INTRODUCTION
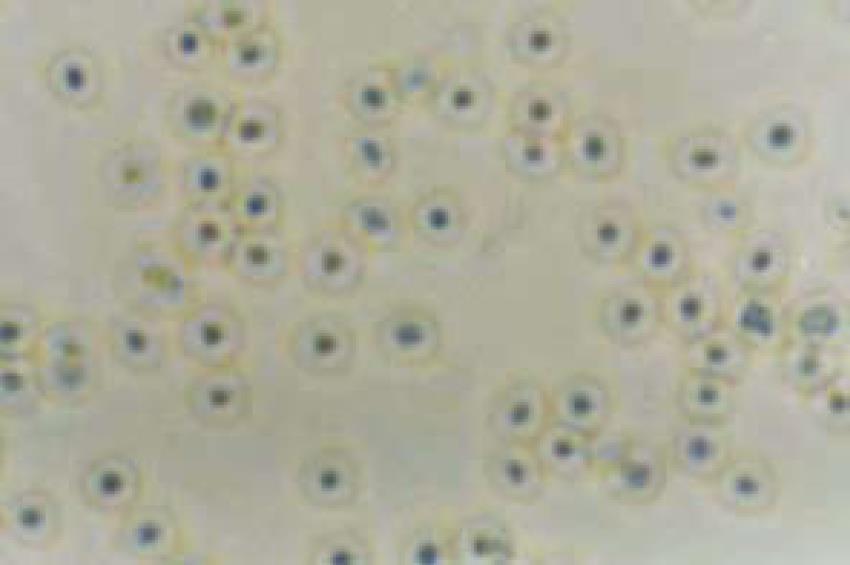
Figure 1 Culture of normal human gallbladder epithelial cells (HGBEC) (× 400).
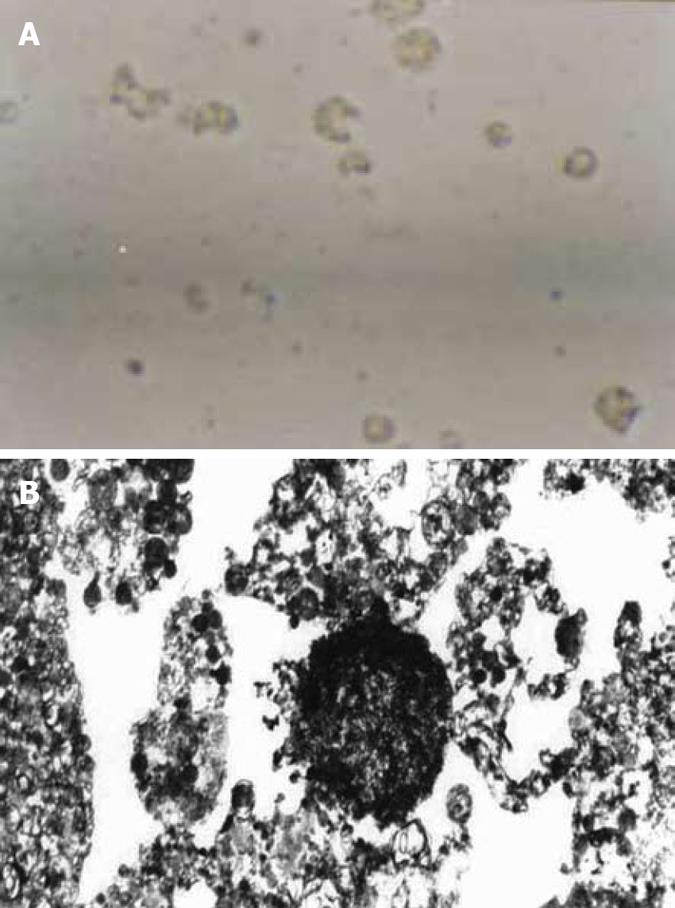
Figure 2 Four d after addition of enriched H pylori.
A: dead HGBEC (× 400); B: HGBEC characterized by karyopyknosis, vacuoles and incomplete cell structure (× 6000).
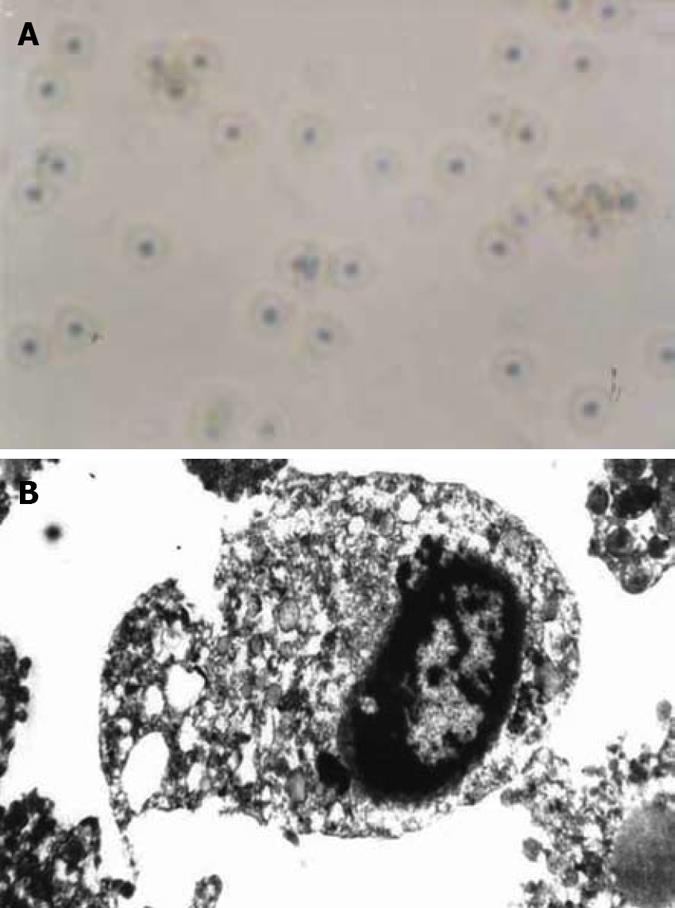
Figure 3 Four days after addition of H pylori sonicated extracts.
A: Most HGBEC were normal, however, a few HGBEC died (× 400); B: Basically normal HGBEC and some cellular plasm under the vacuole (× 6000).
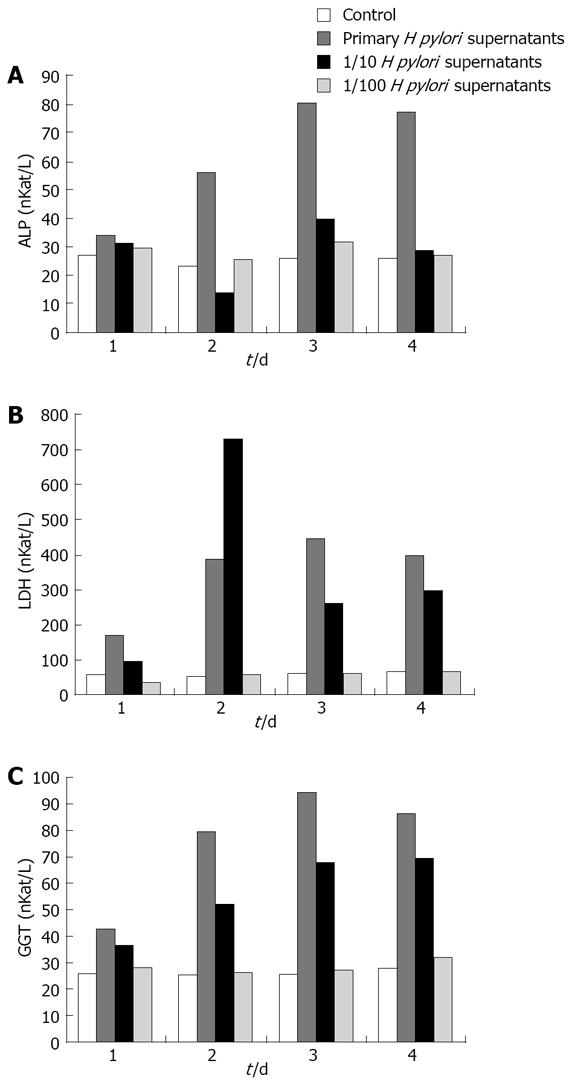
Figure 4 Effect of different concentrations of H pylori supernatants on ALP, LDH and GGT in the supernatants of HGBEC culture.
A: ALP; B: LDH; C: GGT.
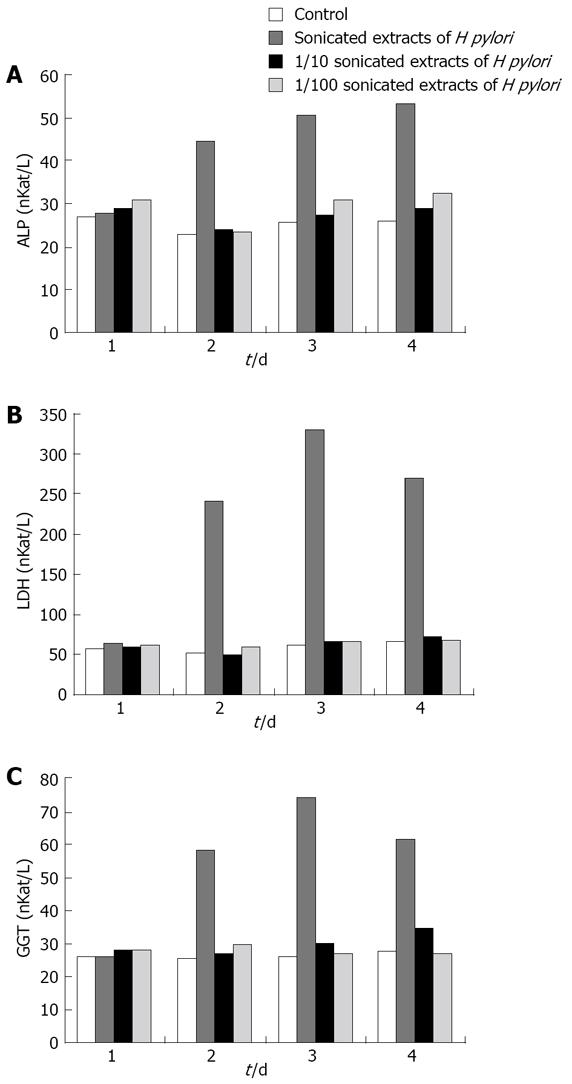
Figure 5 Effect of different concentrations of sonicated extracts of H pylori on HGBEC supernatant ALP, LDH and GGT.
A: ALP; B: LDH; C: GGT.
Cholecystitis can be caused by many factors[1-6], of which Helicobacter pylori (H pylori) has been reported to cause gallbladder mucosa injury in the colonial region and inflammatory reaction[7-9]. In this study, in order to ascertain if the damage to gallbladder mucosa relates to H pylori, we explored the damaging effect of H pylori on human gallbladder epithelial cells (HGBEC) using an enriched liquid and a primary culture of HGBEC to show that H pylori is an important clinical etiological factor leading to cholecystitis.
MATERIALS AND METHODS
Materials
H pylori colonies extracted from the gallbladder were selected and transferred to a modified Brucella broth liquid medium, with a density of up to 107/L, and placed into a triangular filter which was then sealed with a vacuum lipid and connected to a rubber tube for air exchange. The rubber tube was clipped with hemostatic forceps so that the oxygen inside the filter was maintained at 50 mL/L. The rubber tube was then immobilized in a type THI-82 homeothermia oscillator (Shanghai Yuejin Medical Instrument Corporation) at 37°C, l50 r/min, and observed with regard to bacterial opacity at 24, 48 and 72 h. The extracted bacteria were used to determine the A660 value using a 721 spectrophotometer (1.0 A660 was approximately equal to l × 1011/L) and the density of H pylori calculated. The bacterial status was observed via smeared Gram staining. The optimal growth period of H pylori was determined by the density and morphology of the bacteria. Thereafter, the liquid culture was terminated to allow the bacteria to adjust to 1011/L for centrifugation, after which supernatants and precipitates were collected and frozen at -30°C until use. H pylori grew well in the liquid culture medium and reached a live bacterial density of 1012-1013/L after shaking for 24-48 h, when the bacterial morphology was typical, but was atypical with less live bacteria and many spheroids after 72 h. Therefore, H pylori cultivated for 48 h was considered suitable for this study. HGBEC during their optimal growth period (6 d after primary culture) taken from the HGBEC growth curve was considered suitable for this study[10] (Figure 1). HGBEC cultured and supplemented with Brucella broth were used as controls.
Methods
An enriched liquid containing 1012/L of H pylori was diluted to 1011/L of H pylori using ultrapure water. The collected supernatants, following centrifugation, were diluted to a final density of 1/10 and 1/100 using ultrapure water, filtered using a 0.22 μm filter coat and frozen at -70°C until use. The supernatants and ultrapure water were used to dilute the bacteria to a density of 1011/L, where an ultrasound cell breaker, with an output of 80 W for l min/time was employed, until Gram staining showed that all H pylori were broken. H pylori were diluted to 1/10 and 1/100, filtered using a 0.22 μm filter coat and frozen at -70°C until use. At the bacterial logarithmic growth period, different concentrations of supernatants from enriched H pylori, H pylori break liquid as well as Brucella broth, each 150 μL, were added and the morphological and anchoring changes in the cells were observed on days 1, 2, 3 and 4. Supernatants of the cell culture were kept at -30°C, and the cells were collected at the most typical stage and immobilized with 30 g/L glutaral for electron microscopic observation. Finally, assays to determine the levels of alkaline phosphatase (ALP), lactate dehydrogenase (LDH) and glutamyltransferase (GGT) in the cell culture supernatants were performed. The results were expressed as nKat/L.
Statistical analysis
SSAP 10.0 software was used for statistical analyses. Data were processed using the Student’s t test and expressed as mean ± SD. P < 0.05 was considered statistically significant.
RESULTS
Morphological changes in HGBEC
From the second day after addition of H pylori supernatants, electron microscopy showed that cell division decreased and the anchored HGBEC loosened leading to dissociation. On day 3, the cell number significantly decreased, with rarefaction of the cytoplasm, reduced cell division, significant vacuoles, slow motion and deepened nuclei chromatin. On day 4, the cells ruptured into fragments and shrank, showing karyopyknosis, light color and different sizes, loss of adhesiveness and increased cell death (Figure 2A). The addition of diluted supernatants of H pylori to HGBEC alleviated the cell damage and prolonged survival time and anchoring time compared with HGBEC with added primary H pylori. The addition of 1/100 H pylori supernatants did not significantly change cell morphology, suggesting that the addition of 1/100 H pylori supernatants induced no obvious damage to HGBEC. The cell changes in the controls (Brucella broth added) were in accordance with the growth of HGBEC receiving no other substance, i.e. at day 7, cell vitality was reduced, cell number decreased, and on day 14, all cultured HGBEC died. Electron microscopy showed the following characteristics in HGBEC supplemented with primary H pylori supernatants for 3 and 4 d. Cells were scattered and reduced in size, with rarefaction and swelling of the cytoplasm, mitochondrion swelling, myelin sheath changes and obvious vacuoles as well as karyopyknosis, assembled chromatin scattered to blocks, incomplete structure of cell nuclei and nuclear membrane in some regions, and even a dense body formed by the whole cell nuclei and hence the cell nuclei disappeared, leading to broken cells (Figure 2B). It was shown that H pylori supernatants had a significant damaging effect on HGBEC in a time- and dose-dependent manner, resulting in obvious vacuoles.
From the second day after the addition of sonicated extracts of H pylori, electron microscopy showed that the primary HGBEC had decreased cell division and had insignificant morphological changes. At day 3, the anchored cells were dissociated, with slow motion of free cells, morphological changes, decreased numbers, increased vacuoles, loss of HGBEC adhesiveness, cell malformation, karyopyknosis and even cell rupture or death, which was less than HGBEC exposed to H pylori supernatants with regard of injury severity. Sonicated extracts of H pylori at 1/10 exerted a slight damaging effect on HGBEC, but at 1/100 showed an insignificant damaging effect (Figure 3A). Under the electron microscope, the cells had irregular morphology and were reduced in size, with cytoplasm rarefaction, vacuoles, dilatation of endoplasmic reticulum and mitochondria, reduced nuclei, aggregation of chromatin and even cell rupture and death, which resulted in a slightly more overall damage compared with H pylori supernatants (Figure 3B).
Enzymological changes in HGBEC culture supernatants
In comparison with the controls, the three enzymological indices were significantly higher following the addition of different concentrations of enriched liquid into the HGBEC culture supernatants (P < 0.01) and still higher on days 2 and 3 following the addition of 1/10 enriched H pylori (P < 0.01). However, only the values for LDH and GGT were significantly higher on day 4 than those for the controls (P < 0.01). There were no significant differences with respect to the enzymological indices in the supernatants after the addition of 1/100 enriched H pylori, compared with the controls (P > 0.05). With the addition of the stock solution of enriched H pylori and 1/10 diluted fluid, the enzymological indices in the supernatants gradually increased with time (P < 0.01) with exception on days 3 and 4 (Figure 4).
ALP, LDH and GGT in the supernatants of the HGBEC culture significantly increased 2, 3 and 4 d after the addition of sonicated extracts of H pylori, compared with the controls (P < 0.01). The addition of 1/10 and 1/100 sonicated extracts of H pylori produced an insignificant effect on the enzymological indices in the supernatants (P > 0.05). From the second day after the addition of the stock solution of H pylori, the levels of ALP, LDH and GGT increased each day (P < 0.01) with the exception on days 3 and 4 (P > 0.05) (Figure 5).
The above-mentioned results showed that the supernatants of enriched H pylori significantly affected the enzymological indices of cultured HGBEC supernatants. The effect of H pylori on cell damage and permeability weakened with the gradual decrease in concentration of H pylori supernatants. The sonicated extracts of H pylori had similar effects, but were far weaker than those of the enriched H pylori supernatants.
DISCUSSION
The addition of enriched H pylori supernatants resulted in a rapid decrease in cell division, motion and adhesiveness of HGBEC, with obvious vacuoles, cell rupture and necrosis, and even cell death. The sonicated extracts of H pylori had similar effects, but were weaker. The reasons for the above changes may be that H pylori poisonous substances which permeate the liquid medium so that the supernatants of enriched H pylori contain many poisonous substances or metabolites when H pylori are under continual oscillation. When the supernatants enter the cultured HGBEC, the poisonous substances exert effects on the HGBEC which damage the cell membrane, increase permeability, induce rarefaction of cytoplasm, vacuoles, dilatation of mitochondria and endoplasmic reticulum, nuclei membrane and nuclei, leading to a decrease in cell division, disordered metabolism and even cell disruption and death[11-12]. The rarefaction of cytoplasm and obvious vacuoles seen under the optic microscope and electron microscope are due to the actions of H pylori and other cells such as HeLa cells and hepatocytes, which contain vacuolating cytotoxin A (VacA)[13-14]. After VacA disrupts the function of the ion transport protein, which in turn affects the motion of the ion transport protein in HGBEC. The new vacuoles mainly consist of cell endoplasmic reticulum fused with lysosome. The formation of vacuoles in cells is a result of self-phagocytosis. Another H pylori toxin, cytotoxin-associated protein (CagA) has a high homogeneity with sodium passage and can affect the sodium pump of HGBEC which results in cell swelling, disruption and even death. We have hypothesized that the damaging effect of H pylori, isolated from gallbladder, on HEBEC and epithelial cells of gastric mucosa, is similar with respect to the method of action, i.e. by way of enzymes, toxins like VacA and CagA, urease, lipase, phospholipase A, hemolysin and lipopolysaccharide of H pylori[15-19]. In addition, H pylori may damage the epithelial cells of gallbladder mucosa and eventually result in cholecystitis by activating inflammatory cells and secreting inflammatory medium as well as by cellular immunity, humoral immunity and autoimmunity[20-22]. The toxic factors in H pylori can activate factors inhibiting cell proliferation, promote the expression of regulatory genes like bcl-2, bax and fas and finally induce cell apoptosis[11], which is consistent with the apoptosis we observed in HGBEC. The reason why the sonicated extracts of H pylori had a weaker damaging effect on HGBEC than the enriched H pylori, is probably because the main toxic factor within the sonicated extracts (lipopolysaccharide) is not a major toxic factor compared to VacA and CagA[23].
Determination of the ALP, LDH and GGT levels in HEBEC culture supernatants showed the functional status of HGBEC and the integrity of cell membranous structures, as gallbladder epithelial cells containing ALP, LDH and GGT. Our study has clearly demonstrated that the addition of sonicated extracts or supernatants of H pylori to cultured HGBEC can, to a certain degree, raise the activity of these three enzymes in cell culture supernatants. With the decrease in cell vitality and enhanced disruption, the increased level of the three enzymes in cell culture supernatants was not due to cell function enhancement and an increase in enzyme synthesis, but to cell damage which resulted in the destruction of membranous structures and enzyme leaking into the supernatants, suggesting that the supernatants and sonicated extracts of H pylori can damage cell membranous structures, cause cell disruption and raise the levels of ALP, LDH and GGT in cell culture supernatants. The assayed enzyme levels in cell culture supernatants increased with time, with the exception on days 3 and 4, which reflected the increased enzyme leakage from the cells with aggravated HGBEC injury. Following cell disruption and death, the enzymes in the cells leaked and the content showed no increase in the cell culture supernatants, suggesting that H pylori can damage gallbladder epithelial cells and may be one of the factors leading to clinical cholecystitis.