oncogenes and loss of suppressor genes. In trying to understand the mechanism of WCA at the RNA expression level, we used cDNA microarray and real-time quantitative PCR techniques. The expression profiles in paired WCA-treated gastric cancer and control samples were screened by cDNA array analysis, which represented 14 181 cDNA clusters, and verified by real-time quantitative PCR and immunohistochemistry. There were 24 ESTs up-regulated and 21 ESTs down-regulated in WCA samples. These 45 ESTs contains 35 cloned genes and 11 unknown ESTs. According to the result of cDNA microarray analysis, 19 genes related to the regulation of cell proliferation, differentiation and apoptosis were selected for investigation. The expression level of Stat3 (2-ΔΔCT = 0.16), RIPX (2-ΔΔCT = 0.18), ROD1 (2-ΔΔCT = 0.23) and Bcl-2 (2-ΔΔCT = 0.10) was lower in the WCA group than that in the control group. The expression level of another 15 genes did not differ between these two groups. Moreover, we performed immunohistochemistry to confirm these data at the protein level. The expression of Phospho-Stat3 (Tyr705) and Bcl-2 in the WCA and 5-FU groups was significantly decreased compared with that in the control group.
Jak-Stat pathways play important roles in oncogenesis, tumor progression, angiogenesis, cell motility, immune responses and stem cell differentiation[16]. Stat3 is a key signaling molecule for many cytokines and growth-factor receptors, and is required for murine fetal development. In addition, Stat3 is constitutively activated in a number of human tumors and possesses oncogenic potential and anti-apoptotic activity[1718]. Stat3 is activated by tyrosine phosphorylation at Tyr705, which induces dimerization, nuclear translation and DNA binding. Stat3 isoform expression appears to reflect biological function. The relative expression levels of Stat3 α (86 kDa) and Stat3 β (79 kDa) depends on cell type, ligand exposure or maturation stage of the cells. Phospho-Stat3 (Tyr705) antibody detects endogenous levels of Stat3 only when phosphorylated at Tyr705. Activated forms of Stat3 and Phospho-Stat3 (Tyr705) were found in the nucleus of cancer cells in 30% of human gastric cancer specimens, which suggests that constitutively activated Stat3 signaling supports gastric cancer cell survival in association with survivin expression[19]. Stat3 regulates multiple genes important for apoptosis, cellular proliferation and angiogenesis in gastric cancer, therefore its activation may play an important role in gastric cancer development and progression[20]. The expression level of stat3 mRNA and Phospho-Stat3 protein was lower in the WCA group than that in the control group, which suggests WCA may regulate human gastric cancer cell SGC-7901 apoptosis and/or proliferation through the Stat3 pathway.
The expression level of bcl-2 mRNA was also decreased in the WCA group, as was that of its encoded protein. Bcl-2 is one of the Bcl-2 family that is important in regulation of apoptosis. Previous studies have demonstrated Stat3 regulates Bcl-2 expresssion[21], therefore, we inferred that the decrease in Bcl-2 expression in the WCA group was related to the mechanism of action of WCA, and may be correlated with lower Stat3 expression.
Other two genes: ROD1 (Hs.269988) or RIPX (Hs.7927) we found decreased expressing in WCA group had been seldom studied in solid tumor. In the fission yeast Schizosaccharomyces pombe, the N-arginine dibasic convertase 1 (NRD1) gene that encodes an RNA-binding protein negatively regulates the onset of differentiation. The mammalian homologue of NRD1 is ROD1, which encodes a protein with four repeats of typical RNA-binding domains. When expressed in fission yeast, ROD1 protein functions are similar to those of Nrd1. ROD1 is highly expressed in adult and embryo hematopoietic cells or organs. Overexpression of ROD1 effectively blocks the differentiation of human leukemia cells, without affecting their proliferative ability, which suggests ROD1 plays a critical role in controlling differentiation in mammalian cells[2223]. Andoh et al have reported interaction of Rod1 and Rsp5 may be important for drug resistance[24]. However, the role of ROD1 in the development of human gastric cancer is not clear.
Another name for RIPX is kIAA0871, which is a member of the KIAA gene family[25]. In the progress of the human gene project, KIAA genes have been found continuously. These genes are newly identified long cDNAs that encode large proteins. The large proteins play important roles in biological processes. Further physiological, developmental and genetic studies are necessary[26].
In conclusion, genes involved in the mechanism of WCA are Stat3, Bcl-2, RIPX and ROD1. WCA down-regulates the expression of several genes. How WCA affects the expression of these genes and the roles of these genes themselves need to be explored.
Previous clinical paired comparative studies have indicated patients with gastric carcinoma will benefit from treatment with the Chinese Jianpi herbal recipe Wei Chang An (WCA).
This study was designed to investigate the underlying mechanism of WCA in treatment of gastric cancer by cDNA microarray analysis and real-time quantitative polymerase chain reaction.
Gastric cancer, despite its declining incidence, remains one of the most common causes of cancer-related death throughout the world. The poor outcome is due to the fact that 75% of patients are considered incurable at diagnosis (advanced disease), and therefore, its treatment remains a great challenge. Previous research has shown the tumor suppressive effect of WCA involves multiple biological processes and mechanisms, including enhanced apoptosis and inhibition of proliferation. To understand the further mechanisms of WCA is of benefit to developing a new therapeutic method.
WCA is composed of Atractylodes macrocephala koidz., Poria cocos (Schw.) Wolf, Sargentodoxa cuneata (Oliv.) Rehd. Et Wils. and Prunella vulgaris L. The concentration of the decoction was 120 g/L. High Performance Liquid Chromatography was used for monitoring the stability of WCA.
This is an interesting study. The authors investigated the expression of multiple genes in nude mice grafted with human gastric cancer cell line SGC-7901 and treated with WCA. It was demonstrated that WCA inhibited gastric cancer cell growth in vivo. WCA can induce gastric cancer cell apoptosis and suppress proliferation.
| 1. | Phan AT, Ajani JA. Gastric carcinoma. Curr Oncol Rep. 2004;6:192-198. |
| 2. | Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi E. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24:4991-4997. |
| 3. | Scartozzi M, Galizia E, Verdecchia L, Berardi R, Antognoli S, Chiorrini S, Cascinu S. Chemotherapy for advanced gastric cancer: across the years for a standard of care. Expert Opin Pharmacother. 2007;8:797-808. |
| 4. | Yilmaz U, Oztop I, Alacacioglu A, Yaren A, Tarhan O, Somali I. Irinotecan combined with infusional 5-fluorouracil and high-dose leucovorin for the treatment of advanced gastric carcinoma as the first-line chemotherapy. Chemotherapy. 2006;52:264-270. |
| 5. | Takiuchi H, Goto M, Kawabe S, Ohta S, Katsu K. Second-line chemotherapy in gastric cancer. Gan To Kagaku Ryoho. 2005;32:19-23. |
| 6. | Yang JK, Zhen J, Shen KP. Clinical study on post-operative metastasis prevention of progressive stage of gastric cancer by weichang'an. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2003;23:580-582. |
| 7. | Qiu JX, Jia JS, Yang JK, Zheng J, Zheng JG, Tang LD, Wang N, Shen KP, Pang HF, Ji GR. Probing into the treatment of advanced stage of stomach carcinoma mainly by spleen-strengthening method. Zhongyi Zazhi. 1992;33:23-25. |
| 8. | Zhao AG, Cai Y, Yang JK, Zheng J, Shen KP. Effect of Chinese Jianpi herbs on prognosis of gastric cancer. Shijie Huaren Xiaohua Zazhi. 2005;13:1055-1058. |
| 9. | Zhao AG, Zhao HL, Jin XJ, Yang JK, Tang LD. Effects of Chinese Jianpi herbs on cell apoptosis and related gene expression in human gastric cancer grafted onto nude mice. World J Gastroenterol. 2002;8:792-796. |
| 10. | Zhao HL, Zhao AG, You SF, Gu Y, Tang LD, Yang JK. Growth-inhibiting and anti-metastasis effects of Weichang'an Decoction on orthotopic transplant nude mouse model of human gastric cancer. Zhong Xi Yi Jie He Xue Bao. 2005;3:378-381. |
| 11. | Zheng JG, Zhao AG, Gu Y, You SF, Zhao HL, Tang LD. Inhibition of tumor cell proliferation and induction of apoptosis in orthotopic transplanted gastric cancer SGC-7901 by Wei Chang An. Weichangbing Xue. 2006;11:336-339. |
| 12. | Zhao AG, Zhao HL, Yang JK, Gu Y, Liu J, Tang LD. Establish an orthotopic implanted model of human gastric cancer in nude mice and study the spleen asthenia syndrome in this animal model. Zhongguo Zhongxiyijiehe Xiaohua Zazhi. 2001;9:198-204. |
| 13. | Schmittgen TD, Zakrajsek BA, Mills AG, Gorn V, Singer MJ, Reed MW. Quantitative reverse transcription-polymerase chain reaction to study mRNA decay: comparison of endpoint and real-time methods. Anal Biochem. 2000;285:194-204. |
| 14. | Porter AG, Janicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99-104. |
| 15. | Grutter MG. Caspases: key players in programmed cell death. Curr Opin Struct Biol. 2000;10:649-655. |
| 16. | Greten FR, Weber CK, Greten TF, Schneider G, Wagner M, Adler G, Schmid RM. Stat3 and NF-kappaB activation prevents apoptosis in pancreatic carcinogenesis. Gastroenterology. 2002;123:2052-2063. |
| 17. | Kanai M, Konda Y, Nakajima T, Izumi Y, Kanda N, Nanakin A, Kubohara Y, Chiba T. Differentiation-inducing factor-1 (DIF-1) inhibits STAT3 activity involved in gastric cancer cell proliferation via MEK-ERK-dependent pathway. Oncogene. 2003;22:548-554. |
| 18. | Toyonaga T, Nakano K, Nagano M, Zhao G, Yamaguchi K, Kuroki S, Eguchi T, Chijiiwa K, Tsuneyoshi M, Tanaka M. Blockade of constitutively activated Janus kinase/signal transducer and activator of transcription-3 pathway inhibits growth of human pancreatic cancer. Cancer Lett. 2003;201:107-116. |
| 19. | Kanda N, Seno H, Konda Y, Marusawa H, Kanai M, Nakajima T, Kawashima T, Nanakin A, Sawabu T, Uenoyama Y. STAT3 is constitutively activated and supports cell survival in association with survivin expression in gastric cancer cells. Oncogene. 2004;23:4921-4929. |
| 20. | Gong W, Wang L, Yao JC, Ajani JA, Wei D, Aldape KD, Xie K, Sawaya R, Huang S. Expression of activated signal transducer and activator of transcription 3 predicts expression of vascular endothelial growth factor in and angiogenic phenotype of human gastric cancer. Clin Cancer Res. 2005;11:1386-1393. |
| 21. | Bhattacharya S, Ray RM, Johnson LR. STAT3-mediated transcription of Bcl-2, Mcl-1 and c-IAP2 prevents apoptosis in polyamine-depleted cells. Biochem J. 2005;392:335-344. |
| 22. | Yamamoto H, Tsukahara K, Kanaoka Y, Jinno S, Okayama H. Isolation of a mammalian homologue of a fission yeast differentiation regulator. Mol Cell Biol. 1999;19:3829-3841. |
| 23. | Tu X, Wang CC. Pairwise knockdowns of cdc2-related kinases (CRKs) in Trypanosoma brucei identified the CRKs for G1/S and G2/M transitions and demonstrated distinctive cytokinetic regulations between two developmental stages of the organism. Eukaryot Cell. 2005;4:755-764. |
| 24. | Andoh T, Hirata Y, Kikuchi A. PY motifs of Rod1 are required for binding to Rsp5 and for drug resistance. FEBS Lett. 2002;525:131-134. |
| 25. | Katoh M, Katoh M. Characterization of RUSC1 and RUSC2 genes in silico. Oncol Rep. 2004;12:933-938. |
| 26. | Okazaki N, F-Kikuno R, Ohara R, Inamoto S, Koseki H, Hiraoka S, Saga Y, Seino S, Nishimura M, Kaisho T. Prediction of the coding sequences of mouse homologues of KIAA gene: IV. The complete nucleotide sequences of 500 mouse KIAA-homologous cDNAs identified by screening of terminal sequences of cDNA clones randomly sampled from size-fractionated libraries. DNA Res. 2004;11:205-218. |