Published online Nov 28, 2008. doi: 10.3748/wjg.14.6802
Revised: November 4, 2008
Accepted: November 11, 2008
Published online: November 28, 2008
AIM: To evaluate the effects of interferon-α-2b (IFN-α-2b) on expression of cyclooxygenase-2 (COX-2) and vascular endothelial growth factor (VEGF) in human hepatocellular carcinoma (HCC) inoculated in nude mice and to study the underlying mechanism of IFN-α-2b against HCC growth.
METHODS: Thirty-two nude mice bearing human HCC were randomly divided into four groups (n = 8). On the 10th day after implantation of HCC cells, the mice in test groups (groups A, B and C) received IFN-α-2b at a serial dose (10 000 IU for group A, 20 000 IU for group B, 40 000 IU for group C sc daily) for 35 d. The mice in control group received normal saline (NS). The growth conditions of transplanted tumors were observed. Both genes and proteins of COX-2 and VEGF were detected by RT-PCR and Western blot. Apoptosis of tumor cells in nude mice was detected by TUNEL assay after treatment with IFN-α-2b.
RESULTS: Tumors were significantly smaller and had a lower weight in the IFN-α-2b treatment groups than those in the control group (P < 0.01), and the tumor growth inhibition rate in groups A, B and C was 27.78%, 65.22% and 49.64%, respectively. The expression levels of both genes and proteins of COX-2 and VEGF were much lower in the IFN-α-2b treatment groups than in the control group (P < 0.01). The apoptosis index (AI) of tumor cells in the IFN-α-2b treatment groups was markedly higher than that in the control group (P < 0.01). Group B had a higher inhibition rate of tumor growth, a lower expression level of COX-2 and VEGF and a higher AI than groups A and C (P < 0.05), but there was no significant difference between groups A and C.
CONCLUSION: The inhibitory effects of IFN-α-2b on implanted tumor growth and apoptosis may be associated with the down-regulation of COX-2 and VEGF expression. There is a dose-effect relationship. The medium dose of IFN-α-2b for inhibiting tumor growth is 20 000 IU/d.
- Citation: Cao B, Chen XP, Zhu P, Ding L, Guan J, Shi ZL. Inhibitory effect of interferon-α-2b on expression of cyclooxygenase-2 and vascular endothelial growth factor in human hepatocellular carcinoma inoculated in nude mice. World J Gastroenterol 2008; 14(44): 6802-6807
- URL: https://www.wjgnet.com/1007-9327/full/v14/i44/6802.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.6802
| mRNA | Protein | ||||||
| Groups | Dose (IU/d) | COX-2 | VEGF | Groups | Dose (IU/d) | COX-2 | VEGF |
| Control | 0 | 0.59 ± 0.11 | 0.61 ± 0.15 | Control | 0 | 0.75 ± 0.16 | 0.82 ± 0.18 |
| Group A | 10 000 | 0.44 ± 0.09a | 0.52 ± 0.12a | Group A | 10 000 | 0.52 ± 0.09a | 0.61 ± 0.13a |
| Group B | 20 000 | 0.12 ± 0.02ad | 0.17 ± 0.03ad | Group B | 20 000 | 0.23 ± 0.04ad | 0.31 ± 0.05ad |
| Group C | 40 000 | 0.26 ± 0.06a | 0.31 ± 0.07a | Group C | 40 000 | 0.38 ± 0.07a | 0.43 ± 0.08a |
In recent years, studies have proved the inhibitory effects of interferon-α-2b (IFN-α-2b) on many kinds of tumors[1-3], but the effects of IFN-α-2b on expression of cyclooxygenase-2 (COX-2) and vascular endothelial growth factor (VEGF) in hepatocellular carcinoma (HCC) have not been extensively studied. This study investigated the effects of IFN-α-2b on COX-2 and VEGF expression in human HCC implanted in nude mice and the underlying mechanism of its inhibitory effect on the growth of HCC.
HCC cell line HepG2 was stored in our laboratory. Rabbit anti-human COX-2 polyclonal antibody (Santa Cruz, US) was purchased from Beijing Zhongshan Biotechnology Company, Ltd. Mouse anti-human VEGF polyclonal antibody (Neomarkers, USA), TRIzol and RT-PCR kits were from Jinmei Biotech Company, Ltd. Primers of RT-PCR were synthesized by Alpha Biotechnology Company (Wuhan, China). In situ apoptosis detection kits (Boehringer Mannheim, Germany) and ECL chemiluminescence detection kits (Pierce, US) were from Beijing Zhongshan Biotechnology Company, Ltd. IFN-α-2b injection (trade name: Interlong) was from Shenzhen Neptunus Interlong Bio-tech Holdings Company, Ltd (China).
A transplantation tumor model of human HCC in nude mice was established. In brief, thirty-two 4-6 wk old female BALB/c nu/nu mice, weighing 18-20 g, bred in SPF rooms, were obtained from the Experimental Animal Center of Tongji Medical University, Huazhong University of Science and Technology (Wuhan, China). The concentration of single cell suspension of HepG2 cells was adjusted to 5 × 109/L at the exponential growth phase. The cell viability was kept above 95% assessed by Typan blue exclusion. Then, the cell suspension was inoculated subcutaneouly in the back of nude mice (0.2 mL/each mouse). The standard of tumor formation was defined as its nodus diameter (up to 0.5 cm). The rate of tumor formation was 100% 10 d after inoculation. The mice meeting the standard of tumor formation were randomly divided into 4 groups (n = 8).
Each nude mouse meeting the standard of tumor formation in groups A, B and C received 10 000 IU IFN-α-2b, 20 000 IU and 40 000 IU of IFN-α-2b, respectively, once a day for 35 d. Each nude mouse in control group received only 0.1 mL normal saline (NS), once a day for 35 d. Then, the mice were killed. The maximum and minimum diameters of tumor were measured with a sliding caliper and the weight of tumor was recorded. A certain number of samples were stored at -70°C. The rest samples were fixed in 10% formalin, imbedded in paraffin, and cut into serial sections for assay of in situ apoptosis. Volume of tumor (V) = ab2/2, inhibition ratio (%) = [(mean tumor weight of control group-mean tumor weight of test group)/ mean tumor weight of control group] × 100%.
Total RNA was extracted with the TRIzol through one-step method, and its concentration and purity were detected using a DNA/RNA detector (Pharmarcia Company, British). cDNA was synthesized by a reverse transcription reaction of 4 μg of total RNA with oligo-(dt)15 primers and retroviridase at 45°C for 60 min, and stored at -20°C.
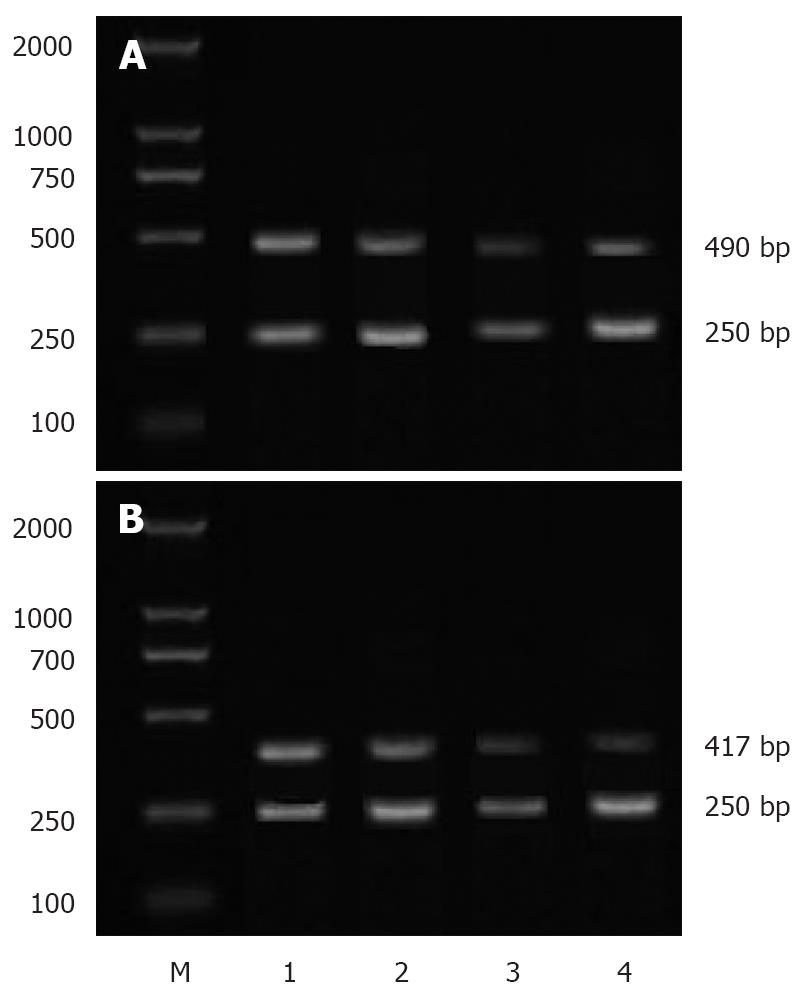
The sequences of primers used for semi-quantitative PCR are as follows: sense: 5'-CAAGTCCCTGAGCATCTACG-3' and anti-sense: 5'-CATTCCTACCACCAGCAACC-3' for COX-2; sense: 5'-TTGCTGCTCTACCTCCAC-3' and anti-sense: 5'-AATGCTTTCTCCGCTCTG-3' for VEGF; sense: 5'-CAGAGCAAGAGAGGCAGCCT-3' and anti-sense: 5'-GGATAGCACAGCCTGGATAG-3' for β-actin. PCR conditions were as follows: 30 cycles at 94°C for 1 min, at 54°C for 30 s, and at 72°C for 1 min for COX-2;27 cycles at 94°C for 1 min, at 56°C for 30 s, and at 72°C for 1 min for VEGF;30 cycles at 94°C for 1 min, at 59°C for 30 s, and at 72°C for 1 min for β-actin. The lengths of PCR products of COX-2, VEGF and β-actin were 490 bp, 417 bp and 250 bp, respectively. After separated by electrophoresis on 1.5% agarose gel, the bands of PCR products were scanned and a densitometric analysis was performed with a MUVB-20 gel analysis system (Ultralum Company, USA). The relative expression levels of COX-2 mRNA and VEGF mRNA were represented as absorbance ratios (COX-2/β-actin and VEGF/β-actin).
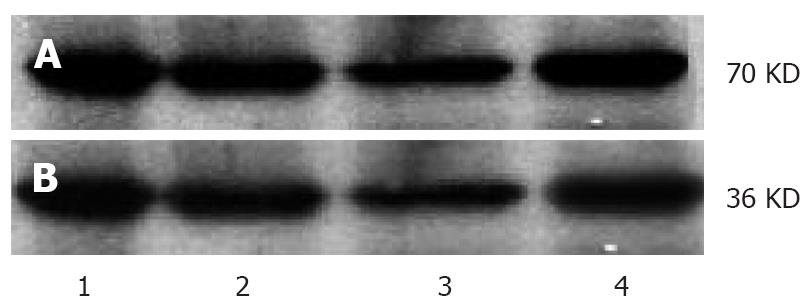
The protein was extracted from tumor tissue through three-detergent methods. An equal quantity of protein was separated from each sample by electrophoresis on 10% SDS-PAGE gel, and then transferred to a nitrocellulose filter (NC filter) with a semidry transfer cell. The NC filter containing protein samples was incubated in a blocking buffer containing 5% nonfat dry milk for 2 h, then with the first antibody at a dilution of 1:250 in a blocking buffer overnight at 4°C. After washed with 0.1% TBS, the membrane was incubated at room temperature for 1 h with the second antibodies at a dilution of 1:5000 in a blocking buffer, then washed three times with 0.1% TBS, visualized, photographed and fixed through ECL according to its manufacturer’s instructions. The absorbance value of protein bands was assessed with an image analysis system.
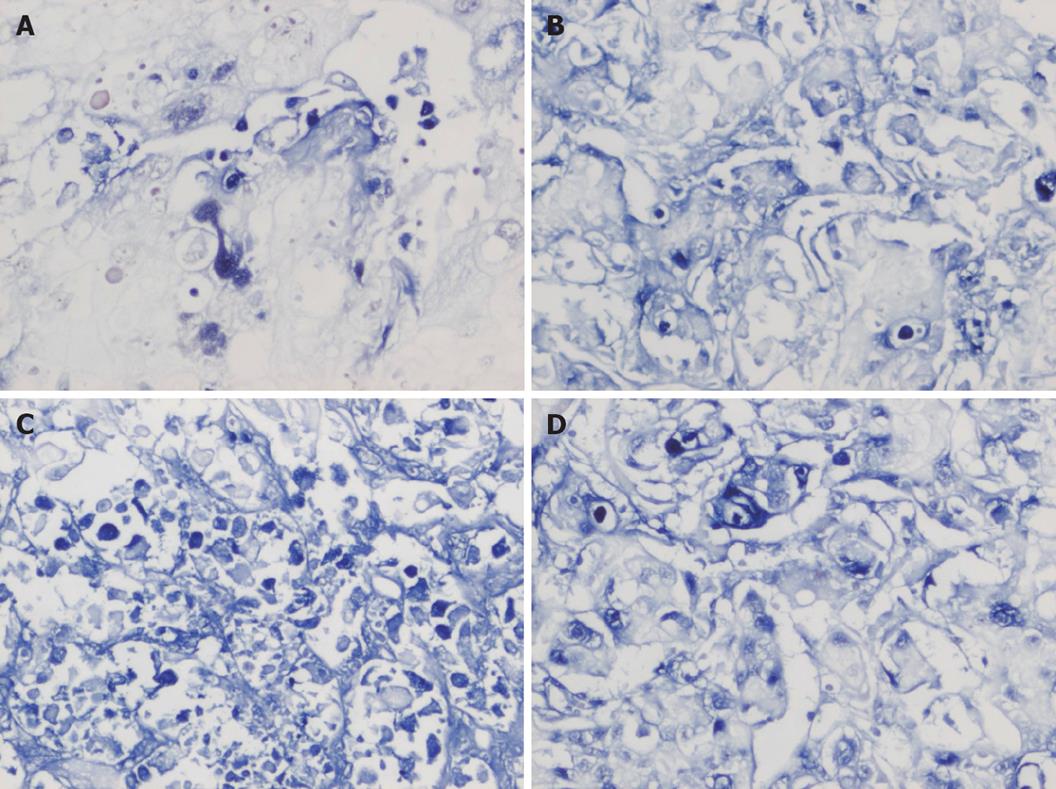
Paraffin-embedded tumor samples were cut into serial 4-5 μm thick serial sections, heated overnight at 58°C, and routinely deparaffinized and rehydrated. After digested by protease K for 15 min, the sections were incubated with TUNEL at 37°C for 1 h. After digoxin- marked alkaline phosphatase antibody was added, the sections were colored with BCIP/NBT, dehydrated with alcoholic, air dried, and mounted. Positive cells were defined as hyacinthine particles distributing in nuclei. Five high power fields were randomly selected from each section. Results were expressed as mean values. Apoptosis index (AI) = [the number of apoptosis cells/ (the number of apoptosis cells+ the number of un-apoptosis cells)] × 100%.
Data were presented as mean ± SD. SPSS 11.5 was employed to analyze the data. The total difference among groups was analyzed by ANOVA. Q-test was used for the comparison between two groups. P < 0.05 was considered statistically significant.
The tumor growth in three IFN-α-2b treatment groups was inhibited at different degrees. The inhibition rate of tumors in groups A, B and C was 27.78%, 65.22% and 49.64%, respectively. The weight and volume of tumors were lower in three IFN-α-2b treatment groups than in control group (P < 0.01). The inhibitory effect was the greatest in group B, and significantly different from that in groups A and C (P < 0.01), but the difference was not statistically significant between groups A and C (Table 1).
RT-PCR showed that the expression levels of COX-2 and VEGF mRNA were lower in three IFN-α-2b treatment groups than in control group (P < 0.01). Meanwhile, their expression levels were significantly lower in group B than in groups A and C (P < 0.05), but the difference was not statistically significant between groups A and C (Table 2, Figure 1).
Western blot assay showed that the expression levels of COX-2 and VEGF protein were lower in three IFN-α-2b treatment groups than in control group (P < 0.01). Statistical analysis revealed similar results of COX-2 and VEGF mRNA in three IFN-α-2b treatment groups (Table 2, Figure 2).
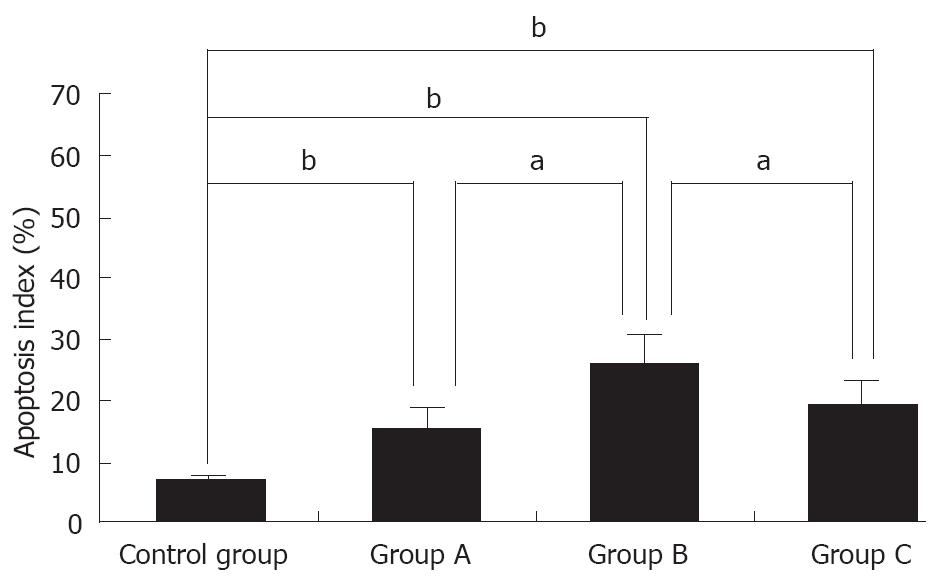
TUNEL assay showed that the AI of tumor cells in the 4 groups was 6.39% ± 1.33%, 15.39% ± 3.09%, 25.53% ± 4.98% and 19.28% ± 3.82%, respectively, and was significantly higher in three IFN-α-2b treatment groups than in control group (P < 0.01). However, the AI in group B was higher than that in groups A and C (P < 0.05), but the difference was not statistically significant between groups A and C (Figures 3-4).
HCC is one of the malignant tumors that can lead patients to death[1], but the molecular mechanism of hepatocarcinogenesis has not been well understood. COX can be divided into two subtypes: COX-1 and COX-2. COX-2 is a rate-limiting enzyme of prostaglandin (PG) biosynthesis, encodes for 603 or 604 amino acids including 17 signal peptides of amino acid residues, contains 4 oligoses and 2 bands (molecular weight = 72 KDa and 74 KDa, respectively) in SDS-PAGE. Oshima et al[2] showed that the expression of COX-2 is up-regulated in colon tumors and its activity plays an important role in the process of tumorigenesis. Besides, the expression of COX-2 is also higher in liver, breast, tongue, prostatic, cutaneous and mouth dermoid cancers, etc[3-5].
It has been recently proved that COX-2 is also expressed in HCC at different degrees and HCC patients with a higher expression level of COX-2 have a higher recurrence rate, early metastasis and worse prognosis[6,7], suggesting that over-expression of COX-2 may be related with a worse prognosis of HCC. The five-year survival rate of HCC patients after operation is only 25%-39%. Some studies indicate that over-expression of COX-2 may promote oncogenesis by regulating the expression of some genes related to cell proliferation and apoptosis[6,7]. The underlying mechanism of COX-2 against oncogenesis may be through influencing many kinds of prostaglandin and thromboxan to bind to their corresponding receptors.
Angiogenesis plays an important role in tumorigenesis and tumor development. If no angiogenesis occurs, the volume of tumor can hardly be larger than 1-2 mm3. The degree of angiogenesis depends on the rate of angiogenesis and anti-angiogenesis factors in tumor cells and adjacent host cells. The angiogenesis factors mainly include bFGF, VEGF, IL-8, MMP-2, MMP-9, etc. Among them, VEGF is most important, and plays a leading role in the growth, generation, recurrence and metastasis of tumors[8-11]. As a dipolymer, glucoprotein has a molecular weight of 34-46 kDa. VEGF exerts its biological effect by binding specifically to Flt1 and KDR/Flk1 exiting on the surface of vascular endothelial cells which belong to RTKIII receptors. A recent study showed that angiogenesis and VEGF play an important role in the recurrence and metastasis of HCC[12]. It was reported that the expression of VEGF is up-regulated in HCC and positively correlated with the recurrence and metastasis of HCC after operation[13].
Interferon (IFN) regulates the activity of cytokines which control cell function and replication, and inhibits the activity of tumor cells in many organs and tissues[14-16]. It has been shown that IFN-α decreases the sensitivity of vascular endothelial cells to VEGF and bFGF, as well as the vascularization of tumors[17].
The results of this study indicate that IFN-α-2b could significantly inhibit the growth of HCC in nude mice. The volume and weight of transplanted tumors decreased evidently in IFN-α-2b treatment groups. The expression levels of COX-2 and VEGF mRNA and protein as well as the AI increased significantly in IFN-α-2b treatment groups, indicating that the expression levels of COX-2 and VEGF are closely related with the growth of tumor and apoptosis of tumor cells. IFN-α-2b can down-regulate the expression levels of COX-2 and VEGF, but its mechanism remains unknown. Singer et al[18] reported that IFN-α exerts its effects through the NF-κB signal transduction pathway, and inhibitors of NF-κB can decrease the expression level of COX-2. A study about the effect of IFN-α on a signal transduction pathway showed that IFN-α could reduce the activity of NF-κB[19], suggesting that IFN-α-2b can decrease the activity of the NF-κB signal transduction pathway, inhibit the expression of COX-2 and the growth of HCC, and induce apoptosis. It has been shown that IFN-α can down-regulate the expression levels of angiogenesis factors, such as bFGF, VEGF, MMP-9 and IL-8, in different tumors of humans, then inhibit angiogenesis and the growth of tumors[20]. Tsujii et al[21] investigated the correlation between COX-2 and angiogenesis of colon carcinoma and found that colon carcinoma cells could secrete angiogenesis factors, such as VEGF, bFGF, TGF-β1 and PDGF, at a high concentration. Inhibitors of COX-2 can significantly depress the expression of angiogenesis factors, indicating that COX-2 can promote the growth of tumors, which is closely related with its effect on promoting angiogenesis. We hold that IFN-α-2b can inhibit HCC growth by inducing apoptosis of tumor cells through down-regulation of COX-2 expression and by inhibiting angiogenesis of tumors through down-regulation of VEGF expression. COX-2 may influence the expression of VEGF. The signal transduction between COX-2 and VEGF still needs to be further studied.
In this study, the inhibitory effect of IFN-α-2b on HCC growth in nude mice showed a dose-effect relationship. The effect of IFN-α-2b was greater in group B than in groups A and C. However, there was no significant difference between groups A and C. Huang et al[22] have reported a similar result in nude mice bearing carcinoma of prostate after treatment with pegylated IFN-α-2b (PEG-IFN-α-2b). At present,no common view is available on the dose of IFN α. Wang et al[23] investigated the treatment of nude mice bearing orthotopically transplanted HCC with large doses of IFN-α (3 × 105 U/d and 6 × 105 U/d) and showed that IFN-α could inhibit metastasis and angiogenesis of HCC at a certain degree. Because the signal transduction of IFN is mediated by the JAK/STAT pathway, SOSC protein could down-regulate the signals of IFN by blocking up the JAK/STAT pathway. Although studies are available on the activity of STAT3 inhibited by SOSC3[24-26], further study is needed to show the maximally tolerated dose of IFN-α and its correlation with the signal transduction of STAT1.
It has been shown that nonsteroidal anti-inflammatory drugs (NSAIDs) and INF-α have a synergistic effect on the HCC cell line HepG2. NSAIDs up-regulate the transcription of ISRE, increase the activity and inhibitory effect of IFN-α on tumors, thus providing a new treatment modality for HCC with IFN α[27].
In recent years, the inhibitory effect of interferon-α-2b (IFN-α-2b) on many tumors has been proved, but its effect on the expression of cyclooxygenase-2 (COX-2) and vascular endothelial growth factor (VEGF) in hepatocellular carcinoma (HCC) has rarely been reported.
HCC is one of the malignant tumors that can lead to death of patients, but the molecular mechanism of hepatocarcinogenesis remains poorly understood.
Studies have proved the inhibitory effects of interferon-α-2b (IFN-α-2b) on many kinds of tumors. However, the effects of IFN-α-2b on expression of cyclooxygenase-2 (COX-2) and vascular endothelial growth factor (VEGF) in hepatocellular carcinoma (HCC) have not been extensively studied. This study investigated the effects of IFN-α-2b on COX-2 and VEGF expression in human HCC implanted in nude mice and the underlying mechanism of its inhibitory effect on the growth of HCC.
This study investigated the effects of IFN-α-2b on COX-2 and VEGF expression in human HCC implanted in nude mice and the underlying mechanism of its inhibitory effect on the growth of HCC. The results show that IFN-α-2b can be used as an effective agent in the treatment of HCC.
The study is well designed. The results of this study are interesting and seem to be reliable.
| 1. | Chen CJ, Yu MW, Liaw YF. Epidemiological characteristics and risk factors of hepatocellular carcinoma. J Gastroenterol Hepatol. 1997;12:S294-S308. |
| 2. | Oshima M, Murai N, Kargman S, Arguello M, Luk P, Kwong E, Taketo MM, Evans JF. Chemoprevention of intestinal polyposis in the Apcdelta716 mouse by rofecoxib, a specific cyclooxygenase-2 inhibitor. Cancer Res. 2001;61:1733-1740. |
| 3. | Hussain T, Gupta S, Mukhtar H. Cyclooxygenase-2 and prostate carcinogenesis. Cancer Lett. 2003;191:125-135. |
| 4. | Peng JP, Su CY, Chang HC, Chai CY, Hung WC. Overexpression of cyclo-oxygenase 2 in squamous cell carcinoma of the hypopharynx. Hum Pathol. 2002;33:100-104. |
| 5. | Sung YK, Hwang SY, Kim JO, Bae HI, Kim JC, Kim MK. The correlation between cyclooxygenase-2 expression and hepatocellular carcinogenesis. Mol Cells. 2004;17:35-38. |
| 6. | Shiota G, Okubo M, Noumi T, Noguchi N, Oyama K, Takano Y, Yashima K, Kishimoto Y, Kawasaki H. Cyclooxygenase-2 expression in hepatocellular carcinoma. Hepatogastroenterology. 1999;46:407-412. |
| 7. | Koga H, Sakisaka S, Ohishi M, Kawaguchi T, Taniguchi E, Sasatomi K, Harada M, Kusaba T, Tanaka M, Kimura R. Expression of cyclooxygenase-2 in human hepatocellular carcinoma: relevance to tumor dedifferentiation. Hepatology. 1999;29:688-696. |
| 8. | Folkman J. Seminars in Medicine of the Beth Israel Hospital, Boston. Clinical applications of research on angiogenesis. N Engl J Med. 1995;333:1757-1763. |
| 9. | Fidler IJ, Ellis LM. The implications of angiogenesis for the biology and therapy of cancer metastasis. Cell. 1994;79:185-188. |
| 10. | Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353-364. |
| 11. | Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995;146:1029-1039. |
| 12. | Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242-248. |
| 13. | El-Assal ON, Yamanoi A, Soda Y, Yamaguchi M, Igarashi M, Yamamoto A, Nabika T, Nagasue N. Clinical significance of microvessel density and vascular endothelial growth factor expression in hepatocellular carcinoma and surrounding liver: possible involvement of vascular endothelial growth factor in the angiogenesis of cirrhotic liver. Hepatology. 1998;27:1554-1562. |
| 14. | Baron S, Dianzani F. The interferons: a biological system with therapeutic potential in viral infections. Antiviral Res. 1994;24:97-110. |
| 15. | Hertzog PJ, Hwang SY, Kola I. Role of interferons in the regulation of cell proliferation, differentiation, and development. Mol Reprod Dev. 1994;39:226-232. |
| 16. | Gutterman JU. Cytokine therapeutics: lessons from interferon alpha. Proc Natl Acad Sci USA. 1994;91:1198-1205. |
| 17. | Dinney CP, Bielenberg DR, Perrotte P, Reich R, Eve BY, Bucana CD, Fidler IJ. Inhibition of basic fibroblast growth factor expression, angiogenesis, and growth of human bladder carcinoma in mice by systemic interferon-alpha administration. Cancer Res. 1998;58:808-814. |
| 18. | Singer CA, Baker KJ, McCaffrey A, AuCoin DP, Dechert MA, Gerthoffer WT. p38 MAPK and NF-kappaB mediate COX-2 expression in human airway myocytes. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1087-L1098. |
| 19. | Manna SK, Mukhopadhyay A, Aggarwal BB. IFN-alpha suppresses activation of nuclear transcription factors NF-kappa B and activator protein 1 and potentiates TNF-induced apoptosis. J Immunol. 2000;165:4927-4934. |
| 20. | Slaton JW, Karashima T, Perrotte P, Inoue K, Kim SJ, Izawa J, Kedar D, McConkey DJ, Millikan R, Sweeney P. Treatment with low-dose interferon-alpha restores the balance between matrix metalloproteinase-9 and E-cadherin expression in human transitional cell carcinoma of the bladder. Clin Cancer Res. 2001;7:2840-2853. |
| 21. | Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93:705-716. |
| 22. | Huang SF, Kim SJ, Lee AT, Karashima T, Bucana C, Kedar D, Sweeney P, Mian B, Fan D, Shepherd D. Inhibition of growth and metastasis of orthotopic human prostate cancer in athymic mice by combination therapy with pegylated interferon-alpha-2b and docetaxel. Cancer Res. 2002;62:5720-5726. |
| 23. | Wang L, Wu WZ, Sun HC, Wu XF, Qin LX, Liu YK, Liu KD, Tang ZY. Mechanism of interferon alpha on inhibition of metastasis and angiogenesis of hepatocellular carcinoma after curative resection in nude mice. J Gastrointest Surg. 2003;7:587-594. |
| 25. | Alexander WS, Starr R, Fenner JE, Scott CL, Handman E, Sprigg NS, Corbin JE, Cornish AL, Darwiche R, Owczarek CM. SOCS1 is a critical inhibitor of interferon gamma signaling and prevents the potentially fatal neonatal actions of this cytokine. Cell. 1999;98:597-608. |
| 26. | Suzuki A, Hanada T, Mitsuyama K, Yoshida T, Kamizono S, Hoshino T, Kubo M, Yamashita A, Okabe M, Takeda K. CIS3/SOCS3/SSI3 plays a negative regulatory role in STAT3 activation and intestinal inflammation. J Exp Med. 2001;193:471-481. |
| 27. | Giambartolomei S, Artini M, Almerighi C, Moavero SM, Levrero M, Balsano C. Nonsteroidal anti-inflammatory drug metabolism potentiates interferon alfa signaling by increasing STAT1 phosphorylation. Hepatology. 1999;30:510-516. |
Peer reviewer: Trond Berg, Professor, Department of Molecular Biosciences, University of Oslo, PO Box 1041 Blindern, Oslo 0316, Norway
S- Editor Li JL L- Editor Wang XL E- Editor Zheng XM