INTRODUCTION
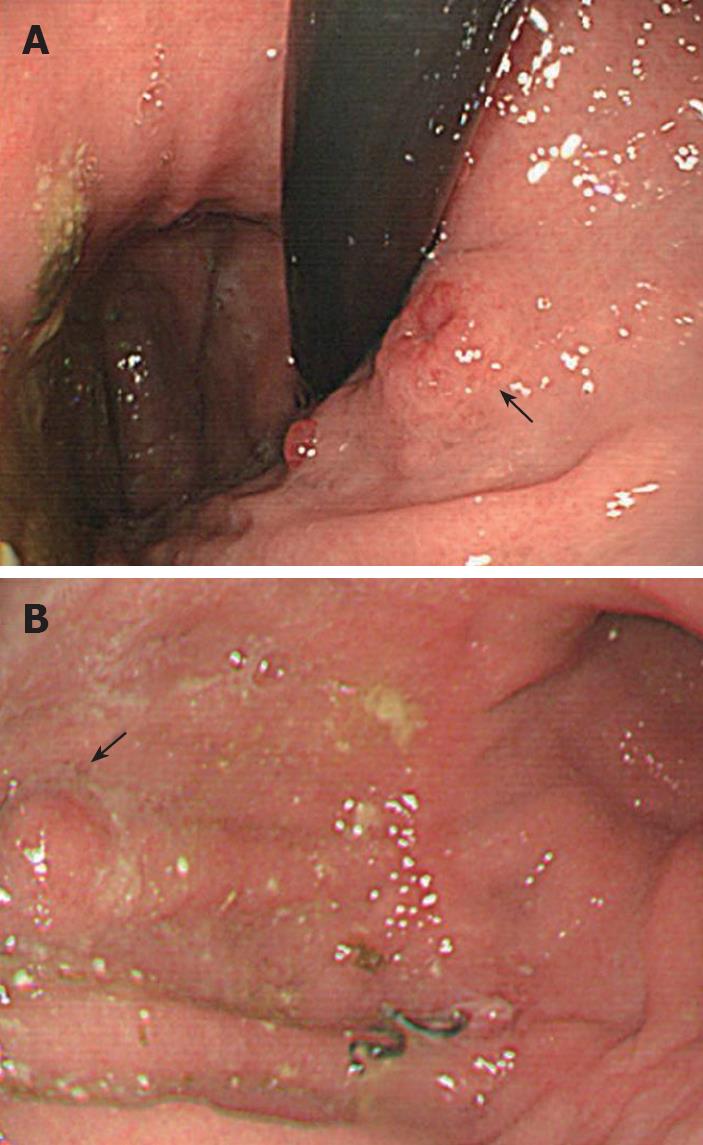
Figure 1 Gastroscopic findings.
A: 1.2 cm ulcerated polyp in the cardia (arrow); B: 0.6 cm polyp on the anterior wall of the corpus near the previous anastomosis (arrow).
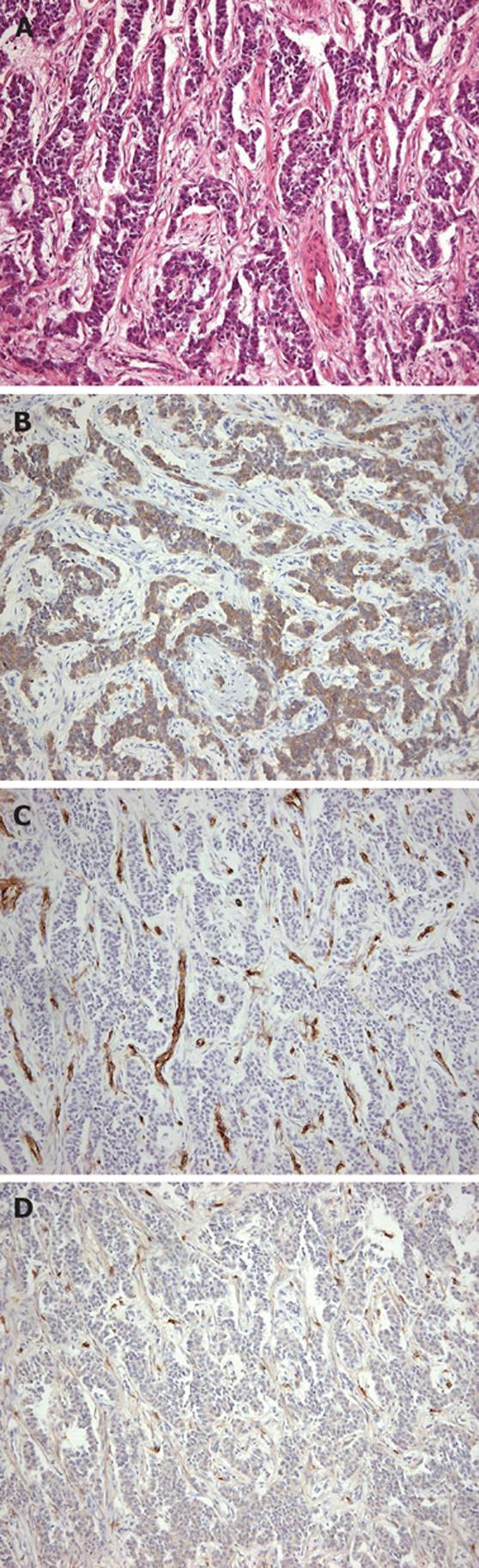
Figure 2 Carcinoid tumor.
A: Uniform round-to-oval cells in trabeculae, nests, and gland-like structures (hematoxylin and eosin, × 100); B: Positive immunohistochemical staining for synaptophysin (× 100); C: Negative immunohistochemical staining for CD 34 (× 100); D: Negative immunohistochemical staining for CD 117 (× 100).
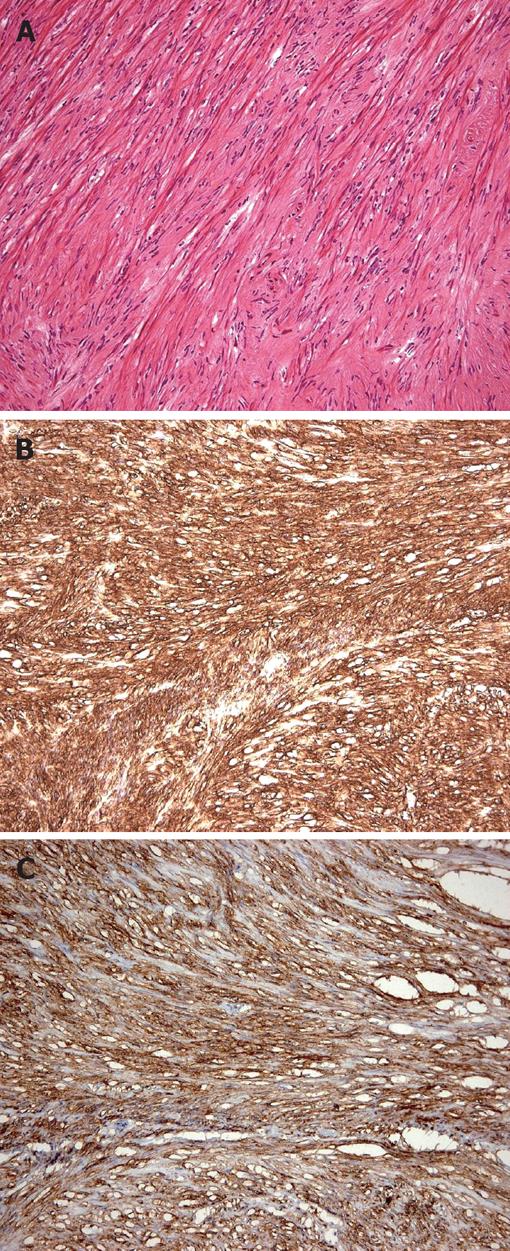
Figure 3 Recurrent gastrointestinal stromal tumor.
A: Composed of spindle cells (hematoxylin and eosin × 100); B: Positive immunohistochemical staining for CD34 (× 100); C: Positive immunohistochemical staining for CD 117 (× 100).
Gastrointestinal stromal tumors (GIST) are the most frequent mesenchymal tumors in the gastrointestinal tract. They may coexist with other types of cancers with a reported frequency from 4.5% to 33%[1,2]. The most common location of a GIST associated with other cancers is the stomach[1], and the most frequently associated tumor type is gastrointestinal adenocarcinoma[1,2]. Infrequently, other primary cancers have been reported along with GIST, including lymphoma, leukemia, breast cancer, prostate cancer, pancreatic adenocarcinoma, or lung cancer[1-4]. GIST has been reported to occur simultaneously with ileal carcinoid tumor[5]. We report a patient with both GIST and carcinoid tumor in the stomach, an association that has not been previously reported.
CASE REPORT
A 65-year-old woman had undergone subtotal segmental gastrectomy for a 5.5 cm GIST in the lower gastric corpus. The diagnosis was based on immunohistochemical staining that was diffusely positive for CD-117 and focally positive for CD-34 and smooth muscle actin; it was negative for desmin. The patient received periodic follow-up gastroscopy. One year after surgery, she was found to have two distinct sessile polypoid lesions in the cardia and lower body of the stomach (Figure 1). The lesion in the cardia, about 2 cm distal to the gastroesophageal junction was a 1.2 cm polyp with a central ulceration. The other lesion was a 0.6 cm mass on the anterior wall near the earlier anastomosis. Immunohistochemical staining of a biopsy specimen of the larger polyp was strongly positive for chromogranin-A, weakly positive for synaptophysin, and negative for CD56, neuron-specific enolase, and cytokeratin 7. Gastric carcinoid was therefore diagnosed. There was inflammation, but no Helicobacter pylori (H pylori) was found. The normal gastric body glands were atrophic and replaced by pyloric and intestinal glands. Abdominal ultrasonography, computerized tomography, barium small bowel series, and colonoscopy were performed to exclude other possible gastrointestinal tumors; there were no other abnormalities found. The patient denied any symptoms of diarrhea, palpitation, facial flushing, or weight loss, and there was no family history suggestive of similar disorders or multiple endocrine syndromes. Her abdominal examination was normal except for the previous surgical scar. The fasting serum gastrin level was 1920 pg/mL (normal 25-125 pg/mL). The 24-h urinary 5-HIAA level was normal. These findings were consistent with a type 1 hypergastrinemic gastric carcinoid tumor. Total gastrectomy was performed because the patient was concerned about further recurrences of gastric tumors. The pathologist reported two carcinoid tumors in the cardia and lower corpus near the previous anastomosis. There was no immunohistochemical evidence of GIST in the two carcinoid tumors (Figure 2). One dissected lymph node had metastatic carcinoid tumor. A 0.6 cm focal GIST was also found on the lesser curvature; and there was no carcinoid component on immunohistochemical staining (Figure 3). The pathologic slides from the first surgery were reviewed and immunohistochemical staining repeated, which showed no evidence of carcinoid tumor. Three weeks after the total gastrectomy, the patient’s fasting gastrin level had returned to normal (72.40 pg/mL). She recovered uneventfully and has remained well for six months after surgery.
DISCUSSION
This patient had metachronous gastric GIST and gastric carcinoid tumors, a previously unreported phenomenon. The tumors were confirmed by pathologic examination, including immunohistochemical studies, to be totally distinct neoplasms.
Gastrointestinal carcinoids comprised 73% of all carcinoid tumors in a large series, the most common sites being in the small bowel, appendix and rectum[6]. Gastric carcinoids are less common, reported as being only 8.7% of enteric carcinoids. Among all gastric cancers, carcinoids are quite rare, with a reported incidence of only 0.35%-1.77%[7]. Gastric carcinoid tumors may present with anemia caused by bleeding from an ulcerative mass, abdominal pain, or with a classical carcinoid syndrome. Histologically, the tumors have uniformly round cells growing in rosettes, cords, or trabecular patterns. Immunohistochemical stains are usually positive for chromogranin A or C and synaptophysin but negative for CD117. The latter marker distinguishes carcinoid from GIST, which is positive for CD117.
Gastric carcinoid tumors can be classified into four types: type 1, with enterochromaffin-like cells, chronic atrophic gastritis, achlorhydria, hypergastrinemia, and often pernicious anemia; type 2, with enterochromaffin-like cells, Zollinger-Ellison syndrome, multiple endocrine neoplasia type 1, and hypergastrinemia; type 3, with enterochromaffin-like cells but which is gastrin-independent and occurs sporadically; and type 4, with miscellaneous non-enterochromaffin-like endocrine cells[8-10]. The prognosis of types 1 and 2 gastric is better than that of types 3 or 4 gastric carcinoid tumors. The 5-year and 10-year survival rates were 96.1% and 73.9% for type 1 disease, compared with only 33.3% and 22.2% for type 4 gastric carcinoid tumors[9]. For type 1 gastric carcinoids, limited surgery, such as endoscopic mucosal resection[11] or partial gastrectomy is adequate, along with appropriate treatment for the hypergastrinemia[9,10,12]. Radical resection is recommended for types 3 and 4[9,10]. Our patient had type 1 gastric carcinoid with hypergastrinemia but no associated symptoms, such as Zollinger-Ellison syndrome or multiple endocrine neoplasia type 1. If she had had only the type 1 gastric carcinoid, more conservative surgery might have been considered. The history of the previous GIST, however, influenced the choice of total gastrectomy.
In a retrospective review of 200 patients with gastrointestinal GIST (with 78% or 39% in the stomach), DeMatteo et al[13] reported an overall 5-year survival rate of about 35%, but it depended on the extent of resection. For patients undergoing complete resection, the 5-year survival was 54%, with those patients surviving a median of 66 mo compared with 22 mo for those who had incomplete resection. The recurrence rate was 40% (median 24 mo’ follow-up). The median disease-specific survival for pateints with local recurrence was 12 mo. Metastases usually occurred with tumors larger than 5 cm or with a mitotic index of more than 10 mitoses per 50 high power fields. The authors recommended complete surgical resection for GIST if possible.
In estimating our patient’s prognosis, it is difficult to know if it should be predicted based on the locally recurrent GIST or the carcinoid metastatic to a lymph node. Carcinoid metastases to regional lymph nodes cannot be reliably predicted by tumor size or depth of invasion, their impact on survival is uncertain[14], and there is no recommended adjuvant treatment. However, type 1 carcinoid is fairly indolent and is less likely than the GIST to limit our patient’s survival. We intend to monitor the patient at 6-12 mo intervals with computed tomography for GIST and using plasma chromogranin measurements and octreoscan for carcinoid. Adjuvant therapy with imatinib is an option for treating residual GIST, with evaluation of c-Kit and PDGFRA mutations a useful tool to predict efficacy[15,16]. There was also a clinical trial conducted by Yao et al[17], testing the imatinib in 27 carcinoid patients with a modest clinical response of median overall survival of 36 mo. However, our patient’s recurrent GIST was totally removed by gastrectomy, and the mitotic index was low. There would be no way of assessing treatment response and therefore no clear benefit, so imatinib is not indicated in this patient unless the GIST is found to recur on follow-up.
In conclusion, our patient had an apparently unique combination of two unusual gastric tumors. Accurate diagnosis of her GIST and carcinoid tumors, based on immunohistochemical studies, was important both for deciding on treatment and follow-up and in estimating prognosis. However, as this case clearly illustrates, a number of individual patient variables affect those decisions and estimates. Inevitably we must rely on clinical judgment which, while informed by what the evidence says about the diseases in general, must also take into account a patient’s particular characteristics, needs, and preferences.