INTRODUCTION
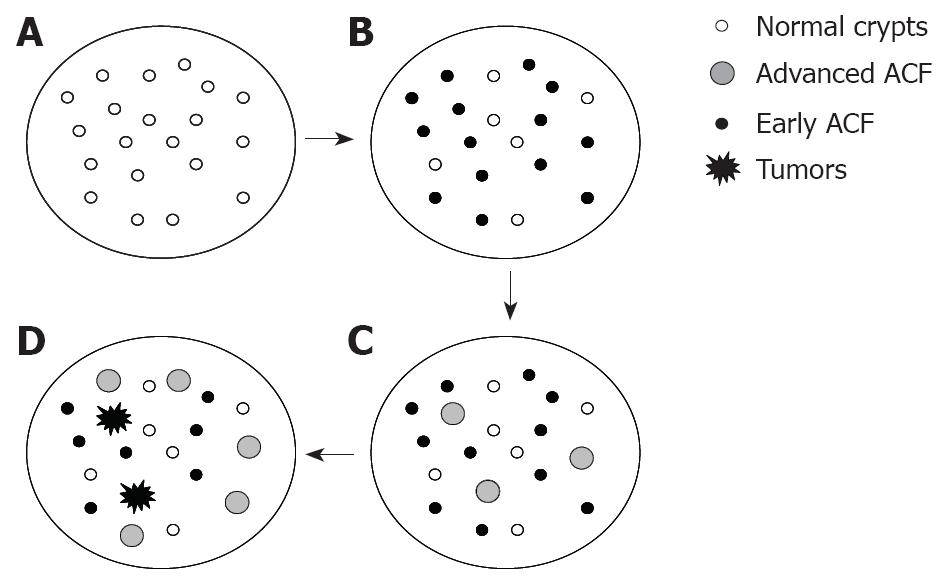
Figure 1 A pictorial representation of the dynamics of ACF during azoxymethane-induced rat colon carcinogenesis.
A: Colon with normal crypts prior to AOM injection; B: Colon bearing early ACF at approximately 8-wk post AOM injections; C: Colon bearing early and advanced ACF at approximately 12-wk post AOM injections; D: Colon bearing early and advanced ACF and tumors at approximately 24-wk post AOM injections. As the population of ACF (inclusive of all sub-types) increase with time after AOM injections, a relative decline in the specificity as a biological marker is observed.
Aberrant crypt foci (ACF) have been identified and defined as putative precancerous lesions of the colon in both experimental models and humans[1-4]. In animal models, ACF as a biological marker have been functional in identifying both naturally-occurring and synthetically-derived compounds, as well as foods and nutritive agents for their ability to prevent or control the process of colon carcinogenesis[5-7]. On the other hand, they have also been vital in understanding toxic effects of various compounds[8]. The use of the rodent ACF as a biomarker has been successful in chemoprevention studies mainly due to the fact that they are: (a) preneoplastic lesions; (b) morphologically distinguishable from normal crypts; (c) induced in colons of several animal models; (d) remarkable similarity between human and rodents; (e) rapidly detectable; (f) easily modified by genetic changes or drug/dietary interventions; (g) amenable in short term experimental modules (8 wk); (h) classifiable according to their progression; and (i) generally present in larger populations. Whether addressing aspects of cancer prevention/control or toxicity, studies have essentially focused on the ability to modulate the incidence of total ACF or incidence of ACF displaying advanced biological features related to size, dysplasia and crypt multiplicity. It has been widely accepted that the incidence of ACF in rodents correlates strongly with the final tumor outcome[9,10]. Several factors such as dose of carcinogen, route of administration, time of intervention during the carcinogenic state, age and sex of the animals, location and appearance of lesions in the colon, as well as the choice of animal models play a critical role in understanding the modulation of ACF, tumor outcomes, and their incidental correlation[11].
For colon cancer chemoprevention studies, the ability of compounds to regress or inhibit the incidence of total or subtypes of ACF has been utilized using either short-term (6-8 wk) or long-term (12-54 wk) carcinogen-injected rodent studies. While interpreting data arising from these studies, especially outcome of ACF and their sub-types as well as tumors, two points need special consideration. Firstly, it has to be understood that ACF, which are dynamic in nature, are amenable to growth and expansion, and may differ in number and growth features at different end-points. Secondly, in the course of their dynamics, ACF undergo several genetic changes, plausibly acquire novel phenotypes conducive to their progression, and discard those that render them normal or make them susceptible to regression[12,13]. Detailed review of the molecular features of ACF can be found in the papers by Cheng and Lai[12] and Alrawi et al[14], and recent advances in human ACF and their potential as biomarkers of human colorectal cancer have been elegantly discussed by Orlando et al[15], Stevens et al[16] and Gupta et al[17]. Depending on their growth/developmental stage, ACF are heterogeneous in their molecular make-up[12-14]. The growth and development of ACF on one hand could be regressed or inhibited, while on the other augmented by various compounds; both depend on the ACF dynamics as well as their heterogeneity[7].
ACF DYNAMICS AND CHEMOPREVENTION
In screening compounds for their efficacy to prevent or control colon cancer, the popular choices of in vivo models are male rats of F344, Sprague-Dawley or Wistar strains that are approximately 5-6 wk old, at least 180-200 g in weight, and injected s.c. with two weekly doses of AOM at 15 mg/kg body[5,11]. In such protocols, the test compounds are added to the diet either at the time of the first AOM injection, or after a period of 4-8 wk. While in the former protocol, the emphasis is to determine the efficacy to prevent the appearance and growth of ACF at the initiation and post-initiation stages; the latter focuses on changes in ACF that have been established for a given period at the post-initiation and promotion stages[7]. In these models, two week after the second injection, the colonic epithelial cells are initiated, and a huge population of ACF that are primal (1-3 crypts per foci) in appearance are observed in the colons[13,18]. With time (promotion stages), the cells of the aberrant crypt within the same foci clonally grow and expand to morphologically distinctive larger aberrant crypts, or those that could be classified as either intermediate (foci with 4-6 crypts) or advanced lesions. These changes are generally seen 12-wk post-carcinogen injection. With time, a decline in the incidence of primal lesions and the gradual increase in the incidence of other ACF types such as large, intermediate or advanced are noted. Towards and beyond 24 wk post-carcinogen injection which can be categorized as the progression stage, the incidence of advanced lesions reaches a peak and the appearance of microadenomas, adenomas and adenocarcinomas is evident. Another important criterion for understanding the early stages of colon carcinogenesis is the differential growth dynamics of ACF in different regions of the colon[19,20]. Spatial distribution of ACF differs among the proximal, mid and distal sections of the colon of AOM-injected F344 rats, with more incidence of ACF in the mid sections compared to the distal colon[20]. In understanding the ability of compounds to modulate the incidence of ACF and their subtypes, several comparisons can be made. For instance, the incidence of ACF or their subtypes could be compared between an experimentally-treated group and the controls (placebo, vehicle-treated) at a single time point. Otherwise, a comparison between time-frames within a single treatment group could be made. Comparisons of ACF incidence made between different colonic sections, but within same time point or same treatment group would give regional differences in modulation. An ideal method would be detailed simultaneous comparisons between time-points, treatment groups and colonic regions on the incidence of ACF and subtypes to give a cross-sectional review of the data. Similarly, the incidences of adenomas and adenocarcinomas can be compared. The entire data such as the incidences of ACF and their subtypes, adenomas and adenocarcinomas have to be considered holistically before making conclusions on the modulating efficacy of a compound.
HETEROGENEITY OF ACF AND CHEMOPREVENTION
The initiation of a single epithelial cell within the crypt to undergo histogenetic changes and gradually proliferate to form morphologically distinct foci is the first and foremost stage in the genesis of ACF in the colon. Monoclonality in ACF, including those without dysplasia, put ACF at the earliest identifiable stage of this carcinogenic process[21]. These colon precancerous lesions progressively advance by acquiring novel genotypes and phenotypes segregating these lesions into different histological and morphological sub-types identified by: (a) size, (b) degree of dysplasia, (c) crypt multiplicity, and (d) a combination of dysplasia and crypt multiplicity. Segregation of ACF into sub-types is vital to understand the disease process and to delineate the novel changes exhibited at the microscopic level in conjunction with the given appearance and characteristics of tumors. As ACF progress from one stage to another, epithelial cells within the ACF undergo genetic changes that lead to their radical behavior to skip growth inhibition or apoptosis and favor proliferation and growth[11,12]. Their goal seems to be achieving a “neoplastic bliss”. Those molecular features that result in neoplastic transformation appear sequentially, and are due to a complex interplay/crosstalk of several signal transduction pathways. Purported chemopreventive agents retard or cease the growth and/or inhibit the development of ACF or their subtypes. Compounds are known to inhibit preneoplastic changes by blocking key molecular pathways in the colonic epithelial cells. As a result, either the progression within the preneoplastic stages, or the conversion of preneoplasia to neoplastic transformation is halted. The response of a single crypt within an ACF with multiple crypts (those with four or more crypts per foci) may differ in the modulatory influence of a compound. In such a case (within multicrypt foci), although all the crypts are proposed to have developed by clonal expansion of a single transformed cell, each crypt plausibly branches itself from others by acquiring differential genotypic changes, influenced largely by the environment. The rate at which the changes are acquired by different crypts within a focus may differ considerably owing to a biological heterogenous subtype within the ACF. Hence, within one ACF, each crypt may succumb differently to growth inhibition by a particular compound. However, signals from those crypt cells that undergo inhibition may be transferred to cells that may be avoiding the inhibitory function of the compound, making the latter initiate growth inhibition. Eventually, a complex dialogue within a single ACF is created, and the crypt eventually succumbs to the inhibitory actions of the compound. Genetic and molecular heterogeneity of crypts can also be evident as histological differences such as hyperplastic and dysplastic crypts[12].
LIMITATIONS OF THE AOM-INDUCED RAT ACF AS A BIOMARKER
Limitations to the use of ACF and their sub-types as a biomarker to identify cancer preventive agents is compromised as the disease progresses from early to late stages. Increased biological segregation from normal crypts increases the specificity to the diseased state but the ability of early lesions, such as the ACF to predict the differences between two or more experimental groups are limited by the fact that their number also declines. With time, ACF undergo the selection process; while some regress, remodel or even get eliminated; others progress forward to the next stage. For example as shown in Figure 1, consider a pool of AOM-injected rat colonic ACF with different sub-types at varied duration (8, 12 and 24 wk post-initiation). In 8 wk, ACF with 1, 2 or 3 crypts per foci appear, and at this time point the incidence of total ACF (irrespective of their crypt multiplicity) becomes a valid biomarker. In subsequent weeks, ACF with higher crypt multiplicities or those with advanced biological features appear, while those with lower crypt multiplicities regress. So, at this time point, incidences of higher crypt multiplicities or those displaying advanced features could be considered more specific. In more advanced stages of the carcinogenic process, the value or sensitivity of ACF as a biomarker to predict tumor outcome diminishes; however, their specificity as an indictor of the stage remains uncompromised. The use of ACF and its sub-types as biomarkers thus depend much on the stage of colon carcinogenesis. Only at early stages, total number of ACF and ACF displaying different biological features may be considered as a valid biomarker. At very early stages the relevance of utilizing a parameter involving advanced lesions such as aberrant crypts per ACF (AC/ACF) becomes invalid, as at early stages to begin with there may not be a significant pool of ACF with varied features. The description as ‘very early’, ‘early’ and ‘advanced’ stages of colon carcinogenesis have been used cautiously and have considered the AOM-induced rats as the model system (described in the former Section).
ACF with severe dysplasia have been identified as the actual precursors of colonic adenomas and adenocarcinomas[22]. The recent findings of the presence of β-catenin accumulated aberrant crypts (BCAC)[23], mucin-depleted crypts (MDF)[24], and flat-dysplastic ACF (FDACF)[25] in carcinogen-treated rat colons are significant advances in the identification of events leading to tumor development. The morphological features of these lesions resemble those of advanced ACF, as described earlier[10,13,14]; strongly suggesting that they plausibly are subtypes of advanced ACF. However, it is still unclear as to the exact evolutionary sequence of BCAC, MDF or FDACF, and their relevance to the human situation. These specific ACF-subtypes are purported to accurately predict tumor outcome[20-22]. However using them as valid biomarkers to screen chemicals/agents for potential colon cancer chemoprevention requires further scrutiny. Indeed, the simplistic methylene blue staining method in assessing ACF in whole mounts of colon remains a valuable tool in screening compounds for their colon cancer chemopreventive potential.
CONCLUSION
ACF are unique biological markers for the purpose of assessing potential colon cancer chemopreventive efficacy of compounds during the early stages of colon carcinogenesis. Interpreting the ability of compounds to retard or inhibit the incidence of ACF is becoming evidently complex. Sometimes, ACF data seem not to correlate with that of tumor outcome; in such cases, a single variable such as ACF incidence at a given time point to that of tumor outcome may have to be taken for correlative consideration. Incorporation of the cross-sectional criteria described in the earlier sections may facilitate a realistic correlation between the incidences of ACF and tumor outcome. Thus, in interpreting ACF data, their dynamics and heterogeneity need to be considered in order to formulate stronger correlations to tumor outcomes. A recent trend in the identification of ACF subtypes are certainly useful in understanding their biology, but their use as biomarkers in efficacy studies requires caution, especially when the relation between their specificity and predictability at a given time of the carcinogenic process is taken in to account. More studies are encouraged to identify the molecular repertoire of ACF that categorize them as preneoplastic lesions of the colon. Abrogating the growth and development of preneoplastic lesions emphasizes an early strategy on cancer prevention or control. Defining the role of ACF in colon carcinogenesis is vital to understand the relevance as a biological marker in the study of chemopreventive agents. The short-term AOM-induced rat ACF thus remains an ideal early biomarker for the identification of various chemopreventive agents against colon cancer.
Peer reviewer: Peter L Lakatos, MD, PhD, Assistant Professor, 1st Department of Medicine, Semmelweis University, Koranyi S 2A, Budapest H1083, Hungary
S- Editor Li LF L- Editor O'Neill M E- Editor Lin YP