Published online Dec 7, 2008. doi: 10.3748/wjg.14.6936
Revised: September 9, 2008
Accepted: September 16, 2008
Published online: December 7, 2008
AIM: To investigate the effects and possible mechanisms of Wy14643 on hepatic ischemia-reperfusion (I/R) injury in rats.
METHODS: Thirty male Sprague-Dawley rats weighing 220-280 g were randomly divided into five experimental groups: sham group (G1, n = 6): a sham operation was performed (except for liver I/R); I/R-untreated group (G2, n = 6): rats underwent liver ischemia for 90 min followed by reperfusion for 4 h; and I/R + Wy14643 groups (G3, G4, G5; n = 6): after the same surgical procedure as in group 2, animals were pretreated with Wy14643 at the dose of 1, 5 and 10 mg/kg 1 h before ischemia, respectively. Hepatic ischemia-reperfusion (I/R) was induced by clamping blood supply to the left lateral and median lobes of the liver for 90 min, and atraumatic clamp was removed for 4 h reperfusion. Blood samples and liver tissues were obtained at the end of reperfusion to assess serum and hepatic tissue homogenate aminotransferase (ALT), aspartate aminotransferase (AST), myeloperoxidase (MPO), serum interleukin-1β (IL-1β) and tumor necrosis factor alpha (TNF-α), as well as activity of superoxide dismutase (SOD) and content of malondialdehyde (MDA) in the hepatic tissue homogenate.
RESULTS: Hepatic I/R induced a significant increase in the serum levels of ALT, AST, TNF-α, IL-1β and MPO, as well as the levels of ALT, AST and MDA in the liver tissue homogenate, which were reduced by pretreatment with Wy14643 at the dose of 1, 5 and 10 mg/kg, respectively. The activity of SOD in the liver tissue homogenate was decreased after hepatic I/R, which was enhanced by Wy14643 pretreatment. In addition, serum and liver tissue homogenate ALT and AST in the Wy14643 10 mg/kg group were lower than in the Wy14643 1 mg/kg and 5 mg/kg groups, respectively.
CONCLUSION: Wy14643 pretreatment exerts significant protection against hepatic I/R injury in rats. The protective effects are possibly associated with enhancement of anti-oxidant and inhibition inflammation response.
- Citation: Xu SQ, Li YH, Hu SH, Chen K, Dong LY. Effects of Wy14643 on hepatic ischemia reperfusion injury in rats. World J Gastroenterol 2008; 14(45): 6936-6942
- URL: https://www.wjgnet.com/1007-9327/full/v14/i45/6936.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.6936
Interruption of hepatic inflow is a common procedure during trauma surgery, liver transplantation, and resectional surgery. However, the resulting period of hepatic ischemia and subsequent reperfusion can lead to liver injury and dysfunction through the initiation of a biphasic inflammatory response[1]. An excessive inflammation response is considered as a key mechanism of ischemia-reperfusion injury[2]. The acute phase of this response is characterized by activation of Kupffer cells and their subsequent production and release of reactive oxygen species, which may contribute to liver dysfunction and cell injury during reperfusion[3]. Proinflammatory cytokines, chemokines, and activated complement factors are responsible for neutrophil recruitment and the subsequent neutrophil-induced oxidant stress during the later reperfusion phase[4]. In addition, accumulated neutrophils release oxidants and proteases that directly injure hepatocytes and vascular endothelial cells and may also obstruct hepatic sinusoids resulting in hepatic hypoperfusion[5].
Peroxisome proliferator-activated receptor-α (PPAR-α) is one of the three subtypes of the nuclear receptor PPAR family[6]. Activation of PPAR-α, by either natural ligands, such as polyunsaturated fatty acids and eicosanoids, or synthetic ligands, such as fibrates, Wy14643, stimulates target-gene transcription via the formation of heterodimeric transcription factor complexes with the retinoid X receptor[7]. PPAR-α has a wide range of effects on metabolism, cellular proliferation and the immune response[8]. Beyond metabolic effects, PPARα activation also induces anti-inflammatory and antioxidant effects in different organs. Several studies indicated that Wy14643 protected organs such as heart, kidney and brain against ischemia-reperfusion injury[9-12]. PPAR-α represses the expression of inflammatory-response genes via a mechanism termed ligand-dependent transrepression[13]. Direct binding of PPAR-α to NF-κB p65 was demonstrated by in vitro assays, suggesting that transrepression might be involved in direct interference with transcriptional activation by NF-κB, thus preventing the synthesis and release of cytokines (interleukin-1 and tumor necrosis factor α)[14,15]. Furthermore, PPAR-α activation induces the expression and activation of antioxidant enzymes such as superoxide dismutase(SOD), catalase and glutathione peroxidase[16,17]. PPAR-α is highly expressed in the liver, particularly in hepatic parenchymal cells, which can protect hepatocytes in the mice hepatic ischemia-reperfusion injury model[1]. But the effect of Wy14643 on hepatic ischemia-reperfusion injury was not clear. In the present study, we determined whether PPAR-α activation by the selective agonist Wy14643 may reduce hepatic I/R injury in rats through modulation of oxidative stress and inflammatory response.
Wy14643, selective PPARα agonist, was purchased from Cayman Chemical (USA) and used as 1, 5 and 10 mg/kg homogenized in 20 mg/L ethanol via intraperitoneal (ip) route.
Ethanol was used to form a homogenized drug. Each dose was homogenized in 1ml ethanol and injected via ip. A previous study has found that 20 mg/L ethanol was not harmful to the liver[18].
Male Sprague-Dawley rats (weighing 220-280 g) were used in these experiments. Temperature and relative humidity were kept at 22 ± 2°C and 50% ± 5%, respectively. All rats were obtained from the Center of Experimental Animals in Anhui Medical University. They were allowed free access to a commercial standard chow and water ad libitum before the experimental procedure began. All rats were acclimatized to our animal facility for at least 1 wk before experiment. Stressful stimuli were avoided. This project was approved by the Committee for Research and Animal Ethics of Anhui Medical University.
Rats were randomly divided into five experimental groups each containing six rats: (1) Sham (except for hepatic I/R), (2) Ischemia-reperfusion (I/R), (3) I/R + Wy14643 (1 mg/kg), (4) I/R + Wy14643 (5 mg/kg), and (5) I/R + Wy14643 (10 mg/kg). Partial hepatic ischemia was induced as described previously[1]. Briefly, each rat was weighed and anesthetized by intraperitoneal administration of 1.0 g/kg ethylurethanm. Anesthetized rats were placed onto a thermostatically controlled heating pad, a rectal temperature probe was inserted, and body temperature was monitored and maintained at 37°C. The abdomen was shaved and disinfected with 75% ethanol. A midline incision was performed; the first porta hepatis was exposed. An atraumatic clip was used to prevent blood supply to the left lateral and median lobes of the liver. After 90 min of partial hepatic ischemia, the clip was removed to recover hepatic reperfusion for 4 h. Sham control rats underwent the same protocol without vascular occlusion. Abdominal incision was closed in layers with 4-0 dexon and 2-0 nylon during reperfusion stage in order to prevent the loss of body fluid and quantity of heat.
After 4 h of reperfusion, blood samples were drawn from aorta ventralis. The liver was carefully dissected out from its attachment, and totally excised. All rats were then sacrificed by hemorrhage. Blood samples were centrifuged with 4000 r/min for 15 min at 4°C, following collection of supernatant liquid, and were kept at -20°C for biochemical analyses which were duplicated. Ischemia liver tissue was also divided into two parts, one part was fixed in 10 g/L glutaraldehyde to observe hepatic ultrastructure, the other part was snap frozen in liquid nitrogen. The samples were stored at -80°C until assayed.
Ischemia liver samples fixed in 10 g/L Glutaral, dehydrated, drying and surface gilding according to standard procedures. Electron microscope was used to assess the degree of hepatic damage.
Serum and hepatic tissue homogenate ALT and AST, a marker of hepatocellular injury, were measured using commercial available kit and the result was expressed as U/L as well as U/mg protein, respectively.
Total protein concentrations in liver homogenate samples were determined by the Coomassie blue method.
MPO activity in serum and hepatic tissue was detected by a spectrophotometric method, reflecting the number of polymorphonuclear neutrophils (PMN) in the liver. This method uses 3, 3’, 5, 5’-tetramethyl benzidine (TMB) as an oxidizable dye, and the reaction was started by adding hydrogen peroxide (H2O2) in the medium.
The assay kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Serum TNF-α and IL-1β levels were determined with double antibody sandwich ABC-ELISA using a rat TNF-α and IL-1β kit method according to the manufacturer’s instructions. The samples were compared with the standard curve and expressed as pg/mL. All assay kits were purchased from Shanghai Senxiong Technologyindustry Co. Ltd, China.
SOD activity was measured through the inhibition of nitroblue tetrazolium (NBT) reduction by O2-generated by the xanthine/xanthine oxidase system. One SOD activity unit was defined as the enzyme amount causing 50% inhibition in 1 mL reaction solution per milligram tissue protein and the result was expressed as U/mg protein.
Liver homogenate malondialdehyde (MDA) concentration was measured using the thiobarbituric acid (TBA) method. The amount of lipid peroxides (LPO) was measured as the production of MDA, which in combination with TBA forms a pink chromogen compound whose absorbance at 532 nm was measured. The result was expressed as nmol/mg protein.
All assay kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
All data were expressed as mean ± SD. The statistical significance of differences between groups was analyzed using the one-way analysis of variance (ANOVA) and methods of LSD with the SPSS11.5 for Windows XP statistical software package. The P values less than 0.05 were considered statistically significant.
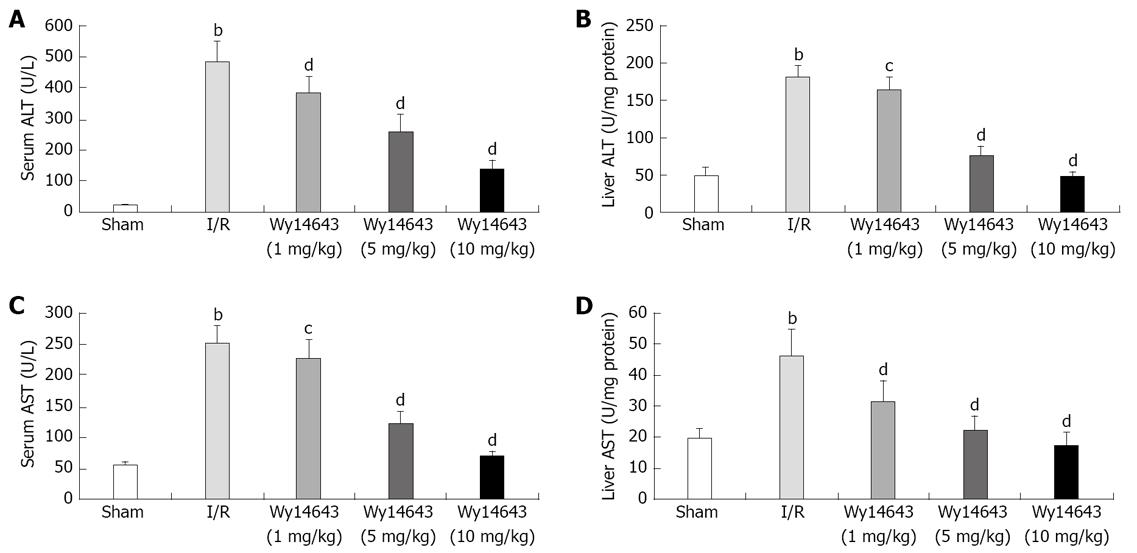
Liver function was examined by measuring serum and liver tissue levels of ALT and AST. Hepatic I/R caused a marked elevation of serum and liver tissue ALT as well as AST activity (serum ALT: 20 ± 4 U/L vs 485 ± 69 U/L, liver tissue homogenate ALT: 50 ± 10 U/mg protein vs 181 ± 16 U/mg protein, P = 0.000, P = 0.000, serum AST: 56 ± 5 U/L vs 252 ± 28 U/L, liver tissue homogenate AST: 20 ± 3 U/mg protein vs 46 ± 8 U/mg protein, P = 0.000, P = 0.000). The increase in serum and liver tissue homogenate ALT as well as AST activity induced by hepatic I/R was significantly attenuated by administration of Wy14643 at dose of 1, 5 and 10 mg/kg (serum ALT: 485 ± 69 U/L vs 386 ± 49 U/L, 259 ± 56 U/L, 139 ± 29 U/L, P = 0.001, P = 0.000, P = 0.000, liver tissue homogenate ALT: 181 ± 16 U/mg protein vs 164 ± 17 U/mg protein, 75 ± 13 U/mg protein, 47 ± 6 U/mg protein, P = 0.031, P = 0.000, P = 0.000, serum AST: 252 ± 28 U/L, 227 ± 30 U/L, 124 ± 19 U/L, 70 ± 8 U/L, P = 0.048, P = 0.000, P = 0.000, liver tissue homogenate AST: 46 ± 8 U/mg protein, 31 ± 7 U/mg protein, 22 ± 5 U/mg protein, 17 ± 4 U/mg protein, P = 0.000, P = 0.000, P = 0.000). The results demonstrated that Wy14643 has the dose-dependent protective effects on liver injury, and that alteration of ALT and AST activity in serum and liver tissue was coincident (Figure 1).
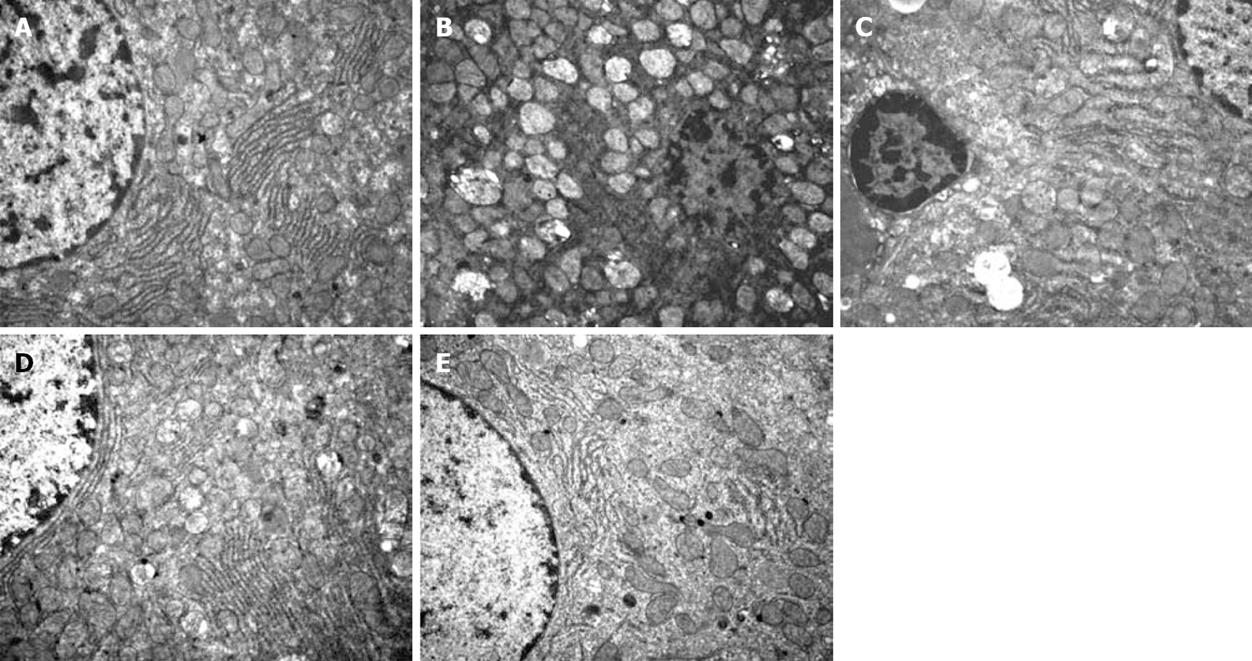
The ultrastructural structure of cells was normal in the sham group. After 90 min of hepatic ischemia followed by reperfusion for 4 h, compared with the sham group, the occluded liver tissue from the I/R group was markedly damaged, with mitochondrion swollen, vacuolar degeneration and, mitochondrial crista destruction, marked decrease of rough endoplasmic reticulum and nucleus structure destruction under the electron microscope. Pretreatment of rats with 1, 5 and 10 mg/kg Wy14643 resulted in a significant amelioration of hepatic injury (Figure 2).
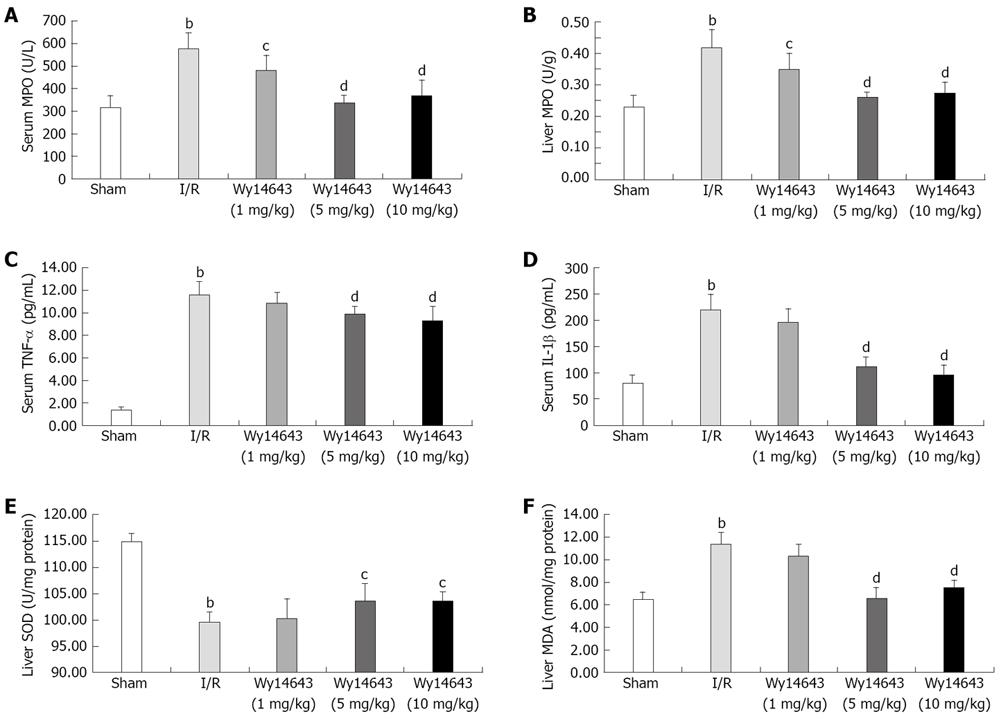
MPO activity in serum and liver tissue was detected, reflecting the number of polymorphonuclear neutrophils (PMN). The serum and liver tissue homogenate MPO activity was significantly increased after hepatic I/R compared with the sham group (serum MPO: 313 ± 55 U/L vs 574 ± 70 U/L, P = 0.000; liver tissue homogenate MPO: 0.23 ± 0.04 U/g vs 0.42 ± 0.06 U/g, P = 0.000). Pretreatment with Wy14643 at dose of 1, 5 and 10 mg/kg led to the marked reduction of MPO content compared with I/R group (serum MPO: 574 ± 70 U/L vs 479 ± 63 U/L, 334 ± 33 U/L, 365 ± 72 U/L, P = 0.012, P = 0.000, P = 0.000; liver tissue homogenate MPO: 0.42 ± 0.06 U/g vs 0.35 ± 0.05 U/g, 0.26 ± 0.02 U/g, 0.27 ± 0.03 U/g, P = 0.010, P = 0.000, P = 0.000). All these showed that Wy14643 reduced infiltration of leukocytes into the inflammatory sites (Figure 3).
The TNF-α and IL-1β content was significantly higher after reperfusion in I/R group than in sham group (TNF-α: 1.37 ± 0.21 pg/mL vs 11.63 ± 1.11 pg/mL, P = 0.000; IL-1β: 78 ± 16 pg/mL vs 220 ± 30 pg/mL, P = 0.000), Pretreatment with Wy14643 1, 5 and 10 mg/kg resulted in the reduction in dependent-dose manner compared with the I/R group (TNF-α: 11.63 ± 1.11 pg/mL vs 10.83 ± 0.94 pg/mL, 9.89 ± 0.60 pg/mL, 9.29 ± 1.20 pg/mL P = 0.132, P = 0.002, P = 0.000; IL-1β: 220 ± 30 pg/mL vs 195 ± 27 pg/mL, 112 ± 17 pg/mL, 95 ± 20 pg/mL, P = 0.070, P = 0.000, P = 0.000) (Figure 3).
The levels of liver SOD lowered significantly after hepatic I/R compared with the sham group (SOD: 114.81 ± 1.13 U/mg protein vs 99.52 ± 1.68 U/mg protein, P = 0.000), after administration of Wy14643 1, 5 and 10 mg/kg, SOD activity in liver was elevated (99.52 ± 1.68 U/mg protein vs 100.09 ± 3.75 U/mg protein, 103.45 ± 3.08 U/mg protein, 103.45 ± 1.73 U/mg protein, P = 0.706, P = 0.014, P = 0.014) (Figure 3).
The MDA content as an index of lipid peroxidation became significantly higher after reperfusion in I/R group than in sham group (MDA: 6.48 ± 0.64 nmol/mg protein vs 11.36 ± 1.10 nmol/mg protein, P = 0.000). Pretreatment with Wy14643 1, 5 and 10 mg/kg caused marked reduction compared with the I/R group (MDA: 11.36 ± 1.10 nmol/mg protein vs 10.37 ± 0.99 nmol/mg protein, 6.59 ± 0.97 nmol/mg protein,7.50 ± 0.66 nmol/mg protein, P = 0.066, P = 0.000, P = 0.000) (Figure 3).
The present study provides noticeable evidence that the selective PPAR-α agonist Wy14643 protects the rat liver from hepatic I/R injury. This protective effect is demonstrated by reducing ALT and AST levels and is associated with an inhibition of oxidative stress and inflammatory response. An increase in serum and liver homogenate ALT and AST levels has been suggested to be an effective indicator of impaired liver parenchymal cells with hepatic I/R. Our results indicate that Wy14643 results in a marked reduction of ALT and AST levels with dose-dependent manner in the rat hepatic tissue homogenate compared with the sham group.
Hepatic ischemia-reperfusion is known to induce formation of reactive oxygen, as well as excessive inflammatory response, which is clearly recognized as a key mechanism of injury during reperfusion[2,19].
Hepatic ischemia activates Kupffer cells, which are the main sources of vascular reactive oxygen formation during the initial reperfusion period[20]. In addition to Kupffer cell-induced oxidant stress, with increasing length of the ischemic episode, components of NADPH oxidase are recruited to the cell surface leading to the priming for enhanced reactive oxygen formation[21]. Furthermore, ROS can activate diverse downstream signaling pathways, such as the transcription factor nuclear factor-κB (NF-κB), thus regulating expression of genes encoding a variety of proinflammatory proteins[22]. SOD, an oxygen radical scavenger which converts superoxide anion radicals present in the upper stream of reactive oxygen metabolism cascade, protects cells against damage. Lipid peroxidation, mediated by free oxygen radicals, is believed to be an important cause of destruction and damage to cell membranes. Membrane peroxidation can lead to changes in membrane fluidity and permeability and also to enhanced rates of protein degradation, eventually resulting in cell lysis[23]. MDA levels have been extensively used as markers of lipid peroxidation and lipid peroxidation damage in tissues, including cells and body fluids in both clinical and experimental studies[24,25]. In our study, we confirm that hepatic I/R caused significant increase of MDA, accompanied by SOD decrease. Administration of Wy14643 ip 1 h before I/R decreased lipid peroxidation in rats subjected to I/R and, at the same time, offered protection against SOD decrease. This finding is in agreement with other reports showing that Wy14643 enhances expression of antioxidant enzymes such as SOD and catalase in the rat liver[26].
TNF-α and IL-1β derived from activated Kupffer cells play an important role in the pathogenesis of hepatic ischemia-reperfusion injury. These cytokines are capable of up-regulating adhesion molecules and causing polymorphonuclear neutrophils to adhere to endothelial cells. This causes microcirculation disturbance, which is thought to be a major mechanism of ischemia-reperfusion injury, including the “no-flow phenomenon”[27]. NF-κB is activated during I/R of the liver, and plays an important and complex role in the gene expression of proinflammatory cytokines (TNF-α and IL-1), which will lead to the tissue injury[28]. PPAR-α agonists may have the anti-inflammatory action, which are thought to be mediated through negative regulation of the transcription factors NF-κB and activator protein-1, resulting in decreased expression of their target genes[29,30]. Cuzzocrea et al[31] found that administration of Wy14643 before the onset of gut ischemia significantly reduced intestinal I/R injury in rats. The improved outcome was accompanied by reductions in neutrophil infiltration, proinflammatory cytokine(TNF-α and IL-1β) expression. Similarly, renal and liver I/R injury was reduced after Wy14643 pretreatment, partially due to its anti-inflammatory effects[1,32]. In mice, PPAR-α ligands attenuate cisplatin-induced ARF by repressing inflammation via inhibition of NF-κB binding activity, which attenuate neutrophil inflation and cytokine release(TNF-α and IL-1β)[33]. It is reported that Wy14643 lowers levels of TNF-α and IL-1β in plasma[34,35]. It is observed that pretreatment with PPAR-α agonists Wy14643 significantly decreased TNF-α and IL-1β in serum. Thus, the results showed that its reduction represents a further mechanism for hepatic protection by PPAR-α agonists.
MPO is an enzyme restricted mainly to polymorphonuclear neutrophils (PMNs), reflecting the number of PMNs in the serum and liver. The evidence indicated that Wy14643 reduced infiltration of the reperfused intestine with polymorphonuclear neutrophils[31]. Our result shows that Wy14643 significantly decreased the serum and liver MPO activity compared with the I/R group. That is to say, Wy14643 reduced infiltration of leukocytes into the inflammatory sites to decrease hepatic I/R injury. In the present study, the significant increase of MPO activity in the hepatic tissue homogenate after hepatic I/R is consistent with a previous study[36].
In summary, our results show that Wy14643 pretreatment protects liver injury induced by hepatic I/R. The protection effects are probably associated with enhancement of antioxidant capacities and inhibition of inflammation responses. However, the precise mechanisms of PPAR-α agonists need to be further investigated in the oxidative stress and inflammatory response implicated in the pathogenesis of hepatic I/R injury.
The peroxisome proliferator-activated receptor-alpha (PPAR-α) is a member of the nuclear receptor family of ligand-dependent transcription factors. Several studies indicated that PPARα protected organs such as heart, kidney and brain against ischemia-reperfusion injury. PPARα activation also induces anti-inflammatory and antioxidant effects. In addition, PPAR-α is highly expressed in the liver, particularly in hepatic parenchymal cells, which can protect hepatocytes in the mouse model of hepatic ischemia-reperfusion injury.
In recent years, more attention has been paid to the effects of PPAR-α on the important organs such as heart, brain, liver and kidney. PPAR-α is a transcription factor that in some in vitro systems has been linked with down-regulation of proinflammatory mediators, thus implicating a potential role for PPAR-α in the regulation of inflammatory processes. Meanwhile, PPAR-α enhances expression of antioxidant enzymes such as SOD and catalase.
Wy14643 was shown to enhance antioxidant capacities and inhibit inflammation responses in some studies. The present study analyzed serum and hepatic tissue homogenate indexes such as ALT, AST, MPO, SOD, MDA,TNF-α and TNF-α. Ultrastructural alterations of liver tissue were also observed. These results all indicated that Wy14643 significantly mitigated hepatic I/R injury in a dose-dependent manner.
This study has indicated that PPAR-α agonist Wy14643 pretreatment protects liver injury induced by hepatic I/R. The protective effects may be associated with enhancement of antioxidant capacities and inhibition of inflammation responses. This may present a novel and attractive approach to prevent hepatic I/R injury.
The research is important because authors demonstrated that Wy14643 pretreatment exerts significant protection against hepatic I/R injury in rats. The protective effects are possibly associated with enhancement of anti-oxidant and inhibition inflammation response.
Peer reviewer: Adriana M Torres, Professor of Pharmacology, Suipacha 531, Rosario 2000, Argentina
S- Editor Tian L L- Editor Ma JY E- Editor Lin YP
| 1. | Okaya T, Lentsch AB. Peroxisome proliferator-activated receptor-alpha regulates postischemic liver injury. Am J Physiol Gastrointest Liver Physiol. 2004;286:G606-G612. |
| 2. | Jaeschke H. Mechanisms of reperfusion injury after warm ischemia of the liver. J Hepatobiliary Pancreat Surg. 1998;5:402-408. |
| 3. | Jaeschke H, Farhood A. Neutrophil and Kupffer cell-induced oxidant stress and ischemia-reperfusion injury in rat liver. Am J Physiol. 1991;260:G355-G362. |
| 4. | Jaeschke H, Bautista AP, Spolarics Z, Spitzer JJ. Superoxide generation by neutrophils and Kupffer cells during in vivo reperfusion after hepatic ischemia in rats. J Leukoc Biol. 1992;52:377-382. |
| 5. | Jaeschke H, Smith CW. Mechanisms of neutrophil-induced parenchymal cell injury. J Leukoc Biol. 1997;61:647-653. |
| 6. | Fruchart JC, Duriez P, Staels B. Peroxisome proliferator-activated receptor-alpha activators regulate genes governing lipoprotein metabolism, vascular inflammation and atherosclerosis. Curr Opin Lipidol. 1999;10:245-257. |
| 7. | Berger J, Moller DE. The mechanisms of action of PPARs. Annu Rev Med. 2002;53:409-435. |
| 8. | Moraes LA, Piqueras L, Bishop-Bailey D. Peroxisome proliferator-activated receptors and inflammation. Pharmacol Ther. 2006;110:371-385. |
| 9. | Tabernero A, Schoonjans K, Jesel L, Carpusca I, Auwerx J, Andriantsitohaina R. Activation of the peroxisome proliferator-activated receptor alpha protects against myocardial ischaemic injury and improves endothelial vasodilatation. BMC Pharmacol. 2002;2:10. |
| 10. | Yue TL, Bao W, Jucker BM, Gu JL, Romanic AM, Brown PJ, Cui J, Thudium DT, Boyce R, Burns-Kurtis CL. Activation of peroxisome proliferator-activated receptor-alpha protects the heart from ischemia/reperfusion injury. Circulation. 2003;108:2393-2399. |
| 11. | Li S, Gokden N, Okusa MD, Bhatt R, Portilla D. Anti-inflammatory effect of fibrate protects from cisplatin-induced ARF. Am J Physiol Renal Physiol. 2005;289:F469-F480. |
| 12. | Collino M, Aragno M, Mastrocola R, Benetti E, Gallicchio M, Dianzani C, Danni O, Thiemermann C, Fantozzi R. Oxidative stress and inflammatory response evoked by transient cerebral ischemia/reperfusion: effects of the PPAR-alpha agonist WY14643. Free Radic Biol Med. 2006;41:579-589. |
| 13. | Kielian T, Drew PD. Effects of peroxisome proliferator-activated receptor-gamma agonists on central nervous system inflammation. J Neurosci Res. 2003;71:315-325. |
| 14. | Delerive P, Martin-Nizard F, Chinetti G, Trottein F, Fruchart JC, Najib J, Duriez P, Staels B. Peroxisome proliferator-activated receptor activators inhibit thrombin-induced endothelin-1 production in human vascular endothelial cells by inhibiting the activator protein-1 signaling pathway. Circ Res. 1999;85:394-402. |
| 15. | Delerive P, De Bosscher K, Besnard S, Vanden Berghe W, Peters JM, Gonzalez FJ, Fruchart JC, Tedgui A, Haegeman G, Staels B. Peroxisome proliferator-activated receptor alpha negatively regulates the vascular inflammatory gene response by negative cross-talk with transcription factors NF-kappaB and AP-1. J Biol Chem. 1999;274:32048-32054. |
| 16. | Marx N, Sukhova GK, Collins T, Libby P, Plutzky J. PPARalpha activators inhibit cytokine-induced vascular cell adhesion molecule-1 expression in human endothelial cells. Circulation. 1999;99:3125-3131. |
| 17. | Blanquart C, Barbier O, Fruchart JC, Staels B, Glineur C. Peroxisome proliferator-activated receptors: regulation of transcriptional activities and roles in inflammation. J Steroid Biochem Mol Biol. 2003;85:267-273. |
| 18. | Daglar GO, Kama NA, Atli M, Yuksek YN, Reis E, Doganay M, Dolapci M, Kologlu M. Effect of 5-lipoxygenase inhibition on Kupffer cell clearance capacity in obstructive jaundiced rats. J Surg Res. 2001;96:158-162. |
| 19. | Jaeschke H. Preservation injury: mechanisms, prevention and consequences. J Hepatol. 1996;25:774-780. |
| 20. | Jaeschke H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am J Physiol Gastrointest Liver Physiol. 2003;284:G15-G26. |
| 21. | El-Benna J, Dang PM, Gougerot-Pocidalo MA, Elbim C. Phagocyte NADPH oxidase: a multicomponent enzyme essential for host defenses. Arch Immunol Ther Exp (Warsz). 2005;53:199-206. |
| 22. | Crack PJ, Taylor JM. Reactive oxygen species and the modulation of stroke. Free Radic Biol Med. 2005;38:1433-1444. |
| 23. | Garcia JJ, Reiter RJ, Guerrero JM, Escames G, Yu BP, Oh CS, Munoz-Hoyos A. Melatonin prevents changes in microsomal membrane fluidity during induced lipid peroxidation. FEBS Lett. 1997;408:297-300. |
| 24. | Trimarchi H, Mongitore MR, Baglioni P, Forrester M, Freixas EA, Schropp M, Pereyra H, Alonso M. N-acetylcysteine reduces malondialdehyde levels in chronic hemodialysis patients--a pilot study. Clin Nephrol. 2003;59:441-446. |
| 25. | Freudenthaler SM, Schreeb KH, Wiese A, Pilz J, Gleiter CH. Influence of controlled hypoxia and radical scavenging agents on erythropoietin and malondialdehyde concentrations in humans. Acta Physiol Scand. 2002;174:231-235. |
| 26. | Toyama T, Nakamura H, Harano Y, Yamauchi N, Morita A, Kirishima T, Minami M, Itoh Y, Okanoue T. PPARalpha ligands activate antioxidant enzymes and suppress hepatic fibrosis in rats. Biochem Biophys Res Commun. 2004;324:697-704. |
| 27. | Andus T, Bauer J, Gerok W. Effects of cytokines on the liver. Hepatology. 1991;13:364-375. |
| 28. | Ali S, Mann DA. Signal transduction via the NF-kappaB pathway: a targeted treatment modality for infection, inflammation and repair. Cell Biochem Funct. 2004;22:67-79. |
| 29. | Reiterer G, Toborek M, Hennig B. Peroxisome proliferator activated receptors alpha and gamma require zinc for their anti-inflammatory properties in porcine vascular endothelial cells. J Nutr. 2004;134:1711-1715. |
| 30. | Staels B, Koenig W, Habib A, Merval R, Lebret M, Torra IP, Delerive P, Fadel A, Chinetti G, Fruchart JC. Activation of human aortic smooth-muscle cells is inhibited by PPARalpha but not by PPARgamma activators. Nature. 1998;393:790-793. |
| 31. | Cuzzocrea S, Di Paola R, Mazzon E, Genovese T, Muia C, Caputi AP. WY 14643, a potent exogenous PPAR-alpha ligand, reduces intestinal injury associated with splanchnic artery occlusion shock. Shock. 2004;22:340-346. |
| 32. | Sivarajah A, Chatterjee PK, Hattori Y, Brown PA, Stewart KN, Todorovic Z, Mota-Filipe H, Thiemermann C. Agonists of peroxisome-proliferator activated receptor-alpha (clofibrate and WY14643) reduce renal ischemia/reperfusion injury in the rat. Med Sci Monit. 2002;8:BR532-BR539. |
| 33. | Li S, Basnakian A, Bhatt R, Megyesi J, Gokden N, Shah SV, Portilla D. PPAR-alpha ligand ameliorates acute renal failure by reducing cisplatin-induced increased expression of renal endonuclease G. Am J Physiol Renal Physiol. 2004;287:F990-F998. |
| 34. | Muia C, Mazzon E, Crisafulli C, Di Paola R, Genovese T, Caputi AP, Cuzzocrea S. ROLE of endogenous peroxisome proliferator-activated receptor-alpha (PPAR-alpha) ligands in the development of gut ischemia and reperfusion in mice. Shock. 2006;25:17-22. |
| 35. | Genovese T, Mazzon E, Di Paola R, Muia C, Crisafulli C, Caputi AP, Cuzzocrea S. Role of endogenous and exogenous ligands for the peroxisome proliferator-activated receptor-alpha in the development of bleomycin-induce lung injury. Shock. 2005;24:547-555. |
| 36. | Yuan GJ, Ma JC, Gong ZJ, Sun XM, Zheng SH, Li X. Modulation of liver oxidant-antioxidant system by ischemic preconditioning during ischemia/reperfusion injury in rats. World J Gastroenterol. 2005;11:1825-1828. |