INTRODUCTION
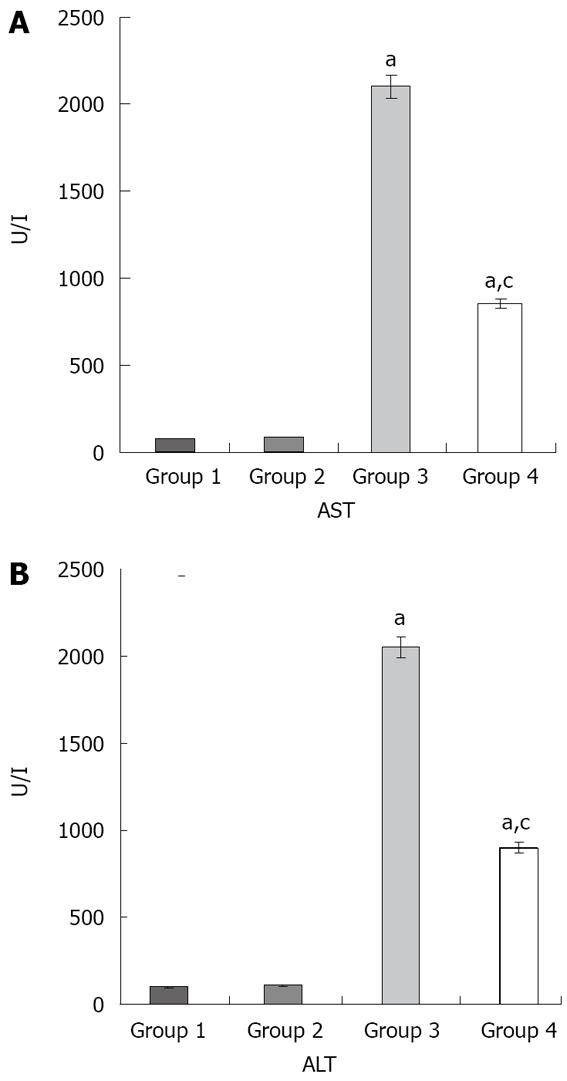
Figure 1 Effects of liver I/R and resvera-trol on liver function.
A: The AST values; B: The ALT values aP < 0.05 compared with Group 1 and 2; cP < 0.05 compared with Group 3. The values are the mean ± SE. ALT: Alanine aminotransferase; AST: Aspartate aminotransferase.
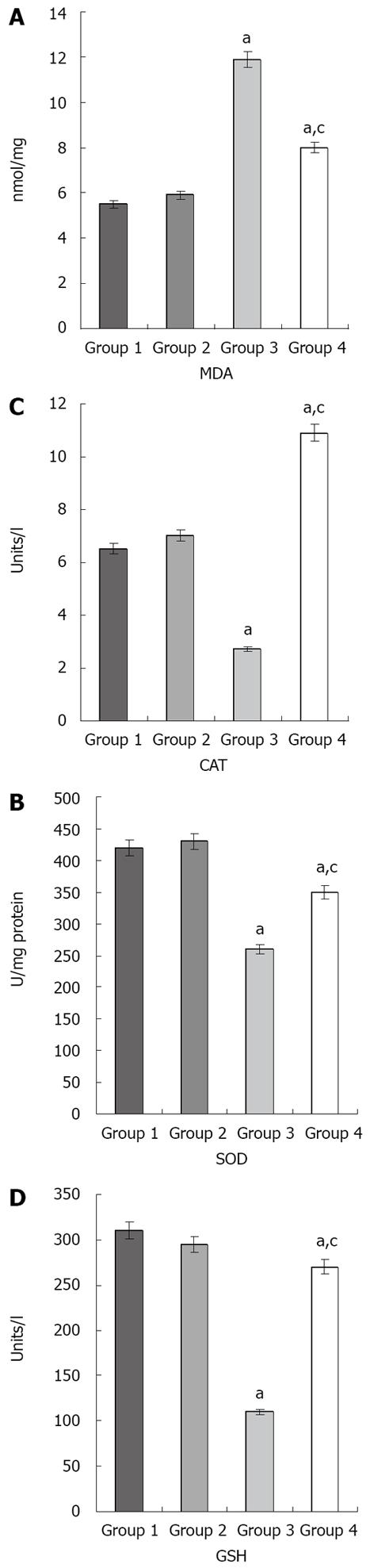
Figure 2 Effects of ischemia/reperfusion and resveratrol on the MDA (A), SOD (B), CAT (C), and GSH (D) levels in liver tissue.
aP < 0.05 compared with groups 1 and 2. cP < 0.05 compared with group 3. The values are the mean ± SE. MDA: Malondialdehyde; SOD: Superoxide dismutase; CAT: Catalase; GSH: Glutathione peroxidase.
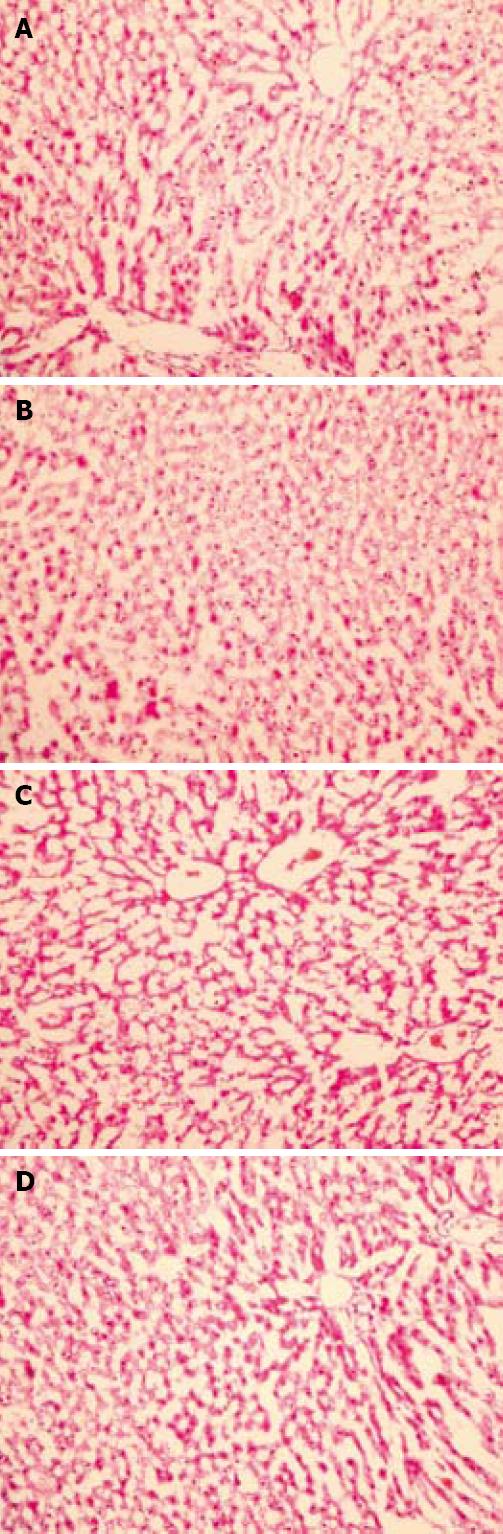
Figure 3 Effect of resveratrol on hepatic tissue injury after I/R evaluated by histological examination.
In groups 1 and 2, normal findings were obtained on histological examination (A and B, respectively) (HE, × 200); C: In group 3, swollen hepatocytes with marked vacuolization and congestion were observed (HE, × 200); D: In group 4, the hepatocytes and sinusoids showed normal morphology, reflecting a well preserved liver parenchyma (HE, × 200).
Hepatic injury caused by ischemia/reperfusion (I/R) has been proposed as a key clinical problem associated with liver transplantation and major liver surgery[1]. Additionally, hepatic I/R injury also occurs in diverse situations, including heart failure, liver trauma, and blood occlusion to the liver[2,3]. The production of reactive oxygen species (ROS), including superoxide, hydrogen peroxide, and hydroxyl radical, has been demonstrated in reperfusion injury[4]. I/R activates Kupffer cells (KC), the resident macrophages in the liver, to generate ROS, proinflammatory cytokines, and chemokines and to upregulate inducible nitric oxide synthase[5].
Resveratrol (3,4,5 tri-hydroxystilbene) is a naturally occurring phytoalexin present in high concentrations in the skin and seeds of grapes[6]. It occurs naturally in a trans-or cis-isoform[7-9]. Resveratrol has been reported to have several biologic effects such as a potent antioxidative effect via prevention of lipid peroxidation[10,11], anti-platelet activity[12], an estrogenic activity[13], and anti-inflammatory activity attributed to cyclooxgenase inhibition[6,14]. Previously, it was suggested that resveratrol stimulated nitric oxide production in endothelial cells, has a vasodilatory effect on blood vessels[15], and improves the energy metabolism system after spinal cord trauma[16].
The aim of this study was to evaluate the possible protective effect of resveratrol against I/R-induced hepatic injury, using biochemical and histological parameters.
MATERIALS AND METHODS
Forty male Sprague-Dawley rats weighing 240-290 g were used in the study. All of the experimental protocols were performed according to the guidelines for the ethical treatment of experimental animals.
Animals and experimental protocol
The rooms where the animals were housed were windowless and were under controlled temperature (22 ± 2°C) and lighting conditions. The rats were housed individually in cages, and allowed free access to standard rat chow and water before and after the experiments. The animals were fasted overnight before the experiments, but were given free access to water. They were anesthetized using 100 mg/kg ketamine and 20 mg/kg xylazine body weight (ip), The right femoral vein was cannulated to administer drugs and saline.
The animals were randomized into four groups (n = 10 in each group): (1) controls: Unmanipulated animals, rats not subjected to any surgical procedure or liver manipulation; (2) sham group: Rats subjected to the surgical procedures described below, except for liver I/R, and administered saline vehicle and maintained under anesthesia for an equivalent duration (i.e. 45 min plus 45 min); (3) I/R group: Rats subjected to the surgical procedures described below and underwent liver ischemia for 45 min followed by reperfusion for 45 min (n = 10); (4) I-R/Resveratrol group: Rats received resveratrol 10 mg/kg dissolved in 2 mL 50% alcohol (Sigma Chemicals, St. Louis, MO, USA) by infusion pump 15 min before liver reperfusion.
Liver ischemia/reperfusion
As described previously[17], the ligament attachments connecting the liver, diaphragm, abdominal wall, and neighboring organs were divided. After the organ was carefully isolated, the liver hilus was exposed to find the common hepatic artery and portal vein. A vascular microclamp was used to interrupt the blood supply to three-quarters of the liver for 45 min, and this was followed by 45 min of reperfusion. Other rats were subjected to a sham operation (sham-operated), which was identical to the surgical procedure used for the I/R group rats without clamping; the rats were kept under anesthesia for the same length of time. At the end of the experiments, the rats were killed with an overdose of sodium pentobarbital.
Measuring serum liver enzymes
The abdominal aorta was punctured and 5 mL of blood was taken and placed in heparinized tubes. Plasma was separated by centrifugation (3000 r/min for 10 min at room temperature) for biochemical studies. The activities of alanine aminotransferase (ALT, a specific marker for hepatic parenchymal injury), and aspartate aminotransferase (AST, a nonspecific marker for hepatic injury) in plasma were determined in units per liter using standard auto-analyzer methods on an Abbott Aeroset (Abbott Laboratories, Abbott Park, IL, USA). Just before the rats were sacrificed, the livers were removed for histopathological evaluation.
Histopathological study
The livers were divided into two pieces. One was immediately placed in 10% formalin solution, left overnight, and then embedded in paraffin blocks. The blocks were cut into 4-μm sections and stained with hematoxylin-eosin, using standard protocols. The severity of hepatic injury in the sections was evaluated using a point-counting method on an ordinal scale as follows: grade 0, minimal or no evidence of injury; grade 1, mild injury consisting of cytoplasmic vacuolation and focal nuclear pyknosis; grade 2, moderate to severe injury with extensive nuclear pyknosis, cytoplasmic hypereosinophilia, and loss of intercellular borders; and grade 3, severe necrosis with disintegration of hepatic cords, hemorrhage, and neutrophil infiltration[18].
Biochemical analyses
The other piece of liver was washed in ice-cold 0.9% saline solution, weighed, and stored at -70°C. Tissue homogenates were prepared as 0.1 g/mL in 250 mmol/L sucrose, 1 mmol/L EDTA, 1 mmol/L dl-dithiothreitol, and 15 mmol/L Tris HCl (pH 7.4), using an all-glass Potter Elvehjem homogenizer (Selecta, Barcelona, Spain). Each homogenate was centrifuged for 20 min at 800 r/min. The resulting supernatant fraction was used to determine enzyme activities. The protein concentrations in the supernatant were determined using the Bradford method[19].
Malondialdehyde determination
Liver malondialdehyde (MDA) levels were determined using the method of Wasowicz et al[20] based on the reaction of MDA with thiobarbituric acid at 95 to 100°C. Fluorescence intensity was measured in the upper n-butanol phase using fluorescence spectrophotometry (F-4010; Hitachi, Tokyo, Japan) adjusted for excitation at 525 nm and emission at 547 nm. The arbitrary values obtained were compared with a series of standard solutions (1,1,3,3-tetramethoxypropane). The results are given in nanomoles per gram of wet tissue.
Superoxide dismutase, catalase, and glutathione peroxidase determination
Superoxide dismutase (SOD) activity was measured using the xanthine-oxidase-cytochrome c method, as described by McCord and Fridovich[21]. The final concentrations in the cuvettes were 50 mmol/L potassium phosphate (pH 7.8), 0.1 mmol/L EDTA, 10 mmol/L cytochrome c, 50 mmol/L xanthine, 50 or 2 mmol/L cyanide, 1 U catalase (CAT), and 0.05-0.1 mg of tissue. The reaction was initiated by adding 1 U xanthine-oxidase. The inhibition of xanthine-oxidase was followed spectrophotometrically at 550 nm. One unit of SOD activity was defined as the amount of enzyme that produced 50% inhibition of the control rate of cytochrome c reduction.
CAT activity was assayed according to the method of Beers and Sizer[22]. The final concentrations in the cuvettes were 500 mmol/L potassium phosphate (pH 7), 100 mmol/L H2O2, and 0.05-0.1 mg of tissue. The decrease in the absorbance at 240 nm after adding the substrate was followed spectrophotometrically.
GSH activity was assayed using a coupled enzyme system in which oxidized glutathione (GSSG) reduction was coupled to NADPH oxidation by glutathione reductase[23]. The assay mixture contained 50 mmol/L potassium phosphate (pH 7.5), 1 mmol/L EDTA, 1 mmol/L NaN3, 1 mmol/L reduced glutathione (GSH), 0.2 mmol/L NADPH, 1 U glutathione reductase, and tissue (0.05-0.2 mg). After a 5-min preincubation period (20-25°C), the reaction was initiated by adding 0.25 mmol/L H2O2. The decrease in absorbance at 340 nm was followed spectrophotometrically.
Protein assays
The protein content of the homogenates was determined using the procedure of Lowry et al[24].
Statistical analysis
Data were entered and analyzed on an IBM-compatible personal computer using SPSS version 9.0. All values were expressed as the mean ± SE. The significance of the data obtained was evaluated using analysis of variance (ANOVA). Differences between means were analyzed using the post-ANOVA test (Tukey’s b). P < 0.05 was considered significant.
RESULTS
The ALT and AST levels were significantly increased in groups 3 and 4 in comparison with groups 1 and 2 (P < 0.05 in all cases). Moreover, the ALT and AST levels were significantly decreased in group 4 compared to group 3 (P < 0.05) (Figure 1).
The MDA, SOD, CAT, and GSH values for the different groups are shown in Figure 2. In group 3, MDA was significantly increased compared with groups 1, 2, and 4 (P < 0.05 in all cases). In addition, SOD, CAT, and GSH significantly decreased in group 3 compared with groups 1, 2, and 4 (P <0.05 in all cases).
The liver histopathological scores were 0.1 ± 0.2, 0.1 ± 0.2, 2.8 ± 0.1, and 1.1 ± 0.2 in groups 1 to 4, respectively. The histopathological scores were higher in groups 3 and 4 than in groups 1 and 2 (P < 0.05 in all cases). Moreover, the histopathological score was lower in group 4 than in group 3 (P < 0.05). Normal findings were obtained on histological examination of rats in groups 1 or 2 (Figure 3A and B). In group 3, focal necrosis with sinusoidal congestion and neutrophil accumulation were observed at the midzonal region (Figure 3C). In contrast, these changes were markedly attenuated in resveratrol-treated livers (Figure 3D).
DISCUSSION
Hepatic ischemia and reperfusion injury is observed following major liver surgery, transplantation, trauma and sepsis and may cause metabolic and structural hepatic damage[25,26]. This remains a significant problem in surgical procedures, and is a limitation of liver transplantation[27]. Several mechanisms have been considered to explain I/R injury of the liver. Activation of KC with enhanced formation of ROS and secretion of inflammatory cytokines and proteolytic enzymes are considered to play an important role in liver reperfusion injury[28,29]. Additionally, migration of activated polymorphonuclear leukocytes at the injury site may prolong the injury as a result of the production of inflammatory cytokines, adhesion molecules, and ROS[30,31]. The formation of ROS may initiate oxidative stress which can lead to lipid peroxidation[32]. Removal of oxidative stress is the primary intervention to decrease tissue injury. Superoxide dismutase, catalase, glutathione, α-tocopherol, and carotene are known endogenous antioxidants, but none of these endogenous antioxidants are sufficient to compensate oxidative stress. Several antioxidants have been studied and reported to decrease oxidative stress in hepatic and renal tissue in experimental obstructive jaundice, septic shock, and I/R models.
Resveratrol is a polyphenol phytoalexin (trans-3,5,4- trihydroxystilbene) that possesses diverse biochemical and physiological actions, including estrogenic, antiplatelet, and anti-inflammatory properties[33,34]. Recently, resveratrol was found to be a highly potent antioxidant which could inhibit free radical generation in brain, spinal cord, kidney, liver and red cell membrane[35-38]. It has been shown that resveratrol inhibits lipid peroxidation[39], and prevents apoptotic cell death induced by oxidative stress[40]. The end production of lipid peroxidation includes aldehydes, hydrocarbon gases, and MDA. MDA is a sensitive index of lipid peroxidation[18,41]. Bertelli et al[33] suggested that MDA formation was reduced in the ischemic-reperfused myocardium of resveratrol-treated rats. In an experimental study by Kirimlioglu et al[42], MDA levels in liver tissue and plasma were higher in group B subjected to 70% partial hepatectomy than those of group A treated with resveratrol before and after 70% partial hepatectomy. Although the exact mechanism is not known, it is thought that this was probably due to the antioxidant and inhibitory effects of resveratrol on ROS, which might be a biochemical mechanism related to the anti-inflammatory and anticarcinogenic properties of resveratrol[43]. To control the detrimental effects of ROS, organisms have developed a variety of antioxidant defense systems, especially the endogenous antioxidant enzymes system including SOD, CAT and GSH. The activities of these enzymes are higher in the liver than in other tissues. CAT is an oxidoreductase enzyme, which transforms H2O2 into H2O and O2. It can protect cells from damage induced by ischemia reperfusion by scavenging ROS[44]. GSH is considered to be the principal mitochondrial antioxidant and its depletion markedly enhances the sensitivity of mitochondrial structures to ROS-mediated injury[45]. A decrease in GSH level after reperfusion could reflect ROS-mediated consumption[46]. SOD catalyses dismutation of the superoxide anion (O2-) into H2O2; H2O2 can be transformed into H2O and O2 by CAT; GSH is a selenoprotein, which reduces lipidic or nonlipidic hydroperoxides as well as H2O2 while oxidizing GSH[47]. Plin et al[48] showed that resveratrol protects mitochondria and cells against cold preservation-warm reperfusion (CPWR)-induced injury. Resveratrol improved both mitochondrial coupling and ATP synthesis measured after CPWR as demonstrated by the increase in respiratory control ratio and ADP/oxygen values, respectively. These protective effects could be related to the antioxidant properties of the molecule which has been shown to be able to scavenge ROS[49].
In our study, liver tissue MDA levels in the I/R group increased compared with normal and sham groups. With resveratrol administration, liver tissue MDA levels decreased. Additionally, our results suggest that increased SOD, GSH and CAT activities may be attributed to reactive oxygen products such as superoxide anions and H2O2 in the resveratrol-treated group. These data indicate that resveratrol may provide protection to the liver during I/R injury, in part by improving activities of the endogenous antioxidant enzymes, which scavenge ROS and reduce their effects. Histopathologically, the resveratrol-treated group showed well preserved liver parenchyma with hepatocytes arranged radially around the central vein. In a study by Hassan-Khabbar et al[50], the effect of trans-resveratrol on liver injury induced by I/R was investigated. While Hassan-Khabbar performed measurements after 3 h of reperfusion, in the present study, measurements were performed after less than 1 h. Thus, the present study suggests that the protective effect of resveratrol may occur more rapidly than previously thought.
In conclusion, this study is the first to evaluate the hepatoprotective effects of resveratrol in hepatic I/R. The protective effects of resveratrol may be associated with its antioxidant activity and free radical scavenging activity which are released during the reperfusion period.
COMMENTS
Background
Hepatic injury caused by ischemia/reperfusion (I/R) has been proposed as a key clinical problem associated with liver transplantation and major liver surgery. Furthermore, hepatic I/R injury also occurs in diverse situations, including heart failure, liver trauma, and blood occlusion to the liver. The production of reactive oxygen species has been demonstrated in reperfusion injury. Resveratrol has been reported to have several biologic effects such as a potent antioxidative effect via prevention of lipid peroxidation. Therefore, the possible protective effects of resveratrol against I/R-induced hepatic injury were investigated.
Research frontiers
This study is the first to evaluate the hepatoprotective effects of resveratrol in hepatic I/R. The protective effects of resveratrol may be associated with its antioxidant activity and free radical scavenging activity which are released during the reperfusion period.
Innovations and breakthroughs
This study explained one of the possible mechanisms of the protective effects of resveratrol.This may be associated with its antioxidant activity and free radical scavenging activity released during the reperfusion period.
Applications
In this study, the implication of ROS in the pathology of hepatic I/R injury was explored. Protective effects of resveratrol were determined. This may represent a novel and attractive approach to determining the protective effects of resveratrol therapy on hepatic injury caused by ischemia/reperfusion
Peer review
In the present study, the authors tested the effect of Resveratrol on ischemia/reperfusion-induced liver injury in rats, and found a protective effect.
Peer reviewers: Dr. Martin Hennenberg, Dipl-Biol, Medizinische Klinik & Poliklinik I, Uni-Klinik Bonn, Sigmund-Freud Str. 25, 53105 Bonn, Germany; Adriana M Torres, Professor of Pharmacology, Suipacha 531, Rosario 2000, Argentina
S- Editor Li LF L- Editor Webster JR E- Editor Ma WH