Published online Nov 28, 2008. doi: 10.3748/wjg.14.6824
Revised: November 11, 2008
Accepted: November 18, 2008
Published online: November 28, 2008
AIM: To evaluate the effect of β-blockade on angiotensins in the splanchnic and peripheral circulation of cirrhotic patients and also to compare hemodynamic parameters during liver transplantation according to propranolol pre-treatment or not.
METHODS: Patients were allocated into two groups: outpatients with advanced liver disease(LD) and during liver transplantation(LT). Both groups were subdivided according to treatment with propranolol or not. Plasma was collected through peripheral venipuncture to determine plasma renin activity(PRA), Angiotensin(Ang) I, Ang II, and Ang-(1-7) levels by radioimmunoassay in LD group. During liver transplantation, hemodynamic parameters were determined and blood samples were obtained from the portal vein to measure renin angiotensin system(RAS) components.
RESULTS: PRA, Ang I, Ang II and Ang-(1-7) were significantly lower in the portal vein and periphery in all subgroups treated with propranolol as compared to non-treated. The relationships between Ang-(1-7) and Ang I levels and between Ang II and Ang I were significantly increased in LD group receiving propranolol. The ratio between Ang-(1-7) and Ang II remained unchanged in splanchnic and peripheral circulation in patients under β-blockade, whereas the relationship between Ang II and Ang I was significantly increased in splanchnic circulation of LT patients treated with propranolol. During liver transplantation, cardiac output and index as well systemic vascular resistance and index were reduced in propranolol-treated subgroup.
CONCLUSION: In LD group, propranolol treatment reduced RAS mediators, but did not change the ratio between Ang-(1-7) and Ang II in splanchnic and peripheral circulation. Furthermore, the modification of hemodynamic parameters in propranolol treated patients was not associated with changes in the angiotensin ratio.
- Citation: Vilas-Boas WW, Jr ARO, Ribeiro RDC, Vieira RLP, Almeida J, Nadu AP, Silva ACSE, Santos RAS. Effect of propranolol on the splanchnic and peripheral renin angiotensin system in cirrhotic patients. World J Gastroenterol 2008; 14(44): 6824-6830
- URL: https://www.wjgnet.com/1007-9327/full/v14/i44/6824.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.6824
| Characteristics and measurements | LD with propranolol (n = 9) | LD without propranolol (n = 7) |
| Age (yrs) | 45 ± 2 | 54 ± 5 |
| Sex male/female | 5 (55.6%)/4 (44.4%) | 4 (57%)/3 (43%) |
| Child Pugh Score | 9.8 ± 0.5 | 11.0 ± 0.8 |
| MELD Score | 27.1 ± 1.3 | 29.3 ± 2.1 |
| Albumin (g/dL) | 2.7 ± 0.2 | 2.4 ± 0.3 |
| Bilirubin (mg/dL) | 2.5 (1.3-5.1) | 2.5 (1.2-7.1) |
| Creatinine (mg/dL) | 1.2 (0.8-2.3) | 1.0 (1.0-1.45) |
| INR (International Normalized Ratio) | 1.62 (1.01-6.15) | 1.55 (1.20-2.20) |
| Serum Na+ (mEq/L) | 133.0 ± 1.6 | 126.0 ± 2.7a |
| Characteristics and measurements | LT with propranolol (n = 10) | LT without propranolol (n = 11) |
| Age (yr) | 50.6 ± 3.4 | 50.0 ± 2.6 |
| Sex male/female | 3 (30%)/7 (70%) | 7 (63.6%)/4 (36.4%) |
| Child Pugh Score | 10.5 ± 0.4 | 11.2 ± 0.6 |
| MELD Score | 28.0 ± 1.1 | 29.8 ± 1.6 |
| Albumin (g/dL) | 2.81 ± 0.09 | 2.61 ± 0.15 |
| Bilirubin (mg/dL) | 3.38 ± 1.20 | 3.70 ± 0.87 |
| Creatinine (mg/dL) | 1.0 (1.0-1.45) | 1.05 (0.75-1.50) |
| INR (International Normalized Ratio) | 1.36 (1.32-1.95) | 1.69 (1.24-2.11) |
| Serum Na+ (mEq/L) | 135.2 ± 1.0 | 130.1 ± 1.8a |
| Angiotensin ratios | LT with propranolol (n = 10) | LT without propranolol (n = 11) |
| Ang II/Ang I | 0.46 (0.26-2.64) | 0.19 (0.13-0.24)a |
| Ang-(1-7)/Ang I | 0.27 (0.06-0.91) | 0.05 (0.04-0.11) |
| Ang-(1-7)/Ang II | 0.43 ± 0.08 | 0.52 ± 0.12 |
The Renin-Angiotensin System(RAS) is a multilayered complex system. Previously, Angiotensin (Ang) II was thought to be the principle active peptide, exerting its action through type I and type II receptors[1]. However, our understanding of the RAS has significantly grown[1,2]. Additional active RAS peptides have been identified such as Ang III, with actions similar to Ang II and Ang IV, which exerts its activity at insulin-regulated amino peptidase receptors, and Ang-(1-7), which acts mainly through Mas receptors[1,2]. There is evidence that the RAS acts at the local tissue and even intracellular level through specific receptors by exerting paracrine, endocrine and intracrine functions[1,2]. Further, the system mediates a variety of opposing physiological actions including vasoconstriction/vasodilation, fibrosis/antifibrosis and inflammation/anti-inflammatory[1,2]. Therefore, the RAS is now viewed as a dual system composed of two arms: a vasoconstrictor arm formed by angiotensin converting enzyme(ACE)-Angiotensin (Ang) II-AT1 receptor and a vasodilator arm with ACE2-Ang-(1-7)-Mas receptor. The ACE2-Ang-(1-7)-Mas arm mainly acts as a counter-regulatory mechanism for the vasoconstrictor arm[1]. According to this novel concept, the final functional effect of the RAS may reflect a balance between these two arms[2-5].
This novel view of the RAS makes the evaluation of this system in cirrhosis particularly challenging. In this regard, recent studies have suggested that the RAS seems to be involved in cirrhosis through its two main arms: the first (ACE-Ang II-AT1) by inducing liver fibrosis[6] and maintaining the basal vascular tonus in cirrhosis[7] and the second [ACE2-Ang-(1-7)-Mas] by exerting an anti-fibrotic role[8,9] and probably by participating in the vasodilation of cirrhosis[10].
Non-selective β-adrenergic blockers have been widely used in treatment of portal hypertension in cirrhosis. β-blockers lower portal pressure by reducing portal blood flow as a consequence of a decreased cardiac output (β1-receptor blockade) and arteriolar splanchnic vasoconstriction (β2-receptor blockade)[11]. β-blockers also inhibit renin secretion[12]. However, the effect of propranolol on RAS mediators has still not been quantified, and neither have the hemodynamic changes that might occur during liver transplantation in cirrhotic patients pre-treated with propranolol. Since non-specific β blockade has been a standard approach to controlling the symptoms of portal hypertension and because the RAS seems to influence the outcome of portal hypertension and cirrhosis, it is reasonable to ask if there is a functional relationship between the RAS and beta-receptor system. For this purpose, we have taken in this study the first steps to understand how β1 and β2 blockade affects the RAS in cirrhotic patients. Thus, the aim of the present study was to compare the levels of plasma renin activity (PRA), Ang I, Ang II and Ang-(1-7), measured in the splanchnic and peripheral circulations of cirrhotic patients receiving or not propranolol and to evaluate the effect of previous administration of propranolol on hemodynamic parameters during liver transplantation.
This cross-sectional study used a convenience sample recruited from either the Alfa Institute of Hepatology/Liver Transplantation or the Clinical Primary Care Center of our institution.
Patients diagnosed with hepatic cirrhosis defined through liver histopathology and/or ultrasonography findings were included in this study. Tables 1 and 2 display the Child-Pugh[13] and MELD[14] scores of our patients (all patients were on the waiting list for liver transplantation). The etiology of the liver disease was established in the majority of the subjects (69%), and included alcoholism, virus C, virus B and bile cirrhosis. The cirrhotic patients were allocated to two study groups: one group was composed of patients who had advanced liver disease and were seen in an outpatient clinic (LD, n = 16) and the second group was composed of liver transplant recipients during surgery (LT, n = 21). Each of these two groups was further divided into patients who received propranolol and those who did not. The assistant physician was the only person responsible for the prescription and indication of propranolol treatment and the study protocol did not interfere with any medical prescriptions and recommendations. Thus, patients who were already on treatment with propranolol were then compared to those that did not receive treatment. As shown in Tables 1 and 2, the two subgroups of patients (treated vs non-treated) are comparable in the major demographic characteristics.
The LD group comprised outpatients with ascites and extra-hepatic complications such as encephalopathy and moderate to large esophageal varices (> 5 mm) with risk of bleeding. These patients were using diuretics (furosemide: 40-80 mg/d associated with spironolactone: 25-100 mg/d). Nine of these patients were also receiving propranolol for a mean period of 60 d (40-80 mg/d). The doses of propranolol were titrated to achieve a 20%-25% change in baseline heart rate.
The LT group included hospitalized cirrhotic patients with the same severity of liver disease as compared to LD group based on Child Pugh and MELD scores (Child Pugh: 11.0 ± 0.8 in LD vs 11.2 ± 1.2 in LT and MELD: 29.3 ± 2.1 in LD vs 29.8 ± 3.2 in LT, P > 0.05 for both comparisons). These patients also presented the same clinical and laboratorial features as the LD group and received the same diuretic treatment. The only difference between both groups is the fact that LT patients have been submitted to liver transplantation. Ten of the LT patients were using propranolol (40-80 mg/d) until the time of liver transplantation and their doses were also titrated to achieve a 20%-25% change in baseline heart rate.
Co-morbidities such as diabetes, heart, pulmonary, autoimmune and neurological diseases automatically excluded subjects from the study. Patients receiving chronic treatment with angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, renin inhibitors and corticosteroids were also excluded from the study. During liver transplantation, blood collection was suspended whenever the subject presented acute hemodynamic disarrangements and needed to use a vasoconstrictor.
The Ethics Committee of the Federal University of Minas Gerais approved the study. Informed consent was obtained from all included subjects. The research protocol did not interfere with any medical recommendations or prescriptions. Subject follow-up was guaranteed even in cases of refusal to participate in the study.
Protocol 1 - Evaluation of circulating RAS in outpatients using or not using propranolol: Blood samples for PRA and angiotensin measurements were obtained from LD patients on a single occasion taking into account the inclusion and exclusion criteria for each group. Due to ethical reasons, no changes to the clinical approach were made for study purposes. Blood samples (10 mL) were collected through peripheral venipuncture in the morning after a fasting period of 8 h. All subjects rested in supine position for at least 30 min before blood sampling.
Protocol 2 - Evaluation of RAS and hemodynamic parameters during pre-anhepatic stage of liver transplantation in patients pre-treated with propranolol or not: In the LT group, blood sampling was performed during the pre-anhepatic stage of liver transplantation and samples were obtained from the portal vein (10 mL) to evaluate RAS mediators. Hemodynamic parameters (cardiac output, cardiac index, systemic vascular resistance and systemic vascular resistance index) were determined simultaneously with the blood sampling. These measurements were obtained through invasive continuous monitoring via a Swan-Ganz catheter (CCOMBO/SvO2, 110 cm/7.5 F, Edwards Lifesciences, Irvine, CA, USA), using Dixtal (DX 2020, Dixtal Biomedical, São Paulo, Brazil) and Vigilance (CEDV, Edwards Lifesciences, Irvine, CA, USA) monitors. Anesthesia for liver transplantation was induced by a rapid sequence of etomidate, fentanyl and succinylcholine and maintained by isoflurane (CAM~1.0) and atracurium until the blood sampling.
Blood collection: For all blood collections, samples were drawn into two sets of ice-cooled tubes-one containing 7.5% EDTA for PRA determinations and the other containing a cocktail of protease inhibitors for angiotensin measurements, as previously described[14]. Blood samples were centrifuged at × 2000 g for 20 min at 4°C and plasma stored at -20°C[14].
Plasma extraction and radioimmunoassays: Plasma samples were extracted using Bond-Elut cartridges (Analytichem International, Harbor City, CA), as described elsewhere[15]. PRA as well as Ang I, Ang II and Ang-(1-7) concentrations were determined through radioimmunoassays, as detailed elsewhere[15]. The recovery of 125I-labeled Ang I, Ang II, and Ang-(1-7) was 79.2% ± 2.3%, 86.9% ± 0.8% and 83.5% ± 0.9%, respectively. Results were expressed as nanograms of Ang I generated per mL of plasma per hour (ng Ang I/mL per hour) for PRA and pg/mL of plasma for Ang measurements.
Gaussian distribution of variables was evaluated by the Shapiro normality test. Results were reported as mean ± SE or median, when appropriate. Unpaired t test was used for the comparison of means between groups. Mann-Whitney was used to compare non-parametric data. The level of significance was set at P < 0.05. Graphpad PRISM was used for the statistical analyses.
The outpatients with liver cirrhosis (LD, n = 16) consisted of 9 males and 7 females from 44 to 66 years. The etiologies of liver failure in these subjects were: alcoholism in 4, bile cirrhosis in 1, virus C in 5, virus B in 1 and idiopathic causes in 5 patients. Clinical and laboratorial data revealed high Child Pugh and MELD scores that were very similar between the groups of patients using or not using propranolol (Table 1). Only serum sodium levels were significantly reduced in LD patients not receiving propranolol as compared to those treated with propanolol (Table 1).
Cirrhotic patients during liver transplantation (LT, n = 21) comprised 10 males and 11 females from 36 to 67 years. The etiologies for primary hepatic diseases in this group included virus C in 9, alcoholism in 4, bile cirrhosis in 2, virus B in 1 and idiopathic in 5 patients. The LT group without propranolol revealed reduced serum sodium levels compared to the LT group receiving propranolol. Other demographic characteristics and liver function scores were similar in both subgroups (Table 2).
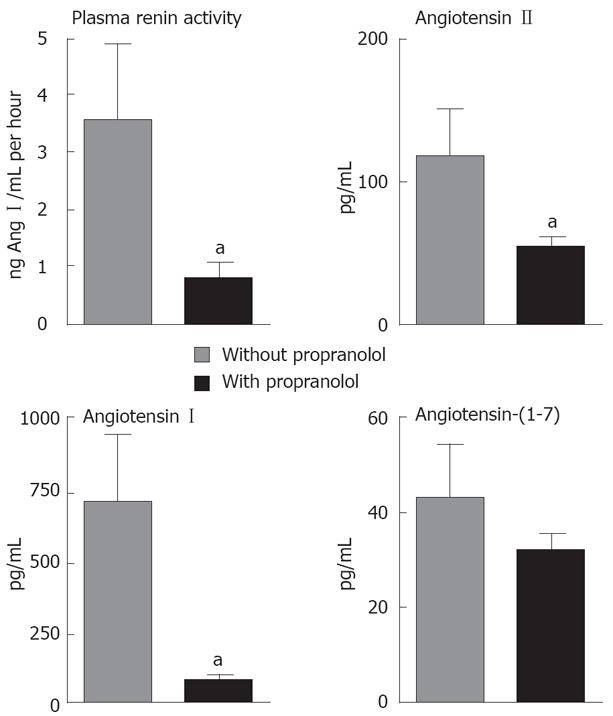
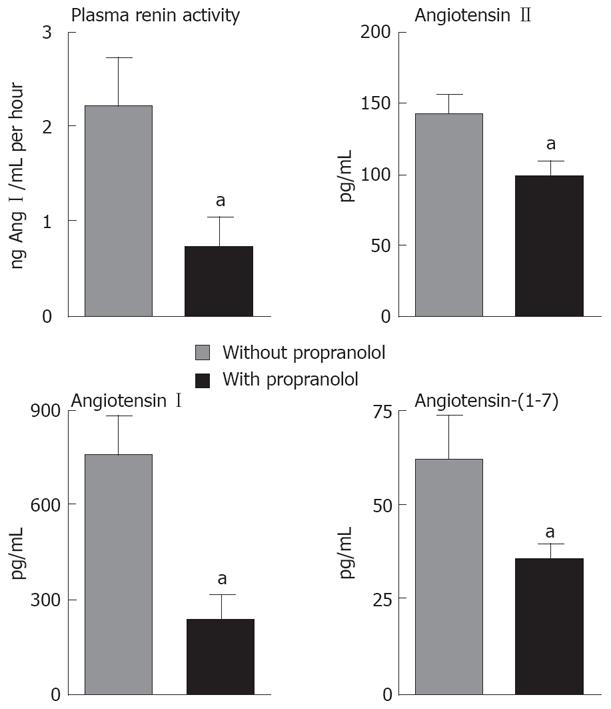
As displayed in Figure 1, PRA and angiotensin I (Ang I) were lower in peripheral circulation of the LD group treated with propranolol in comparison to LD group not receiving propranolol (PRA: 3.54 ± 1.35 ng Ang I/mL per hour vs 0.77 ± 0.28 ng Ang I/mL per hour; Ang I: 717.2 ± 39.0 pg/mL vs 79.8 ± 24.8 pg/mL, P < 0.05 for both comparisons). As shown in Figure 2, the same profile was observed for the LT group pre-treated with propranolol, which also presented a significant reduction of the PRA and Ang I plasma levels in the portal vein when compared to the LT group that was not treated with propranolol (PRA: 2.20 ± 0.51 ng Ang I/mL per hour vs 0.74 ± 0.30 ng Ang I/mL per hour; Ang I: 764.2 ± 117.0 pg/mL vs 235.0 ± 75.6 pg/mL, P < 0.05 for both comparisons).
LD patients receiving propranolol also exhibited a significant reduction in the levels of Ang II in peripheral circulation when compared to LD patients not using propranolol (Ang II: 117.2 ± 33.5 pg/mL vs 53.9 ± 6.5 pg/mL, P < 0.05, Figure 1). The same reduction of Ang II levels was observed in splanchnic circulation (portal vein) of the LT group under β-blockade in comparison to LT group not treated with propranolol (Ang II: 143.4 ± 13.3 pg/mL vs 96.9 ± 12.6 pg/mL, P < 0.05, Figure 2). Plasma levels of Ang-(1-7) were also reduced in splanchnic circulation of the LT group that was previously treated with propranolol in comparison to non-treated LT group (62.3 ± 10.9 pg/mL vs 35.5 ± 3.8 pg/mL, P < 0.05, Figure 2), whereas plasma Ang-(1-7) in peripheral circulation of the LD group did not differ significantly despite treatment or not with propranolol (Figure 1).
Ratios between Ang-(1-7) and Ang I levels, between Ang II and Ang I, and between Ang-(1-7) and Ang II in LD and LT groups are displayed in Tables 3 and 4, respectively. The ratio between Ang-(1-7) and Ang I indirectly reflects ACE2 activity, whereas the ratio between Ang II and Ang I indirectly estimates ACE activity. Both ratios were significantly increased in peripheral circulation of the LD group using propranolol in comparison to LD patients not receiving the β-blocker (P < 0.01, Table 3). Only Ang II/Ang I ratio was increased in splanchnic circulation of the LT group pre-treated with propranolol in comparison to LT patients that had not received propranolol (P < 0.01, Table 4), whereas Ang-(1-7)/Ang I did not significantly differ in splanchnic circulation of LT patients despite the previous treatment or not with propranolol. More importantly, the ratio between Ang-(1-7) and Ang II, which could represent the final functional relationship between RAS mediators, did not differ in either peripheral circulation of the LD group or in splanchnic circulation of the LT group, independently of the previous use or not of the β-blocker.
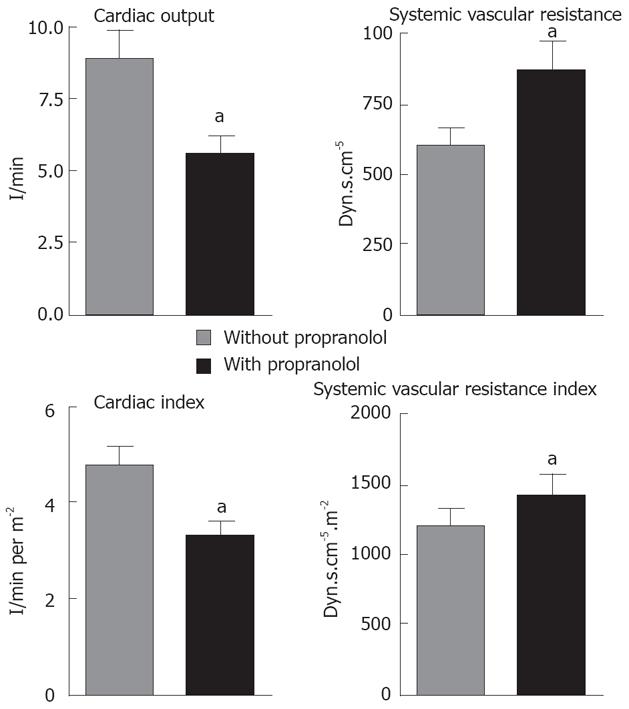
In order to demonstrate that our LT patients pre-treated with propranolol were adequately β blocked, we measured hemodynamic parameters during pre-anhepatic stage of liver transplantation. Accordingly, in LT patients pre-treated with propranolol, the cardiac output (8.9 ± 0.9 L/min vs 5.6 ± 0.6 L/min) and cardiac index (4.7 ± 0.4 L/min per m2vs 3.2 ± 0.3 L/min per m2) were reduced and the systemic vascular resistance (604.2 ± 65.0 × 877.5 ± 106.1 dyn.s/cm5) and its index [(1036 ± 86) × (1399 ± 147)] dyn.s/cm5 per m2) were increased in comparison to patients that had not previously received propranolol (P < 0.05 for all comparisons, Figure 3).
In general, our data showed that chronic treatment with propranolol in cirrhotic patients is characterized by marked changes in the precursors of RAS cascade (Renin and Ang I) with repercussion in RAS two main arms at the splanchnic and peripheral circulation. On the other hand, no changes were detected in the ratio between the two main RAS mediators [Ang-(1-7)/Ang II], which has been used to evaluate the final functional effect of the RAS. In parallel, the chronic use of propranolol produced hemodynamic changes, which were probably able to control the hyperdynamic circulation of cirrhotic patients. Taken together, these findings suggest that the reduction of hyperdynamic circulation produced by chronic treatment with propranolol in cirrhotic patients was associated with an overall RAS inhibition, but it was not due to changes in the balance between the two RAS arms: ACE-Ang-AT1 (vasoconstrictor) versus ACE2-Ang-(1-7)-Mas (vasodilator).
In splanchnic and peripheral circulation, the β-blockade in cirrhotic patients was characterized by reduced PRA and Ang I levels. These RAS components can lead to the synthesis of both Ang II and Ang-(1-7)[5,16]. The cirrhotic patients receiving propranolol have reduced Ang II levels in the splanchnic and in the peripheral circulation as well as reduced Ang-(1-7) levels in the splanchnic circulation. In this regard, Blumenfeld et al[12] previously suggested that β-blockade reduced Ang II levels and PRA in normotensive and hypertensive subjects by inhibiting prorenin processing to renin.
Ratios between angiotensins, especially the relationship between Ang-(1-7) and Ang II, have been used to estimate the final functional RAS effect[2,3,4]. In this study, propranolol use was not able to change the ratio between Ang-(1-7) and Ang II in splanchnic and peripheral circulation of non-compensated cirrhotic patients, although there was an absolute decrease in both angiotensins. In parallel, systemic vascular resistance (SVR) and its index increased and cardiac output (CO) and its index decreased in non-compensated cirrhotic patients treated with propranolol. Similar hemodynamic changes in cirrhotic patients receiving propranolol have already been reported[17-19] and attributed to β-adrenergic blockade[20]. Since the relationship between Ang-(1-7) and Ang II remained unchanged in splanchnic and peripheral circulation of our cirrhotic patients, we could hypothesize that propranolol was not able to interfere with the final functional RAS effect upon vascular tone. Our data also suggest that the activity of the two main RAS enzymes, ACE and ACE2, were probably not reduced by propranolol use in cirrhotic patients, since the ratios between Ang II and Ang I and between Ang-(1-7) and Ang I were increased in peripheral circulation and the ratio between Ang II and Ang I was also elevated in splanchnic circulation. However, we can not exclude the possibility that other factors such as changes in the catabolism of Ang II or Ang-(1-7) could have contributed to the reduction in absolute levels of each peptide.
In cirrhotic patients, arteriolar vasodilation and diuretic administration cause a decreased effective arterial blood volume that stimulates vasopressor systems leading to high levels of PRA, circulating norepinephrine, and vasopressin[21-23]. In this context, propranolol inhibits renin secretion and reduces vasodilation (SVR increase) in cirrhosis[17], leading to a reduction of the relative arterial hypovolemia. These actions could oppose the activated vasopressor systems (RAS, sympathetic nervous system and vasopressin) and may be involved in the amelioration of the hyperdynamic circulation observed in our cirrhotic patients.
It should also be pointed out that we are aware of the limitations of our study design. For example, peripheral blood samples generally represent the cumulative expression of RAS in multiple tissues and may not reliably reflect molecular activity in the splanchnic circulation. For this reason, we did manage to collect samples from the portal vein during liver transplantation. However, it is still difficult to compare these findings to the samples collected in peripheral blood from outpatients. Nevertheless, some aspects of this study may increase the strength of our findings, such as the utilization of strictly defined inclusion and exclusion criteria and the well-established protocol for the measurements of PRA and angiotensins[15].
In conclusion, results obtained with propranolol treatment in cirrhotic patients have been controversial[20,24]. While in advanced liver disease with significant reduction of the hepatic venous pressure gradient propanolol treatment decreased the risk of ascites, spontaneous bacterial peritonitis, hepatorenal syndrome and death[24], in unselected cirrhotic patients the same β-blocker was not able to prevent varices and was associated with an increased number of adverse events[20]. We believe that the use of propranolol in cirrhosis could change the prognosis of patients with hyperdynamic circulation and relative hypovolemia, but it is probably not able to interfere with potentially reversible liver fibrosis. Indeed, the use of propranolol did not alter the balance between the activity of the anti-fibrotic arm of the RAS, ACE2-Ang-(1-7)-Mas[8], and of the pro-fibrotic arm, ACE-Ang II-AT1[6]. For this purpose, many studies have suggested that ACE inhibitors and AT1 receptor blockers seemed to be effective[25-29]. Their mechanisms of action probably involve not only the inhibition of Ang II formation or action but also the augmentation of Ang-(1-7) levels or effects[5,29]. On the other hand, it should be mentioned that, mostly in advanced stages of cirrhosis, the ACE-Ang II-AT1 arm contributes to the maintenance of basal vascular tonus[7] and therefore the use of AT1 receptor blockers or ACE inhibitors as antifibrotic therapies could not be well tolerated. Since propranolol administration seems to improve only the extrahepatic complications of the advanced cirrhotic patients, a possible therapeutic approach for human cirrhosis at this stage could be the combination of AT1 receptor blockers or ACE inhibitors with propranolol. Future studies with more powerful designs are obviously necessary to evaluate whether the use of propranolol at this stage of cirrhosis would enable the administration of AT1 receptor blockers or ACE inhibitors or even receptor Mas agonists to reduce liver fibrosis.
Recent studies have suggested that the Renin Angiotensin System (RAS) seems to be involved in cirrhosis. Non-selective β-adrenergic blockers have been widely used in treatment of portal hypertension in cirrhosis. However, the effect of propranolol on RAS mediators has still not been quantified. Since non-specific β blockade has been a standard approach to controlling the symptoms of portal hypertension and because the RAS seems to influence the outcome of portal hypertension and cirrhosis, it is reasonable to ask if there is a functional relationship between the RAS and beta-receptor system.
This study represents an initial approach in understanding how non-specific β blockade affects RAS in cirrhotic patients by comparing the levels of plasma renin activity, Angiotensin (Ang) I, Ang II and Ang-(1-7), measured in the splanchnic and peripheral circulations of cirrhotic patients receiving or not receiving propranolol and by evaluating the effect of previous administration of propranolol on hemodynamic parameters during liver transplantation.
Chronic treatment with propranolol in cirrhotic patients is characterized by marked changes in the precursors of RAS cascade (Renin and Ang I) with repercussion in RAS two main arms in the splanchnic and peripheral circulation. On the other hand, no changes were detected in the ratio between the two main RAS mediators [Ang-(1-7)/Ang II]. Additionally, the treatment with propranolol seemed to be able to control the hyperdynamic circulation of cirrhotic patients probably due to an overall RAS inhibition, but without changes in the balance between the two RAS arms: ACE-Ang-AT1 (vasoconstrictor) versus ACE2-Ang-(1-7)-Mas (vasodilator).
Our data suggest that a possible therapeutic approach for advanced human cirrhosis could be the combination of AT1 receptor blockers or ACE inhibitors with propranolol. Future studies with more powerful designs are obviously necessary to evaluate whether the use of propranolol at this stage of cirrhosis would enable the administration of AT1 receptor blockers or ACE inhibitors or even receptor Mas agonists to reduce liver fibrosis.
In the current study, the investigators have taken the first steps to understand how β1 and β2 blockade affects RAS in patients. This is important because non-specific β blockade has been a standard approach to controlling the symptoms of portal hypertension.
| 1. | Santos RA, Ferreira AJ, Simões E Silva AC. Recent advances in the angiotensin-converting enzyme 2-angiotensin(1-7)-Mas axis. Exp Physiol. 2008;93:519-527. |
| 2. | Simões E Silva AC, Pinheiro SV, Pereira RM, Ferreira AJ, Santos RA. The therapeutic potential of Angiotensin-(1-7) as a novel Renin-Angiotensin System mediator. Mini Rev Med Chem. 2006;6:603-609. |
| 3. | Matsui T, Tamaya K, Matsumoto K, Osajima Y, Uezono K, Kawasaki T. Plasma concentrations of angiotensin metabolites in young male normotensive and mild hypertensive subjects. Hypertens Res. 1999;22:273-277. |
| 4. | Nogueira AI, Souza Santos RA, Simões E Silva AC, Cabral AC, Vieira RL, Drumond TC, Machado LJ, Freire CM, Ribeiro-Oliveira A Jr. The pregnancy-induced increase of plasma angiotensin-(1-7) is blunted in gestational diabetes. Regul Pept. 2007;141:55-60. |
| 5. | Warner FJ, Lubel JS, McCaughan GW, Angus PW. Liver fibrosis: a balance of ACEs? Clin Sci (Lond). 2007;113:109-118. |
| 6. | Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209-218. |
| 7. | Helmy A, Jalan R, Newby DE, Hayes PC, Webb DJ. Role of angiotensin II in regulation of basal and sympathetically stimulated vascular tone in early and advanced cirrhosis. Gastroenterology. 2000;118:565-572. |
| 8. | Pereira RM, Dos Santos RA, Teixeira MM, Leite VH, Costa LP, da Costa Dias FL, Barcelos LS, Collares GB, Simões E Silva AC. The renin-angiotensin system in a rat model of hepatic fibrosis: evidence for a protective role of Angiotensin-(1-7). J Hepatol. 2007;46:674-681. |
| 9. | Herath CB, Warner FJ, Lubel JS, Dean RG, Jia Z, Lew RA, Smith AI, Burrell LM, Angus PW. Upregulation of hepatic angiotensin-converting enzyme 2 (ACE2) and angiotensin-(1-7) levels in experimental biliary fibrosis. J Hepatol. 2007;47:387-395. |
| 10. | Paizis G, Tikellis C, Cooper ME, Schembri JM, Lew RA, Smith AI, Shaw T, Warner FJ, Zuilli A, Burrell LM. Chronic liver injury in rats and humans upregulates the novel enzyme angiotensin converting enzyme 2. Gut. 2005;54:1790-1796. |
| 11. | Dib N, Oberti F, Cales P. Current management of the complications of portal hypertension: variceal bleeding and ascites. CMAJ. 2006;174:1433-1443. |
| 12. | Blumenfeld JD, Sealey JE, Mann SJ, Bragat A, Marion R, Pecker MS, Sotelo J, August P, Pickering TG, Laragh JH. Beta-adrenergic receptor blockade as a therapeutic approach for suppressing the renin-angiotensin-aldosterone system in normotensive and hypertensive subjects. Am J Hypertens. 1999;12:451-459. |
| 13. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. |
| 14. | Wiesner RH, McDiarmid SV, Kamath PS, Edwards EB, Malinchoc M, Kremers WK, Krom RA, Kim WR. MELD and PELD: application of survival models to liver allocation. Liver Transpl. 2001;7:567-580. |
| 15. | Simões E Silva AC, Diniz JS, Regueira Filho A, Santos RA. The renin angiotensin system in childhood hypertension: selective increase of angiotensin-(1-7) in essential hypertension. J Pediatr. 2004;145:93-98. |
| 16. | Pagliaro P, Penna C. Rethinking the renin-angiotensin system and its role in cardiovascular regulation. Cardiovasc Drugs Ther. 2005;19:77-87. |
| 17. | Andreu V, Perello A, Moitinho E, Escorsell A, García-Pagán JC, Bosch J, Rodés J. Total effective vascular compliance in patients with cirrhosis. Effects of propranolol. J Hepatol. 2002;36:356-361. |
| 18. | Bañares R, Moitinho E, Piqueras B, Casado M, Garcia-Pagan JC, de Diego A, Bosch J. Carvedilol, a new nonselective beta-blocker with intrinsic anti- Alpha1-adrenergic activity, has a greater portal hypotensive effect than propranolol in patients with cirrhosis. Hepatology. 1999;30:79-83. |
| 19. | Moller S, Bendtsen F, Henriksen JH. Effect of beta-adrenergic blockade on elevated arterial compliance and low systemic vascular resistance in cirrhosis. Scand J Gastroenterol. 2001;36:653-657. |
| 20. | Groszmann RJ, Garcia-Tsao G, Bosch J, Grace ND, Burroughs AK, Planas R, Escorsell A, Garcia-Pagan JC, Patch D, Matloff DS. Beta-blockers to prevent gastroesophageal varices in patients with cirrhosis. N Engl J Med. 2005;353:2254-2261. |
| 21. | Henriksen JH, Moller S. Liver cirrhosis and arterial hypertension. World J Gastroenterol. 2006;12:678-685. |
| 22. | Arroyo V, Fernandez J, Gine P. Pathogenesis and treatment of hepatorenal syndrome. Semin Liver Dis. 2008;28:81-95. |
| 23. | Ginès P, Cárdenas A. The management of ascites and hyponatremia in cirrhosis. Semin Liver Dis. 2008;28:43-58. |
| 24. | Bosch J, Abraldes JG, Groszmann R. Current management of portal hypertension. J Hepatol. 2003;38 Suppl 1:S54-S68. |
| 25. | Terui Y, Saito T, Watanabe H, Togashi H, Kawata S, Kamada Y, Sakuta S. Effect of angiotensin receptor antagonist on liver fibrosis in early stages of chronic hepatitis C. Hepatology. 2002;36:1022. |
| 26. | Wei YH, Jun L, Qiang CJ. Effect of losartan, an angiotensin II antagonist, on hepatic fibrosis induced by CCl4 in rats. Dig Dis Sci. 2004;49:1589-1594. |
| 27. | Yoshiji H, Noguchi R, Fukui H. Combined effect of an ACE inhibitor, perindopril, and interferon on liver fibrosis markers in patients with chronic hepatitis C. J Gastroenterol. 2005;40:215-216. |
| 28. | Debernardi-Venon W, Martini S, Biasi F, Vizio B, Termine A, Poli G, Brunello F, Alessandria C, Bonardi R, Saracco G. AT1 receptor antagonist Candesartan in selected cirrhotic patients: effect on portal pressure and liver fibrosis markers. J Hepatol. 2007;46:1026-1033. |
| 29. | Lubel JS, Herath CB, Burrell LM, Angus PW. Liver disease and the renin-angiotensin system: recent discoveries and clinical implications. J Gastroenterol Hepatol. 2008;23:1327-1338. |
Peer reviewer: Mercedes Susan Mandell, MD, PhD, Department of Anesthesiology, University of Colorado Health Sciences Ctr., 12401 E. 17th Ave, B113 Aurora, CO 80045, United States
S- Editor Cheng JX L- Editor Logan S E- Editor Yin DH