INTRODUCTION
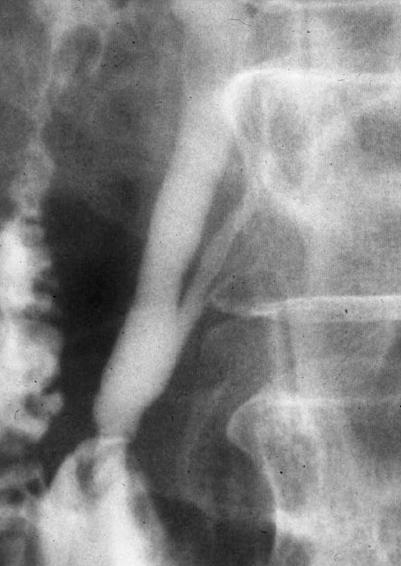
Figure 1 Endoscopic retrograde cholangiopancreatography of a patient with PBM shows a long common channel.
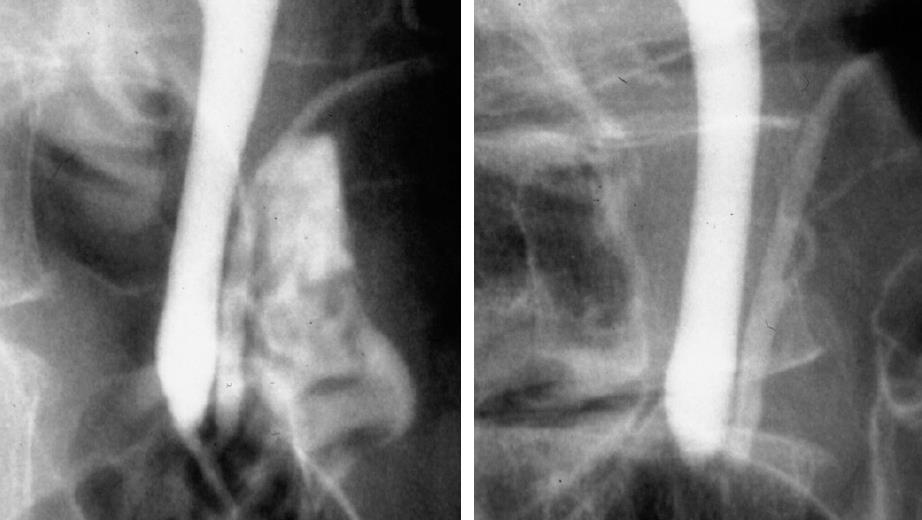
Figure 2 HCPBD.
Cholangiopancreatogram of a patient with high confluence of pancreaticobiliary ducts and a common channel of 9 mm in length (left). The communication between pancreatic and bile ducts was destroyed with sphincter contraction (right).
The main pancreatic duct and the common bile duct open into the duodenum either separately or via a common channel. The incidence of common channel formation ranges from 55%[1] to 82%[2,3]. Dowdy et al[4] have reported that the length of the common channel ranged from 1 mm to 12 mm, with an average length of 4.4 mm. The sphincter of Oddi, which is composed of the sphincter choledochus, the sphincter pancreaticus and the sphincter ampullae, is located at the distal end of the pancreatic and bile ducts and regulates the outflow of bile and pancreatic juice[2,3]. A common channel can be so long that the junction of the pancreatic and bile ducts is located outside of the duodenal wall, as occurs in pancreaticobiliary maljunction (PBM); in such cases, sphincter action does not functionally affect the junction, resulting in two-way regurgitation (biliopancreatic reflux: regurgitation of bile juice into the pancreatic duct, and pancreatobiliary reflux: Regurgitation of pancreatic juice into the common bile duct)[5,6].
Given that the hydropressure within the pancreatic duct is usually greater than that in the bile duct, pancreatic juice frequently refluxes into the biliary duct in PBM[7-9]. Pancreatobiliary reflux can be examined by several methods, and it has become obvious that this reflux can occur in individuals without PBM. Recent advances in diagnosis of pancreatobiliary reflux and its clinical implications were reviewed.
DIAGNOSIS OF PANCREATOBILIARY REFLUX
In PBM patients, pancreatic enzymes, especially amylase, are generally at extremely high levels in the bile within the bile duct and gallbladder obtained percutaneously or immediately after laparotomy[5,10]. It has been reported that, in PBM patients, the amylase level in the bile of the gallbladder was 123.568 ± 180.827 IU/L (mean ± SD), and the amylase level in the bile of the bile duct was 99.018 ± 162.506 IU/L[11]. Levels close to or below the serum amylase level are rarely observed in patients with PBM. However, the normal upper limit of the bile amylase level is unknown.
Pancreaticobiliary reflux in PBM patients can be visualized radiologically using secretin-stimulated dynamic magnetic resonance cholangiopancreatography (MRCP)[12,13]. In normal pancreaticobiliary dynamics, the extrahepatic and intrahepatic bile ducts show no change following secretin injection. On the other hand, in PBM patients, the volume of the extrahepatic bile duct and the gallbladder increases due to regurgitation of pancreatic fluid secreted after secretin injection into the bile duct[12,13]. However, since bile is also secreted after secretin stimulation, enlargement of the gallbladder may imply pancreatobiliary reflux, bile secretion, or both[14].
Pancreatography via the minor duodenal papilla can also demonstrate pancreatobiliary reflux in PBM patients. The contrast medium injected endoscopically via the minor duodenal papilla refluxes into the bile duct through a long common channel without outflow into the duodenum[15].
PANCREATICOBILIARY MALJUNCTION
PBM is a congenital anomaly defined as a junction of the pancreatic and biliary ducts located outside the duodenal wall, usually forming a markedly long common channel (≥ 15 mm) (Figure 1)[5,6]. PBM can be divided into PBM with biliary dilatation (congenital choledochal cyst) and PBM without biliary dilatation (maximal diameter of the bile duct ≤ 10 mm). In PBM patients, since the pancreatic duct and bile duct are joined outside the duodenal wall, the action of the sphincter of Oddi does not functionally affect the junction. Therefore, continuous reciprocal reflux between pancreatic juice and bile occurs, resulting in various pathological conditions in the biliary tract and pancreas[5,6,15].
Given that the hydropressure within the pancreatic duct is usually greater than that in the bile duct, pancreatic juice frequently refluxes into the biliary duct[7-9], which results in a high incidence of carcinoma in the biliary tract. According to a nationwide survey[16] performed in Japan, cancer of the biliary tract was found in 278 (17%) of 1627 of patients with PBM, which was distinctly higher than the incidence of biliary tract cancer in the general population (0.26%-1.8%). In 1239 patients with PBM with biliary dilatation, the occurrence rate of biliary cancer was 11% (131/1239), and cancer of the gallbladder was seen in 85 (65%) out of the 131 patients. In 388 patients with PBM without biliary dilatation, biliary cancer occurred in 147 patients (38%), and 93% (137/147) of the associated biliary cancers were gallbladder cancers.
The etiology of gallbladder cancer in patients with PBM has not been fully clarified. In PBM patients, gallstones do not appear to contribute to carcinogenesis, based on the low rate of gallstone detection[17]. The age of patients with gallbladder cancer associated with PBM without biliary dilatation is significantly younger than in those without PBM[17,18]. The mechanism of carcinogenesis in PBM is considered to be related to stagnation of refluxed pancreatic juice into the biliary tract. Refluxed proteolytic pancreatic enzymes and phospholipase A2 are activated in the biliary tract. Phospholipase A2, which has a direct proliferative effect on the gallbladder mucosa, produces lysophosphatidylcholin, which has a cytotoxic effect[19]. These agents may injure the epithelium of the biliary tract and induce metaplasia or promote cancer progress.
Epithelial hyperplasia of the gallbladder has been reported to be a characteristic pathologic change in PBM patients. The incidence of epithelial hyperplasia of the gallbladder associated with PBM reportedly ranges from 39%[20] to 63%[21], and it is as high as 91%[22] to 100%[23] in PBM without biliary dilatation. Tanno et al[22] have reported that the Ki-67 labeling index of epithelial hyperplasia of the gallbladder with PBM was 6.1% ± 1.5% (mean ± SD), which was significantly greater than the index of 1.2% ± 1.0% in control gallbladder mucosa without PBM. K-ras mutations in the non-cancerous gallbladder epithelium have been detected in 22%[22] to 50%[24] of PBM patients. Considering that increased cell proliferation is linked to the development of cancer by means of tumor promotion and an increased rate of random mutations, the gallbladder mucosa of PBM patients can be considered to be a premalignant region.
The treatment of choice for PBM with biliary dilatation is prophylactic flow-diversion surgery (bile duct resection and bilioenteric anastomosis) before malignant changes can take place in the biliary tract[25]. Although a standard treatment protocol for PBM without biliary dilatation has not been established, prophylactic cholecystectomy is performed in many institutes, as most biliary cancers that develop in cases of PBM without biliary dilatation are gallbladder cancers[16,18].
HIGH CONFLUENCE OF PANCREATICOBILIARY DUCTS
There are some cases with a relatively long common channel that are not classified as PBM because the sphincter of Oddi includes the pancreaticobiliary ductal junction.
Sterling[1] have reported that it varies from 1.2 mm to 8.4 mm, averaging 4.4 mm. Rienhoff and Pickrell[26] have reported that 92 (53%) of 173 cases had a common channel length within 2 mm, 62 (36%) had a common channel length ranging from 3 mm to 5 mm, and 19 (11%) had a common channel length > 6 mm. Based on the above findings, to investigate the clinical significance of a relatively long common channel, we defined a high confluence of pancreaticobiliary ducts (HCPBD) as a common channel length ≥ 6 mm, in which the communication was occluded when the sphincter was contracted (Figure 2)[27-29].
In a series of 3459 patients who underwent endoscopic retrograde cholangiopancreatography (ERCP) in our hospital, 74 patients (2.1%) had PBM, including 41 with biliary dilatation (congenital choledochal cyst) and 65 (1.9%) with HCPBD. Although PBM occurred predominantly in females, there was no difference between genders in patients with HCPBD. The average age at the time of diagnosis was significantly younger in PBM patients with biliary dilatation. Reflux of contrast medium into the pancreatic duct was detected in 12 (86%) of 14 patients with HCPBD who underwent postoperative T-tube cholangiography. The average bile amylase level was elevated to 47 774 IU/L in seven HCPBD patients, but it was lower than that of PBM patients. Compared to controls, the incidences of gallbladder cancer were significantly higher in PBM patients with biliary dilatation (17%) and without biliary dilatation (73%), and HCPBD patients (11%). The average age at the time of diagnosis of the gallbladder cancer patients with HCPBD (64.5 years old) was between that of the PBM patients without biliary dilatation (57.0 years old) and patients without these maljunctions (69.5 years old). The incidence of gallbladder stones in conjunction with gallbladder cancer associated with HCPBD (14%) or PBM (6%) was significantly lower than in those with gallbladder cancers without these maljunctions (62%). Similar to PBM patients without biliary dilatation, hyperplastic change of the gallbladder mucosa with increased epithelial cell proliferative activity was detected in cases of HCPBD. Furthermore, K-ras mutations of the non-cancerous epithelium of the gallbladder were detected in five (28%) of 18 HCPBD cases. A relatively long common channel, as well as a long common channel with PBM, appears to be an important risk factor for the development of gallbladder cancer. However, since there are several differences in gender, age at diagnosis, bile amylase level, and incidence of associated gallbladder cancer between HCPBD and PBM patients, HCPBM should be treated at present as an entity separate from PBM. Although further research is necessary to determine the appropriate management, including prophylactic cholecystectomy, of patients with HCPBD, clinicians should be vigilant regarding the development of gallbladder cancer in such patients.
PANCREATOBILIARY REFLUX IN INDIVIDUALS WITH A NORMAL PANCREATICOBILIARY JUNCTION
By demonstrating elevated levels of pancreatic enzymes in bile sampled from the common bile duct or gallbladder, or by MRCP evidence of biliary duct dilatation after secretin injection, it has been recently recognized that pancreatobiliary reflux can occur with normal pancreaticobiliary junction.
High bile amylase levels are found in some patients without PBM. Anderson et al[30] have reported that the bile amylase level obtained through an indwelling T-tube was higher than the serum amylase level in 21 (81%) of 26 patients with biliary tract disease, and that bile amylase level fluctuated considerably in the same patient. Therefore, they have suggested that intermittent reflux of pancreatic juice might initiate inflammatory changes in the gallbladder and could play a role in gallstone formation by altering the constituents that maintain cholesterol in a soluble state. A high bile trypsin level has also been reported in patients with bile duct stones[31]. Itokawa et al[32] have reported that the amylase level in the bile obtained during ERCP was higher than the serum amylase level in 22 (26%) of 86 patients, and the rate of a high amylase level in the bile was significantly higher in patients who were elderly, had a dilated common bile duct, and in those who had choledocholithiasis. Recently, Sai et al[33] have demonstrated enhanced visualization of the intrahepatic and extrahepatic bile ducts and gallbladder with increased maximal diameter of the extrahepatic bile duct and short axis of the gallbladder on secretin-stimulated dynamic MRCP in four (5%) of 74 patients who had a normal pancreaticobiliary junction on ERCP. The bile amylase level was markedly elevated in all four patients, and three of these four patients had gallbladder cancer. This would suggest that there is a relationship between pancreatobiliary reflux in individuals with a normal pancreaticobiliary junction and gallbladder cancer.
Pancreatobiliary reflux can also occur in cases of sphincter dysfunction[30,34], periampullary diverticula[35], as well as after endoscopic sphincterotomy[36] or endoscopic papillary balloon dilatation[37]. Pancreatobiliary reflux in many cases with normal pancreaticobiliary junction seems to be caused by dysfunction of the sphincter of Oddi. Furthermore, unlike in PBM, pancreatobiliary reflux in individuals with a normal pancreaticobiliary junction occurs not continuously but transiently. Carcinogenesis in the biliary tract is strongly related to stagnation of bile intermingled with refluxed pancreatic juice. Since, in individuals with a normal pancreaticobiliary maljunction, the pancreatic juice refluxes into the common bile duct and is cleared rapidly without stasis, the occurrence of gallbladder cancer poses a problem in these cases. It seems rather obvious that pancreatobiliary reflux might be related to carcinogenesis of the gallbladder. However, the clinical relevance of pancreatobiliary reflux in individuals with normal pancreaticobiliary junction is unknown. Further prospective clinical studies are needed to determine which patients require evaluation of biliary amylase levels and prophylactic cholecystectomy.
CONCLUSION
Pancreatobiliary reflux can be examined using various methods, and it has become clear that reflux can occur in some individuals without PBM. Although the true prevalence and the mechanism of pancreatobiliary reflux in individuals without PBM are unclear, the reflux might be related to biliary carcinogenesis even in individuals with a normal pancreaticobiliary junction. More cases need to be studied in order to determine the clinical implications, including appropriate management.
Peer reviewer: Tom H Karlsen, MD, Institute of Immunology, Rikshospitalet University Hospital, N-0027 Oslo, Norway
S- Editor Cheng JX L- Editor Logan S E- Editor Lin YP