Published online Nov 21, 2008. doi: 10.3748/wjg.14.6743
Revised: September 18, 2008
Accepted: September 25, 2008
Published online: November 21, 2008
AIM: To investigate the pathological characteristics of non-thermal damage induced by pulsed high intensity focused ultrasound (PHIFU) combined with ultrasound contrast agent (UCA), SonoVue (Bracco SpA, Milan, Italy) in rabbit liver VX2 tumor.
METHODS: Liver VX2 tumor models were established in 20 rabbits, which were divided randomly into PHIFU combined with ultrasound contrast agent group (PHIFU + UCA group) and sham group. In the PHIFU + UCA group, 0.2 mL of SonoVue was injected intravenously into the tumor, followed by ultrasound exposure of ISP 5900 W/cm2. The rabbits were sacrificed one day after ultrasound exposure. Specimens of the exposed tumor tissues were obtained and observed pathologically under light microscope and transmission electron microscope. The remaining tumor tissues were sent for 2,3,5-Triphenyltetrazolium chloride (TTC) staining.
RESULTS: Before TTC staining, tumor tissues in both the sham and the PHIFU + UCA groups resembled gray fish meat. After TTC staining, the tumor tissues were uniformly stained red, with a clear boundary between tumor tissue and normal tissue. Histological examination showed signs of tumor cell injury in PHIFU + UCA group, with cytoplasmic vacuoles of various sizes, chromatin margination and karyopyknosis. Electron microscopic examination revealed tumor cell volume reduction, karyopyknosis, chromatin margination, intercellular space widening, the presence of high electron-density apoptotic bodies and vacuoles in cytoplasm.
CONCLUSION: The non-thermal effects of PHIFU combined with UCA can be used to ablate rabbit liver VX2 tumors.
- Citation: Zhou CW, Li FQ, Qin Y, Liu CM, Zheng XL, Wang ZB. Non-thermal ablation of rabbit liver VX2 tumor by pulsed high intensity focused ultrasound with ultrasound contrast agent: Pathological characteristics. World J Gastroenterol 2008; 14(43): 6743-6747
- URL: https://www.wjgnet.com/1007-9327/full/v14/i43/6743.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.6743
High-intensity focused ultrasound (HIFU) is a rapidly developing non-invasive technology for tumor treatment. In recent years it has been widely used for treating a variety of solid tumors, such as liver tumor, bone tumor, and breast cancer. Mechanism for therapeutic actions of HIFU includes thermal effects and non-thermal effects with the latter dominated by cavitational effects. Thermal effects and cavitational effects work together to produce coagulative necrosis in targeted tumor and thus therapeutic purpose is achieved[1]. Glynn et al[2] have reported that cavitational effects of HIFU can be completed in one acoustic cycle, indicating that in the process of coagulative necrosis, cavitational effects precede thermal effects. Furthermore, cavitational effects will inhibit cell growth, increase membrane permeability, damage cellular DNA, promote cell apoptosis and inhibit cell proliferation[3]. Therefore, efforts to reach treatment goals solely by non-thermal effects dominated by cavitational effects have become very meaningful.
Adjusting acoustic parameters of pulsed high intensity focused ultrasound (PHIFU) can control thermal effects and non-thermal effects; short duty cycle and high intensity favor the occurrence of cavitation[4]. Roberts et al[5] used mechanical and cavitational effects generated by pulse focused ultrasound to ablate rabbit kidney and they found that stable and predictable morphological lesions can be achieved by controlling certain parameters. Hall et al[6] used cavitational effects of PHIFU to ablate tissues (histotripsy), and concluded that cavitation causes orderly and predictable histologic changes. Our preliminary study results show that bovine liver can be ablated non-thermally by PHIFU using the following parameters: frequency 0.87 MHz, spatial peak intensity ISP 5900 W/cm2, pulse repetition frequency 100 Hz, and duty cycle 15%[7]. Considering blood circulation, in vivo tissues should be less susceptible to heating under same experimental conditions and acoustic parameters. It has been reported that ultrasound contrast agent (UCA) can enhance cavitational effects[8]. Therefore, we combined PHIFU and UCA to produce non-thermal damage in rabbit liver VX2 tumor in vivo, and investigated the pathological characteristics of the non-thermal damage.
Twenty New Zealand white rabbits of either gender, 3-4 mo old and weighing 2.0 ± 0.16 kg were used in this study. Animals were fasted 24 h before experiments; abdomen was shaved using 10% Na2S; all rabbits were anesthetized by intramuscular injection of 0.1 mL/kg Sumianxin (Institute of Veterinary Medicine, Agriculture and Ranching University of Changchun, China). One block (bout 1 cm3) of active tumor tissues was taken from the rabbit inoculated with VX2 tumor (VX2 squamous carcinoma cell line was offered by Funabashi Farm Company in Kyoto, Japan), washed with normal saline, and then subdivided into small tissue pieces about 1 mm3. The 20 rabbits were fixed in prone position and routinely disinfected. A median incision was made below the Xiphoid process to expose the right lobe of liver, where a hole about 1-2 cm deep was made using ophthalmological forceps; then 2 ready tissue pieces were implanted into each hole. Bleeding points were stopped with gelatin sponge and then the abdominal wall was sutured. Skin incision was disinfected; 1 mL Suxingling was injected intramuscularly. After tumor inoculation, the rabbits were fed for 2-3 wk and then used in this study.
The experiment was approved ethically and scientifically by our university and complied with Practice for Laboratory Animals in China.
SonoVue® (Bracco Company, Italy) was used. After the plastic cap of the vial was removed, 5 mL sterile normal saline was added into the vial. The vial was shaken vigorously for about 20 s before the milky white suspension was ready.
Model JC High Intensity Focused Ultrasound Tumor Therapeutic System [Chongqing Haifu (HIFU) Technology Co. Ltd., China] was used in this study and has been described in detail previously[9]. HIFU parameters used were as follows: ultrasound frequency 0.87 MHz, focal length 150 mm, ISP 5900 W/cm2, pulse repetition frequency 100 Hz, and duty cycle 15%.
Experiment started on the 15th day after VX2 tumor inoculation. The rabbit abdomen was shaved with 10% Na2S one day before experiment. They were anaesthetized by intraperitoneal injection of 3% pentobarbital sodium solution (30 mg/kg body weight) 10 min prior to the experiment and then fixed in prone position on the treatment bed. The 20 rabbits were randomly divided into sham and PHIFU + UCA groups, with 10 rabbits in each group. In the sham group, the target tumor was localized under the assistance of B-mode ultrasonography; after the power generator was turned off, the whole target tumor was irradiated for 90 s. In the PHIFU + UCA group, after the tumor was localized using B-mode ultrasonography, 0.2 mL SonoVue was injected rapidly via ear marginal veins, followed by rapid injection of 1 mL normal saline. Fifteen seconds after the injection, the tumors were exposed to HIFU for 90 s.
Twenty-four hours after the HIFU exposure, rabbits in both groups were killed using excessive anesthetics. The abdomen was opened to excise the liver. Multiple-spot sampling was done from the tumor center to the tumor periphery. One mm3 tumor piece was sampled, double-fixed by glutaraldehyde and osmium acid, embedded in epoxy resin, sectioned ultra-thinly and ultra-structural changes of the targeted tissues were observed under transmission electron microscope. Another 1 mm3 tumor piece was HE stained and observed under light microscope. The remaining tumor tissues were stained at 37°C TTC solution for 15 min and observed grossly.
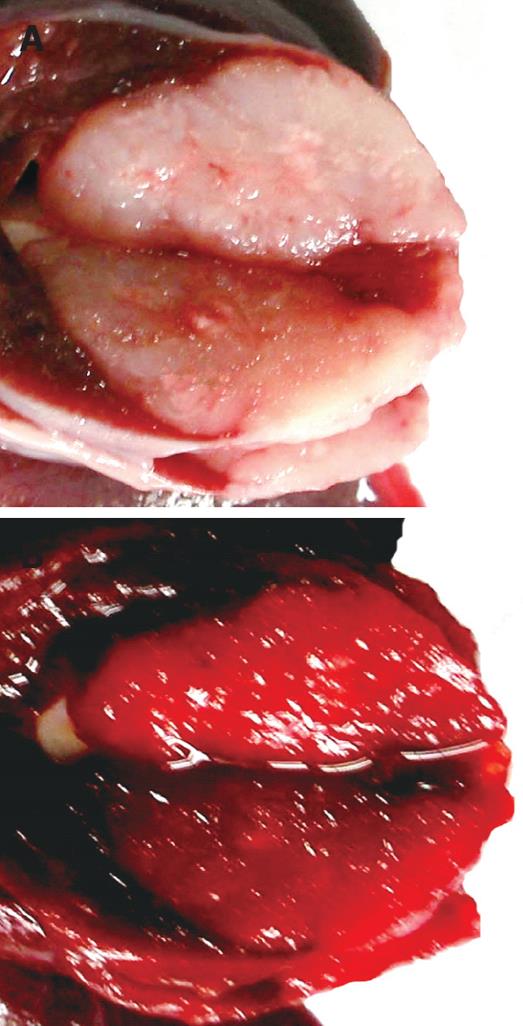
VX2 tumor model was successfully established in all the rabbits. Gross observation found that the implanted tumor was located within hepatic parenchyma, and they were spherical, elliptical or nodular shaped. The tumors resembled the appearance of gray fish meat and the cells were non-encapsulated. There was a clear demarcation between the tumor and the normal surrounding tissues. Before TTC staining, tumor tissues in PHIFU + UCA group resembled gray fish meat and they were demarcated clearly from surrounding normal liver tissues with a sharp boundary (Figure 1A). After the TTC staining the tumor tissues were uniformly stained red without any unstained regions. There was a clear demarcation between the tumor and the normal tissues (Figure 1B).
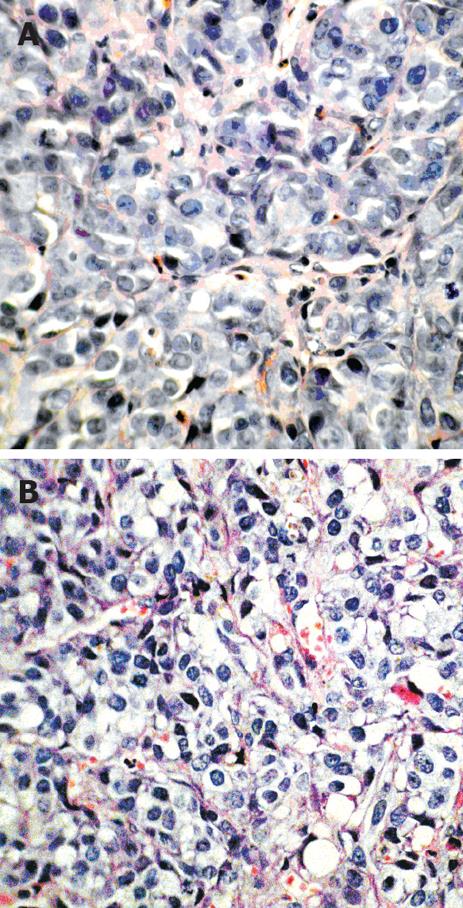
Sham group: Under low power lens, VX2 tumor cells in the sham group appeared mass-flake like or an invasive cancer nest, with reduced connective tissues and an unclear demarcation between the tumor and mesenchymal cells. Under high power lens, tumor cells were large, irregularly arranged with an irregular morphology. The nuclei were large and deeply stained, with great karyoplasmic ratio and increased mitosis (Figure 2A).
PHIFU + UCA group: Tumor cytoplasm in all 10 rabbits was lightly stained, with cytoplasmic vacuoles of various sizes, chromatin margination and karyopyknosis (Figure 2B).
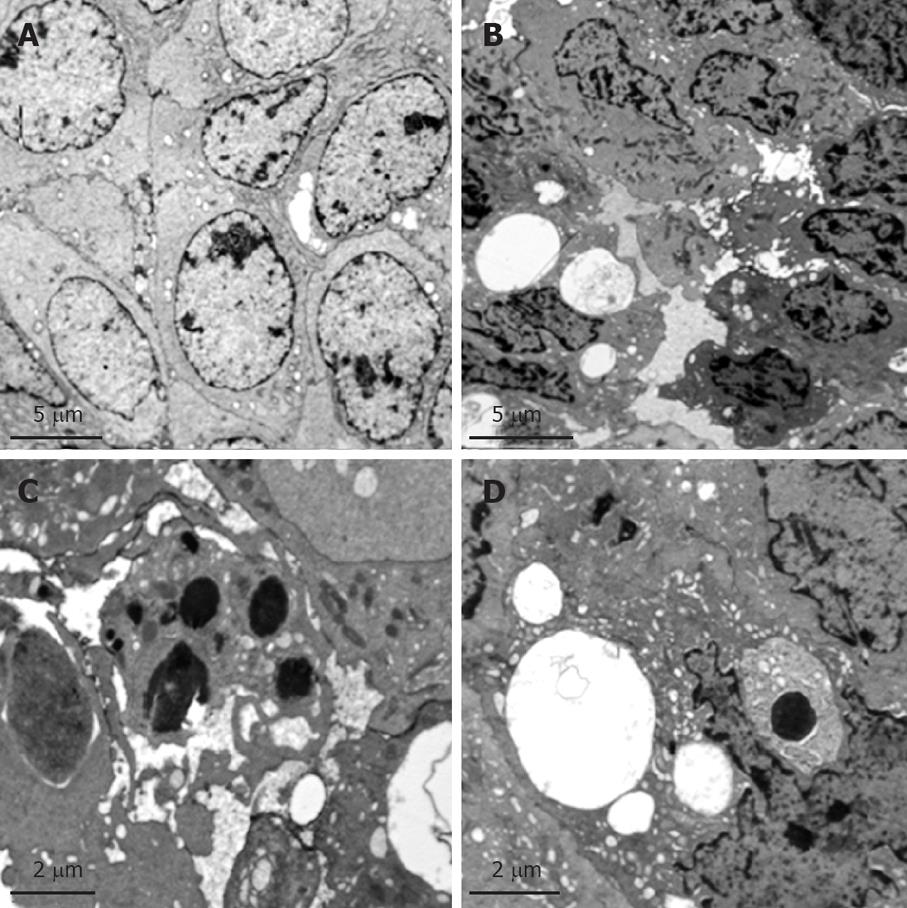
Sham group: Tumor cells in the sham group appeared polymorphic or spindle-shaped of various sizes and with signs of mitosis. The nuclei were large, deformed, with clear nuclear membranes and rich euchromatin. The chromatin particles were large with intranuclear pseudo-inclusions and there were multiple visible nucleoli (Figure 3A).
PHIFU + UCA group: Tumor cells were reduced in size and in some tumor cells, karyopyknosis was revealed. These tumors showed chromatin margination, intercellular space widening (Figure 3B), the presence of high electron-density apoptotic bodies (Figure 3C) and various vacuoles of different sizes in the cytoplasm (Figure 3D).
In recent years, the use of microwave, radio frequency, laser and ultrasound as thermal source to ablate solid tumors has been applied in clinical use[10]. Among these treatments, image-guided high-intensity focused ultrasound (HIFU) in situ ablation of solid tumors is believed to be the most promising non-invasive modality. Ultrasound beams from an extracorporeal source can propagate through overlying tissues and be brought to a tight focus in the target tissue. The temperature at focus will elevate to 65°C instantly (0.5-5 s), causing irreversible coagulative necrosis which is grossly visible and histopathologically confirmed. Coagulative necrosis is induced through a combination of thermal and cavitational effects[1]. Some researchers believe that the thermal ablation mechanism has shortcomings. Due to the individual diversity of tissue characteristics and blood perfusion, the size and morphology of thermal lesion cannot be accurately predicted. The local thermal effects may also damage the adjacent tissues and it is possible that uneven heating in the target region will cause leaping injury[11]. In order to avoid the defects of thermal effects, non-thermal effects of ultrasound may offer an alternative.
When acoustic intensity in the target tissue is higher than 1500 W/cm2, non-thermal effects (mechanical and cavitational effects) will occur. These include forming non-thermal lesion[12], damaging DNA, promoting cell apoptosis, inhibiting cell proliferation[13] and even causing histotripsy[14]. Thermal and non-thermal effects can be effectively controlled by adjusting exposure parameters of PHIFU; short duty cycle and high intensity favor the occurrence of cavitation[4]. PHIFU parameters used in this study, ultrasound frequency 0.87 MHz, ISP 5900 W/cm2, pulse repetition frequency 100 Hz, and duty cycle 15%, have been demonstrated previously to be able to induce non-thermal damage in bovine liver[7]. These parameters are also consistent with non-thermal parameters reported elsewhere[12].
TTC staining assesses dehydrogenase activity and is a staining method for gross specimens. The main principle is that in the presence of NADH, active dehydrogenase in viable tissues will reduce colorless oxidative TTC to red reductive TTC; hence viable tissue can be stained red while necrotic tissue can not. HIFU causes instant temperature rise to above 65°C at focus; at such a high temperature, dehydrogenase is deactivated, hence tissues within the target region cannot be stained. Based on this principle, Chang Shufang et al[15] applied TTC staining to observe HIFU-induced coagulative necrosis grossly. In one of our preliminary studies, the same parameters were used to form non-thermal damage in vitro bovine liver tissues[7]. Considering the cooling effect of blood circulation in vivo animals, it is inferred that temperature elevation under same exposure parameters should be lower in this study. In this study, we found that tissues in PHIFU + UCA group were stained red by TTC, indicating that focal temperature was not high enough to deactivate dehydrogenase, which further proved that temperature in the targeted tissues did not elevate to the level required for formation of thermal coagulation.
Lesions caused by non-thermal effects have characteristic pathological changes quite different from those of thermal lesions. Ashush et al[16] observed the following morphological changes after HIFU cavitation: cell shrinkage, vacuole formation, chromatin condensation, karyorrhexis and the formation of apoptotic bodies. Kieran et al[11] studied non-thermal lesion by changing ultrasound intensity and duty cycle. Their histological observation showed that within certain intensity and duty cycle, vacuoles were formed in the cells, with blanched and dense liquid inside the vacuoles. This study found that PHIFU in combination with UCA can intensify non-thermal effects of HIFU and thus achieve thermal ablation. Light microscopy displayed abundant vacuoles of various sizes in the cytoplasm and chromatin margination and karyopyknosis in some cells. Electron microscopic examination revealed presence of karyopyknosis and chromatin margination in some cells, intercellular space widening, the presence of high electron-density apoptotic bodies and many vacuoles of various sizes in the cytoplasm. These results are consistent with previous reports, indicating that by combining PHIFU with UCA, non-thermal effects can be intensified to achieve non-thermal tumor ablation. Immunohistochemical detection found that PHIFU combined with UCA can promote tumor cell apoptosis and inhibit tumor cell proliferation, which will be reported in another article.
Future studies are needed in many aspects, such as cavitation detection, temperature monitoring and other means to detect non-thermal effects; how to optimize combination between UCA and HIFU exposure parameters; means to control and monitor cavitational lesions; long-term outcomes of non-thermal tumor ablation.
Image-guided high-intensity focused ultrasound (HIFU) in situ ablation of solid tumors is believed to be a promising non-invasive modality. Ultrasound beams from an extracorporeal source can propagate through overlying tissues and be brought to a tight focus in the target tissue. The temperature at focus will elevate to 65°C instantly (0.5-5 s), causing irreversible coagulative necrosis. Mechanism for therapeutic actions of HIFU includes thermal effects and non-thermal effects with the latter dominated by cavitational effects. Due to the individual diversity of tissue characteristics and blood perfusion, the size and morphology of thermal lesion cannot be accurately predicted. The local thermal effects may also damage the adjacent tissues and it is possible that uneven heating in the target region will cause leaping injury. In order to avoid the defects of thermal effects, non-thermal effects of ultrasound may offer an alternative.
Adjusting acoustic parameters of pulsed high intensity focused ultrasound (PHIFU) can control thermal effects and non-thermal effects; short duty cycle and high intensity favor the occurrence of cavitation. Ultrasound contrast agent (UCA) can enhance cavitation effects. Lesions caused by non-thermal effects have characteristic pathological changes quite different from those of thermal lesions. Therefore, the authors combined PHIFU and UCA to produce non-thermal damage in rabbit liver VX2 tumor in vivo, and investigated the pathological characteristics of the non-thermal damage.
Non-thermal damage for tumor is the modality different from thermal damage. Previous studies showed that HIFU could ablate tumors by thermal effects. Short duty cycle and high intensity of PHIFU favor the occurrence of cavitation, and ultrasound contrast agent can enhance cavitation effects. The present study used PHIFU with short duty cycle and high intensity combined with UCA to damage rabbit liver VX2 tumor by non-thermal effect. Histopathology was used to evaluate the result.
The present study showed that the non-thermal effects of PHIFU combined with UCA could be used to ablate rabbit liver VX2 tumors. And this study would help promote a new scheme of therapy for tumors.
This is an interesting investigation, demonstrating the effect of intensity focused ultrasound combined with ultrasound contrast agent on liver metastases.
| 1. | Kennedy JE. High-intensity focused ultrasound in the treatment of solid tumours. Nat Rev Cancer. 2005;5:321-327. |
| 2. | Glynn HR, Ronald AR, Patrick AE, Yang XM. Bubbles and HIFU: the good, the bad, and the ugly. 2nd International Symposium on Therapeutic Ultrasound; 2002 July 29-August 1, Seattle, USA. Washington. 2002;120-131. |
| 3. | Feril LB Jr, Kondo T, Zhao QL, Ogawa R, Tachibana K, Kudo N, Fujimoto S, Nakamura S. Enhancement of ultrasound-induced apoptosis and cell lysis by echo-contrast agents. Ultrasound Med Biol. 2003;29:331-337. |
| 4. | Kieran K, Hall TL, Parsons JE, Wolf JS, Fowlkes JB, Cain CA, Roberts WW. Exploring the acoustic parameter space in ultrasound therapy: defining the threshold for cavitational effects. 6th International Symposium on Therapeutic Ultrasound; 2006 Aug 30-Sep 2; Oxford, UK. 2006;185-190. |
| 5. | Roberts WW, Hall TL, Ives K, Wolf JS Jr, Fowlkes JB, Cain CA. Pulsed cavitational ultrasound: a noninvasive technology for controlled tissue ablation (histotripsy) in the rabbit kidney. J Urol. 2006;175:734-738. |
| 6. | Hall TL, Kieran K, Fowlkes JB, Cain CA, Roberts WW. Temporal trends in the histology of the rabbit kidney after cavitational tissue ablation. 6th International Symposium on Therapeutic Ultrasound;. 2006;191-197. |
| 7. | Liu CM, Chen JY, Ke D, Xu J, Lei L, Li FQ, Wang ZB. Duty cycle affect on in vitro bovine liver treated with pulsed high intensity focused ultrasound. Zhongguo Chaosheng Yixue Zazhi. 2007;23:567-570. |
| 8. | Luo W, Zhou X, Tian X, Ren X, Zheng M, Gu K, He G. Enhancement of ultrasound contrast agent in high-intensity focused ultrasound ablation. Adv Ther. 2006;23:861-868. |
| 9. | Wu F, Wang ZB, Zhu H, Chen WZ, Zou JZ, Bai J, Li KQ, Jin CB, Xie FL, Su HB. Feasibility of US-guided high-intensity focused ultrasound treatment in patients with advanced pancreatic cancer: initial experience. Radiology. 2005;236:1034-1040. |
| 10. | Goldberg SN, Gazelle GS, Mueller PR. Thermal ablation therapy for focal malignancy: a unified approach to underlying principles, techniques, and diagnostic imaging guidance. AJR Am J Roentgenol. 2000;174:323-331. |
| 11. | Kieran K, Hall TL, Parsons JE, Wolf JS Jr, Fowlkes JB, Cain CA, Roberts WW. Refining histotripsy: defining the parameter space for the creation of nonthermal lesions with high intensity, pulsed focused ultrasound of the in vitro kidney. J Urol. 2007;178:672-676. |
| 12. | Parsons JE, Cain CA, Abrams GD, Fowlkes JB. Pulsed cavitational ultrasound therapy for controlled tissue homogenization. Ultrasound Med Biol. 2006;32:115-129. |
| 13. | Tran BC, Seo J, Hall TL, Fowlkes JB, Cain CA. Microbubble-enhanced cavitation for noninvasive ultrasound surgery. IEEE Trans Ultrason Ferroelectr Freq Control. 2003;50:1296-1304. |
| 14. | Xu Z, Hall TL, Fowlkes JB, Cain CA. Effects of acoustic parameters on bubble cloud dynamics in ultrasound tissue erosion (histotripsy). J Acoust Soc Am. 2007;122:229-236. |
| 15. | Chang SF, Gu ML, Wu F, Bai J, Zou JZ, Zhu SY, Li CY, Hu K, Du YH, Li Y. The Application of Triphenyl Tetrazolium Choloride Staining in the Observing of Biological Focal Field of High Intensity Ultrasound. Zhongguo Chaosheng Yixue Zazhi. 2000;16:641-644. |
| 16. | Ashush H, Rozenszajn LA, Blass M, Barda-Saad M, Azimov D, Radnay J, Zipori D, Rosenschein U. Apoptosis induction of human myeloid leukemic cells by ultrasound exposure. Cancer Res. 2000;60:1014-1020. |
Peer reviewer: Mauro Bernardi, Professor, Internal Medicine, Cardioangiology, Hepatology, University of Bologna, Semeiotica Medica-Policlinico S. Orsola-Malpighi-Via, Massarenti 9, Bologna 40138, Italy
S- Editor Zhong XY L- Editor Ma JY E- Editor Ma WH